> Medicine Group. 2020 Dec 16;1(8):421-426. doi: 10.37871/jbres1173.
Measurement of Photon Matter Interaction Parameters for some Iodine Compounds
Adnan Küçükönder1 and Saniye Tekerek2*
2Vocational School of Health Services, Kahramanmaraş Sutçu Imam University, Turkey
- X-ray fluorescence
- Mass attenuation coefficient
- Effective atomic number
- Electron densitiy
- Kerma
In this study, total atomic cross-section (σta), total moleculer cross-section (σtm) total electronic cross-section (σte), effective atomic number (Zeff), effective electron density (Neff) and Kerma (K) were determined both experimentally and theoretically values for some iodine compounds. Experimental mass attenuation coefficient (µ/ρ) values for some iodine compounds were calculated with the data obtained from the test results. The theoretical mass attenuation coefficient values of these compounds were calculated with the WinXCOM data program. Also, we have performed the measurements for the calculations of experimental values mass attenuation coefficient using direct transmission experimental geometry. The transmission photon intensity of halogene iodine compounds were measured in a narrow beam experiment geometry was used 59.543 keV γ-ray from an 241Am radioactive source. The tranmissions spectra from iodine compounds were recorded with a Si (Li) detector having a resolution of 155 eV FWHM at 5.9 keV (55Fe) and coupled to a 1024 channel analyzer through a spectroscopic amplifier.
This study was provided that new insights into the literature since mass attenuation coefficient experimental values of some I compounds have not been determined previously. More research should be done to observe the changes in the chemical structure of iodine compounds with gamma-ray interaction. This study will shed light on further research.
With the development of technology the using of radiation has been increased in the area of medicine and industry. The study of the interaction of gamma radiation with matter has become an important subject in the field of nuclear medicine, radiation protection, radiation physics and chemistry. The mass attenuation coefficient is an important parameter in the field of photon interaction with compounds. The mass attenuation coefficient for different energies and various samples in solid/ liquid/mixtures and alloys was reported by many researcher [1-3]. The mass attenuation coefficient is helpful in deriving many other photon interaction parameters such as molecular cross section, atomic cross section, effective atomic number and effective electron density. The mass attenuation coefficient is a quantity which defineds the extent to which the intensity of gamma photons is reduced as it penetrate the compounds [4]. The mass attenuation coefficients of materials are used prominently in various applications of medical physics, nuclear fields, radiation shielding, radiotherapy, medical applications [5,6].
The purpose of this study were carried out to determine the mass attenuation coefficients and other attenuation parameters for some iodine compounds. XCOM program was used to teoric compute the samples involved. This program is based on the mixture rule [7]. When the material consists of a chemical compound or of a homogeneous mixture mass attenuation coefficient is given by the fractional weighted sum of the mass coefficients of the components [8]. This is known as the rule-of-mixture [9]. This method is widely used in literature to calculate the mass attenuation coefficients for different samples [10]. However, in previous literature studies, the mass attenuation coefficient values for iodine compounds has not been determined experimentally using this method. The experimental data were compared with the theoretical results which were calculated with XCOM program. The values were used to calculate the effective atomic numbers and the electron densities for the iodine compounds. Likewise the effect of iodine concentration on the attenuation parameters of iodine were discussed for iodine compounds. At the same time for the interpretation of high energy photon attenuation via compound samples, knowledge of (Zeff) is obligatory [11,12]. Another important aspect of Zeff is that it allows the atomic numbers of the compounds to be determined [13]. Several investigators calculated the effective atomic number (Zeff) for some compounds [5,13,14]. The studies of photon interaction with matter in terms of mass attenuation coefficient, effective atomic number, and effective electron density led to develope many materials for various applications [14,15]. Also, diverse gamma shielding materials were investigated in the terms of photon interaction parameters [16].
The effective atomic number is a necessary to understand the weakening of X-rays and gamma photons in compounds [17]. The effective atomic number is a numerical informing us about the caracteristic properties of compounds [18].
