> Medicine Group. 2021 Jan 29;2(1):030-033. doi: 10.37871/jbres1183.
Triggered by Covid-19? Large Vascular Occlusion Resulting in Cytokine Storm Syndrome and Kounis Syndrome: A Case Report
Ayfer Ertekin*
- Covid-19
- Cytokine storm
- Kounis syndrome
- Large vessel occlusion
Abstract
It has been widely reported that infections caused by coronaviruses, especially SARS-CoV-2 (Covid-19), can result in cytokine storm syndrome, one of the causes of acute cerebrovascular disease and ‘kounis syndrome’. An 87-year-old male patient, who did not have any chronic diseases apart from hypertensions, was admitted to our emergency department with mental fog and right-sided weakness in the absence of the typical symptoms of Covid-19 (such as fever, cough). In addition to evidence of left middle cerebral artery infarction in Computerized Tomography (CT) of the brain, there were infiltrative findings compatible with Covid-19 in thorax CT. Here, we discuss this case in the light of the literature, assuming that inflammation (cytokine storm) and hypercoagulopathy induced by Covid-19 may have presented with large vessel occlusion and kounis syndrome as a result of increased risk of arterial thrombosis.
Introduction
The primary symptoms of Covid-19 are fever, dry cough and fatigue. However, it is noted that some patients diagnosed with Covid-19 do not show typical respiratory symptoms such as fever and cough at the time of diagnosis. Some infected patients only experience neurological symptoms as initial symptoms; for instance, they may have headache, weakness, unsteady gait, malaise and fatigue, or cerebral hemorrhage, cerebral infarction and other neurological diseases, which may be nonspecific symptoms caused by Covid-19. There is an apparent need for the investigation of the frequency and type of nonspecific findings, particularly as presenting symptoms, in patients with Covid-19 [1].
In a recent study conducted on 214 Covid-19 patients, 78 (36.4%) patients were found to have neurological symptoms such as, headache, dizziness, acute cerebrovascular diseases and loss of consciousness [2]. In previous case series, there have been reports of patients with Covid-19 who developed acute stroke [3], and some estimates have suggested an ischemic stroke frequency of 0.9-2.3% in Covid-19 patients [4].
Additionally, it is well-established that coronavirus infections may result in cytokine storm syndrome, one of the causes of acute cerebrovascular disease [5] and kounis syndrome that also suggest an enhanced activation of the immune system similar to the macrophage-activation syndrome and promoting destabilizing intraplaque inflammation. Activation of the inflammation triggers atherosclerotic plaque instability as defined “Kounis Syndrome” [6].
In this case, we tried to emphasize that patients with Covid-19 may present with large vessel occlusion, since the disease increases the risk of arterial thrombosis in relation with its induction of inflammation (cytokine storm) and hypercoagulability.