Another important parameter calculated was Kerma. Kerma depends on the medium and the mass attenuation coefficient of the material [19]. As known that the value of the Kerma parameter is important in radiation applications [20]. Given working in the air environment, the charged particle transfers energy to the air through ionizations [21].
This study has been used in iodine compounds and is important because of its wide use in applied fields such as radiation physics, health physics and industry [22]. There are various kinds of iodine compounds in nature but in this study some particularly specific iodine compounds were taken into consideration. Iodine (I2) has been used to disinfect water supplies [23]. Sodium Iodate (NaIO3) is a medication supplement [24,25]. As a medication it is used to treat hyperthyroidism, in radiation emergencies, and to protect the thyroid gland. Potassium Iodide (KI) is used for the iodization of salt [25] and in cough medicines. Methyleneiodide (CH2I2) is used in the determination of the refractive indices of minerals [23].
The aim of this study is to determine molecular cross-section, atomic cross-section, electronic cross-section, effective atomic numbers, effective electron density and experimental and theoretical measurement of Kerma by using mass attenuation coefficient for some compounds of iodine elements.
Photon matter interaction parameters was studied in this article (molecular cross section, atomic cross section, effective atomic number [26], effective electron density) was determined using mass attenuation coefficients [4]. The following formulas described were used to calculations of the results.
Values of mass attenuation coefficients were used to determine the total molecular cross section by the following equation (1) [17]:
where is molecular weight, NA is Avogadro’s number, is mass attenuation coefficient.
The mass attenuation coefficient of the compound is calculated by the following equation [27]:
where, I0 is the incident radiation intensity and I is the beam intensity passing through the absorbing sample, ρ is the density of the material, x is the thickness of the compound (cm). ρx is the mass per unit area of the compound (g/cm2). Where m is the mass of the sample (g) and r is the radius of the powder sample (cm).
The total atomic cross section σta can be determined from the following equation (2) [17]:
where; σta is total atomic cross section, σtm is total molecular cross section, ni is total number of elements.
The total electronic cross section (σte) for the individual element is expressed by the following formula (3) [17]
where; fi is fractional abundance of element i with respect to. Ai is atomic weight, Zi is atomic number of the elements that make up the compound respectively.
The effective atomic number (Zeff) is calculated using formula (4) [17]:
The effective electron density Neff can be derived from formula (5) [28]:
In this formula, Neff is the effective electron density, is mass attenuation coefficient of the compound, σte is the total electronic cross section.
Kerma value is obtained by proportioning the mass attenuation coefficient of the compound and mass attenuation coefficients of the air. Kerma for a compound can be expressed as [19]:
Here; is mass attenuation coefficient of the compound, is mass attenuation coefficient of the air.
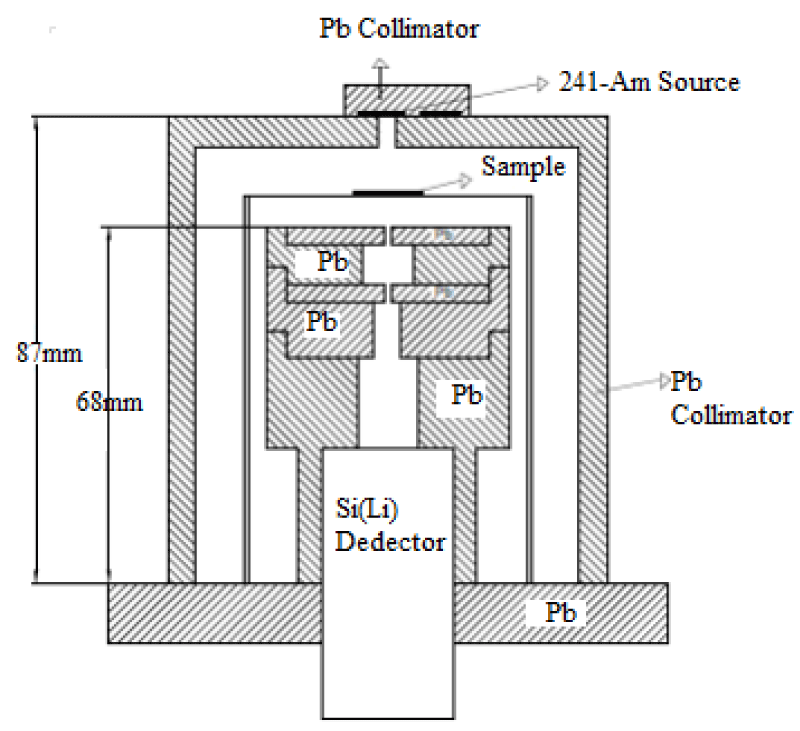
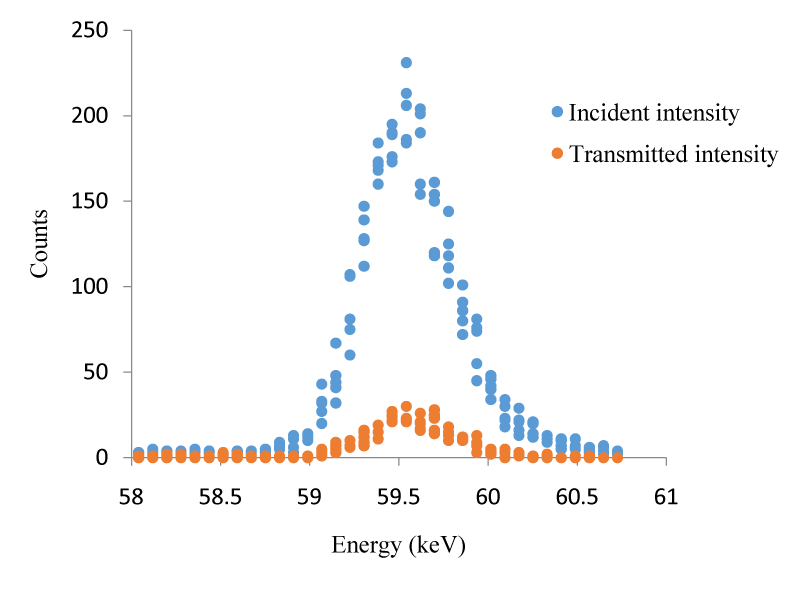
In the first stage of our study the mass attenuation coefficient for some I compounds were calculated. In the present study, a semi solid state detector cooled to liquid nitrogen temperature was used to detect gamma rays passing through the sample. The target compounds were excited by using 59.543 keV photons. In this study the mass attenuation coefficient of this compounds were measured in a narrow beam experiment geometry. The narrow beam transmission method was used to achieve the mass attenuation coefficient. Transmission spectra were measured with a Si (Li) detector with a resolution of 155 eV at 5.9 keV. Digitized Information using the multi-channel analyzer generate the spectrum. Information on this spectrum was recorded on the computer software. For healthy measurements each sample was sieved through 200 mesh and all samples were measured using the same time. The thickness of the specimen was an important parameter in gamma ray interaction for compounds. Samples with a mass thickness of 0.0224-0.1975 g/cm2 were homogenously placed on the Mylar film. The experimental setup used in the present study was shown in Figure 1. Detector shielded with 3 cm thick collimator of lead in order to prevent external background radiation from the environment is shown in figure 1. When a well-collimated narrow beam of photons passes through a homogeneous sample of Mass Thickness (x), the Incident Beam Intensity (I0), the ratio of the transmitted beam Intensity (I), are given by Lambert’s law. This equation assumes that no scattered photons reach the detector, and the collimator used was mandatory. The transmitted counts Intensity (I) r, and (I0) the incident intensity were measured under the same laboratory conditions. The absorption and non-absorption spectrum of CH2I2 was shown in figure 2 on the same chart.
In the light of the results obtained from these analytical procedures, the results were presented in this section. The experimental and theoretical mass attenuation coefficients, atomic, molecular, electronic cross sections, effective atomic numbers, effective electron densities and Kerma of compounds have been listed in tables 1-3.
The theoretical and experimental results of µ/ρ for I2 ranged from 26.46% to 39.48%. The results of NH4I compound had a minimum effective atomic number of 15.294. This might be due to the presence of the H and N element with low Z. CH2I2 compound had the maximum Zeff value was 68.855. The interaction of the high energy photons depends on the central atom (iodine) of the ligands in the compounds. Therefore, the change in the number of effective atoms of the compounds was found to be at most 71.1% and at least 0.87% for the compounds with the ligand group -NH4 and -Ca, respectively. Hg2I2 was found to have a minimum value of 2.261x1023 Neff depending on the presence of Hg. The results obtained for iodine compounds were observed to vary between 26.46% and 39.48% for Kerma compared to the results obtained for pure I. When the results were examined, it was observed that CH2I2 compound had a high Kerma value. It has been observed that KIO4 compound has a minimum kerma value with the worth of 25.026.
When the obtained results were examined, it is seen that there was a small uncertainty between experimental and theoretical values. In theory, the mass attenuation coefficient of a compound was calculated using the Bragg mixing rule method using the mass attenuation coefficients of the elements that make up the compound and the weighting factors of the component elements of the compound. These small inconsistency between experimental and theoretical values were thought to be caused by the chemical structure of the compounds and the effect of the mixing rule method [28]. The overall error in the experimental parameters were the sum of the uncertainties in diffrent factors, namely, as the evaluation of peak areas (2%-6%) and target mass thickness (1.45%-3.20%).
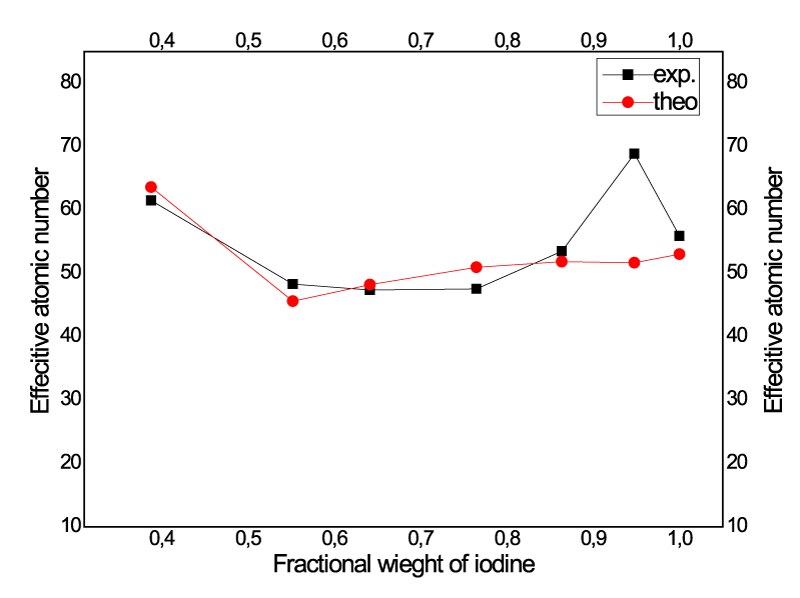
Figure 3 in the change in the number of effective atoms according to the weight fraction in iodine compounds was shown on the graph. As the amount of iodine in the compound increases, it can be seen that there is an increase in Zeff value from figure 3. At the same time given the weight fraction of iodine compounds, CH2I2 has the highest mass attenuation coefficient and the highest Zeff value with the weight fraction of 0.947629. In addition, it was been observed that the photon interaction parameters of these compounds tend to decrease with increasing iodine fractional weight. Further research should be undertaken to explore how iodine compounds is gamma and X ray shielding material. These results of the study useable for shielding applications of iodine compounds. It can be concluded that for CT scans applications the selection alternative contrast compounds can be done based on data of Zeff, Neff consideration of iodine concentration.
The values of the effective atomic number and effective electron density also depend on the atomic number of the elements that make up the compounds. These parameters are affected with the change in chemical structure of its compounds due to its nearby lying ligands. Since iodine has an unfilled outer shell, it is thought to be more sensitive to chemical effects and high energy photon interactions, and more affected by gamma and X-rays. It was observed that the effective electron density of the compounds consisting of elements having generally a small number of atoms is smaller. At the same time, an increase in Kerma value was observed as the mass absorption coefficient of the compounds increased. Moreover, it was found that the photon interaction parameters of the some iodine compounds tend to increase with increasing iodine concentration. The present method can also be extended for measurement of mass attenuation coefficient of remaining other compounds. Since this study has the nature of a manuscript study that serves as a reference to future researchers, the values obtained from the compounds studied in this study can be used as a reference in future studies.
| Table 1: The mass attenuation coefficient µ/ρ (cm2/g), total moleculer cross section σtm (cm2/molecul), total atomic cross section σta (cm2/atom) and effective atomic number theoretically and experimentally values for I compounds. | ||||||||
| Compouns | µ/ρ (cm2/gr) | σtm(cm2/molecul) | σta (cm2 /atom) | Zeff | ||||
| Experimental | Theoretical | Experimental | Theoretical | Experimental | Theoretical | Experimental | Theoretical | |
| I | 7.734 | 53 | ||||||
| CH2I2 | 9.781±0.001 | 7.339 | 435.157 x10-23 | 326.512 x10-23 | 87.031 x10-23 | 65.302 x10-23 | 68.855 | 51.664 |
| I2 | 8.153±0.002 | 7.734 | 343.726 x10-23 | 326.061 x10-23 | 171.863 x10-23 | 163.030 x10-23 | 55.871 | 53.000 |
| CaI2 | 6.982±0.001 | 6.770 | 340.839 x10-23 | 330.490 x10-23 | 113.613 x10-23 | 110.163 x10-23 | 53.465 | 51.842 |
| NH4I | 6.427±0.005 | 6.798 | 154.738 x10-23 | 163.670 x10-23 | 25.789 x10-23 | 27.278 x10-23 | 15.294 | 16.177 |
| Hg2I2 | 5.726±0.004 | 5.922 | 622.992 x10-23 | 644.317 x10-23 | 155.748 x10-23 | 161.079 x10-23 | 61.501 | 63.606 |
| KI | 5.645±0.002 | 6.048 | 155.657 x10-23 | 166.770 x10-23 | 77.828 x10-23 | 83.385 x10-23 | 47.551 | 50.946 |
| NaIO3 | 4.947±0.002 | 5.032 | 162.615 x10-23 | 165.409 x10-23 | 32.523 x10-23 | 33.081 x10-23 | 47.390 | 48.204 |
| KIO4 | 4.680±0.003 | 4.419 | 178.799 x10-23 | 168.827 x10-23 | 29.799 x10-23 | 28.137 x10-23 | 48.297 | 45.604 |
| Table 2: σte (cm2/electron), Neff (electron/gram) atomic parameters theoretically and experimentally values for I compounds | |||||
| Compounds | σte | Compounds | Neff | ||
| Experimental | Theorical | Experimental | Theorical | ||
| CH2I2 | 1.263 x10-23 | 1.263 x10-23 | CH2I2 | 7.738 x10+23 | 5.806 x10+23 |
| I2 | 3.076 x10-23 | 3.076 x10-23 | I2 | 2.650 x10+23 | 2.514 x10+23 |
| CaI2 | 2.124 x10-23 | 2.124 x10-23 | CaI2 | 3.285 x10+23 | 3.185 x10+23 |
| NH4I | 1.686 x10-23 | 1.686 x10-23 | NH4I | 3.811 x10+23 | 4.031 x10+23 |
| Hg2I2 | 2.532 x10-23 | 2.532 x10-23 | Hg2I2 | 2.261 x10+23 | 2.338 x10+23 |
| KI | 1.636 x10-23 | 1.636 x10-23 | KI | 3.448 x10+23 | 3.695 x10+23 |
| NaIO3 | 0.686 x10-23 | 0.686 x10-23 | NaIO3 | 7.208 x10+23 | 7.332 x10+23 |
| KIO4 | 0.617 x10-23 | 0.617 x10-23 | KIO4 | 7.585 x10+23 | 7.162 x10+23 |
| Table 3: Kerma theoretically and experimentally values for I compounds. | ||
| Compounds | Kerma (Ka ) | |
| Experimental | Theoretical | |
| CH2I2 | 52.304 | 39.245 |
| I2 | 43.598 | 41.358 |
| CaI2 | 37.336 | 36.203 |
| NH4I | 34.368 | 36.352 |
| Hg2I2 | 30.620 | 31.668 |
| KI | 30.187 | 32.342 |
| NaIO3 | 26.454 | 26.909 |
| KIO4 | 25.026 | 23.631 |
The study was revealed that present compounds containing high molecular weight elements are good protective materials. As indicated previously the results of the present study useable for medical applications of these compounds. In this study, it is thought that the iodine compounds studied could be used as a good gamma ray shielding material due to the high values of the mass attenuation coefficient. The results give an idea about a comparison of the investigated iodine compounds in terms of radiation shielding. It can be concluded that for computerized tomography scans applications alternative the selection of an appropriate contrast agent can be done based on data of consideration of iodine concentration. The results give an idea about a comparison of the investigated iodine compounds in terms of radiation shielding. Our current research on mass attenuation coefficients of compound photon interaction processes can guide scientists and employees in their fields by filling a gap in available information.
- Gerward L. On the attenuation of X-rays and gamma rays in dilute solutions. Radiat Phys Chem. 1996;48:697. https://bit.ly/2WkonfC
- Singh M, Mudahar GS. Energy dependence of total photon attenuation coefficients of composite materials. Int J Appl Radiat Isot. 1992;43:907. https://bit.ly/3r1iWQD
- Singh VP, Medhatc ME, Badiger NM, Abu Zayed Mohammad Saliqur Rahman. Radiation shielding effectiveness of newly developed superconductors. Radiation Physics and Chemistry. 2015;106:175-183. doi: 10.1016/j.radphyschem.2014.07.013
- Singh S, Kumar A, Singh D, Singh KT, Mudahar GS. Barium–borate–flyash glasses: As radiation shielding materials. Nuclear Instruments and Methods in Physics Research Section B. 2008;266:140-146. https://bit.ly/3qY0rN0
- Içelli O, Erzeneolu S. Effective atomic numbers of some vanadium and nickel compounds for total photon interactions using transmission experiments. J Quant Spectrosc Radiat Transf. 2004;85:115-124. https://bit.ly/3asNm8P
- Singh MP, Sandhu BS, Singh B. Measurement of the effective atomic number of composite materials using Rayleigh to Compton scattering of 279 keV gamma rays. Phys Scr. 2007;76:281-286. https://bit.ly/2Wj4Pbd
- Deslattes RD. Estimates of X-ray Attenuation Coefficients for the Elements and Their Compounds. Acta Crystallogr. 1969;25:89-93. https://bit.ly/2K7X85i
- Kerur BR, Thontadarya SR, Hanumaıah B. Anomalous x-Ray Attenuation Coefficients Around the Absorption Edges Using Mn Kα, and Cu Kα X-Rays. Appl. Radiat. 1994;45:159-163. https://bit.ly/37lJJ2g
- Morabad RB, Kerur BR. Mass attenuation coefficients of X-rays in different medicinal plants. Appl Radiat Isot. 2010;68:271-274. doi: 10.1016/j.apradiso.2009.10.033. Epub 2009 Oct 24. PMID: 19910203.
- Medhat M, Singh V. Mass attenuation coefficients of composite materials by geant4, xcom and experimental data: Comparative study. Radiat Eff Defects Solids. 2014;169:800-807. https://bit.ly/3mrm8S2
- Hine GJ. The effective atomic numbers of materials for various gamma interaction. Physics Review. 1952;85:725-737.
- Mann KS, Singla J, Kumar V, Sidhu GS. Verification of some building materials as gamma-ray shields. Radiat Prot Dosimetry. 2012 Aug;151(1):183-95. doi: 10.1093/rpd/ncr455. Epub 2012 Jan 5. PMID: 22223719.
- Murty RC. Effective Atomic Numbers of Heterogeneous Materials. Nature. 1965;207:398-399. https://go.nature.com/2K9XrfN
- Erik AA, Kavaz E, Ilkbahar S, Kara U, Erik C, Tekin H. Structural and photon attenuation properties of different types of fiber post materials for dental radiology applications. Results Phys. 2019;13:102354. https://bit.ly/3mlaM1X
- Tekin H, Altunsoy E, Kavaz E, Sayyed M, Agar O, Kamislioglu M. Photon and neutron shielding performance of boron phosphate glasses for diagnostic radiology facilities. Results Phy. 2019;12:1457-1464. https://bit.ly/2WhsJnF
- Kurudirek M, Chutithanapanon N, Laopaiboon R, Yenchai C, Bootjomchai C. Effect of Bi2O3 on gamma ray shielding and structural properties of borosilicate glasses recycled from high pressure sodium lamp glass. J Alloy Comp. 2018;745:355-364. https://bit.ly/2LCceQR
- Singh K, Singh H, Sharma V, Nathuram R, Khanna A, Kumar R, Bhatti SS, Sahota HS. Gamma-ray attenuation coefficients in bismuth borate glasses. Nuclear Instruments and Methods in Physics Research Section B. 2002;194:1-6. https://bit.ly/3oSJ66n
- Büyükyıldız M. Effective atomic numbers and electron densities for some lanthanide oxide compounds using direct method in the energy region 1 keV-20 Mev. Bitlis Eren Unv. Sci &Technol. 2016;6:7-12. doi: 10.17678/beujst.07046. https://bit.ly/37piZ0I
- Podgorsak EB. Review of Radiation Oncology Physics, A Handbook for Teachers and students department of medical physics McGill University Health Centre Montréal, Québec, Canada Internatıonal Atomıc Energy Agency Vıenna, AUSTRIA. 2003.
- Demir D, Turşucu A. Studies on mass attenuation coefficient, mass energy absorption coefficient and kerma of some vitamins. Annals of Nuclear Energy. 2012;48:17-20. doi: 10.1016/J.ANUCENE.2012.05.013
- Attix FH. The partition of kerma to account for bremsstrahlung. Health Phys. 1979 Mar;36:347-354. doi: 10.1097/00004032-197903000-00012. PMID: 489286.
- Shivaramu V, Ramprasath V. Effective Atomic Numbers for Photon Energy Dependence of Some Thermoluminescent dosimetric compounds, Nucleer Insturuments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 2000;168:294-304. https://bit.ly/2WirqF5
- Phyllıs AL. Iodine and Iodine Compounds. U.S. Bureau of Mines, Washington, DC, USA TATSUO KAIHO, Godo Shigen Co., Ltd., Tokyo, Japan. 2015. https://bit.ly/2WlY20G
- Council National Research; Studies, Division on Earth and Life; Research, Board on Radiation Effects; Incident, Committee to Assess the Distribution and Administration of Potassium Iodide in the Event of a Nuclear. Distribution and Administration of Potassium Iodide in the Event of a Nuclear Incident. National Academies Press. p.10.ISBN9780309090988. Archived from the original on 2017-09-18.
- Stwertka Albert. A Guide to the Elements. Oxford University Press, USA. p.137. ISBN 9780195150261. Archived from the original on 2017-09-14.
- Manohara SR, Hanagodimath SM, Gerward L. The effective atomic numbers of some biomolecules calculated by two methods: a comparative study. Med Phys. 2009 Jan;36:137-41. doi: 10.1118/1.3030952. PMID: 19235382.
- Gowda S, Krishnaveni S, Gowda R. Studies on effective atomic numbers and electron densities in amino acids and sugars in the energy range 30-1333 keV. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact with Mater Atoms. 2005;239:361-369. doi: 10.1016/j.nimb.2005.05.048
- Jackson FD, Hawkes DJ. X-Ray Attenuation Coefficients of Elements and Mixtures. Physics Reports. 1981; 70(3):169-233. https://bit.ly/3mo5liL
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.