Case Descriptions
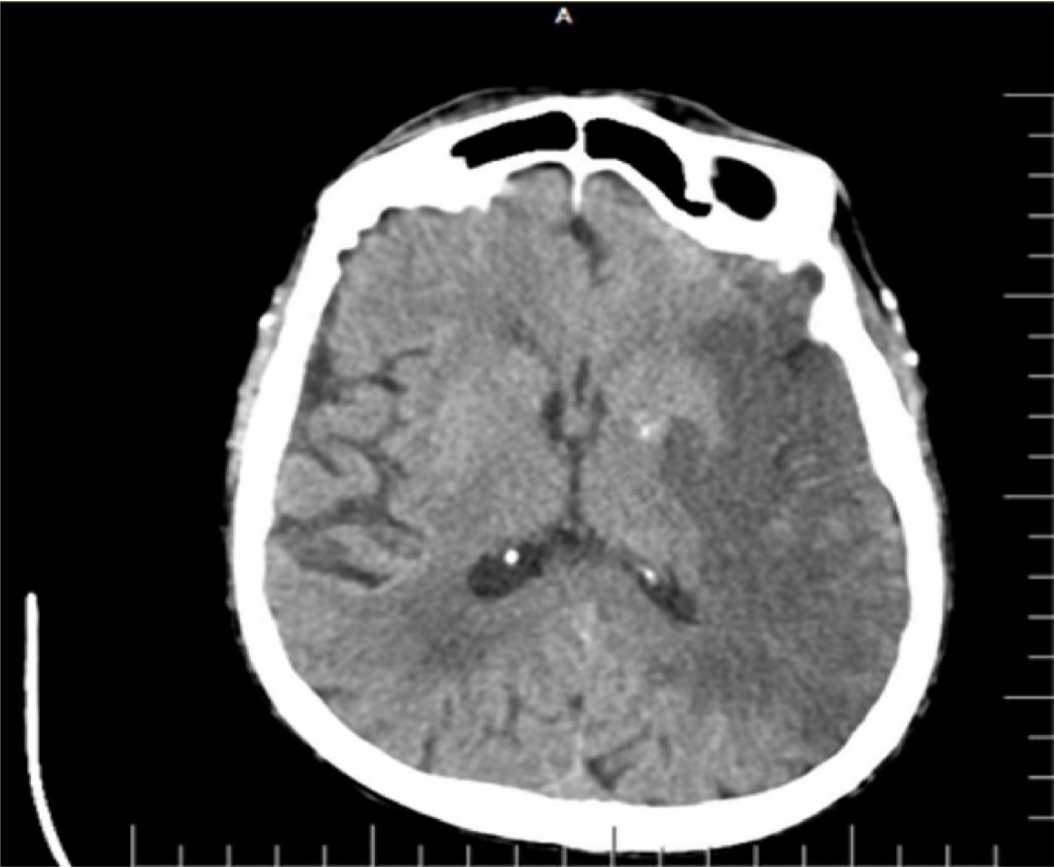
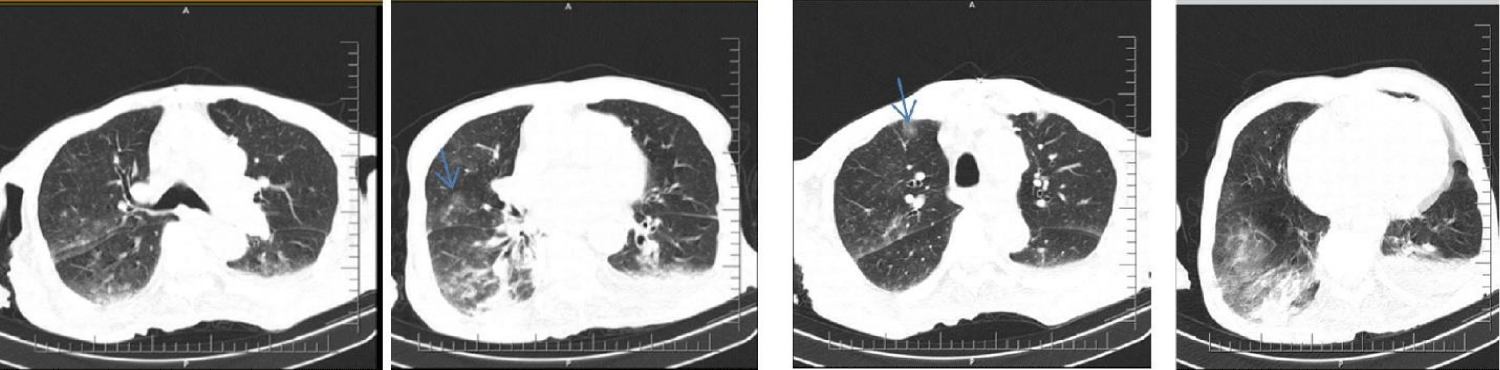
There was no known evidence of any systemic disease other than previously diagnosed hypertension in an 87-year-old male patient. He applied to the emergency department because of right-sided weakness and mental fog. In his neurological examination, consciousness state was evaluated as follows: the patient was in stupor, had no response to painful stimulus on the right, and had positive vulpian sign to the left, while Babinski sign was positive on the right. Vital signs showed a body temperature of 37.5°C and a blood pressure of 180/110. Brain CT was first requested from the patient to exclude intracerebral hemorrhage. Since Covid-19 cases are monitored in a separate unit in the department we work in, thorax CT was ordered in addition to brain CT while the patient was still in the emergency department, despite the lack of Covid-19-related symptoms and the sub febrile body temperature. In the brain CT, there was a hypo dense area compatible with infarct in the territory supplied by the Left Middle Cerebral Artery (MCA) (Figure 1). The patient, who was evaluated to have ischemic stroke after exclusion of intracranial bleeding, did not undergo any procedure since history showed that it was too late for thrombolytic treatment or thrombectomy for left MCA infarction. On thorax CT images, patchy focal infiltrations demonstrating ground glass opacity characteristics, which appeared as consolidation in some areas, were observed in all lobes of the right lung (Figure 2). Due to the pandemic, it was recommended that the patient should be evaluated as a case of Covid-19 viral pneumonia. The patient was admitted to the intensive care unit and was approached with necessary protective equipment and isolation conditions. Oxygen support was provided with a nasal mask under cardiac monitoring. Amlodipine 10 mg/day was started for blood pressure regulation. Acetylsalicylic acid 100 mg/day and Low-Molecular-Weight Heparin (LMWH) 0.6 cc 1x1 treatments were started. Esmolol infusion was initiated average 40-50 cc/hour because blood pressure increased to 220/120 mm Hg and was continued throughout the first 48 hours. After 48 hours, esmolol infusion was discontinued due to the gradual regulation of blood pressure and the medical treatment of the unconscious patient was continued with long-acting oral antihypertensive drugs administered via the nasogastric tube. Consultations were requested from the cardiology and internal medicine departments. Echocardiography revealed the following: ejection fraction = 50%, left atrium dilated, Left Ventricle Hypertrophy (LVH), mild aortic and mitral insufficiency, pulmonary hypertension + mild-moderate tricuspid insufficiency (PAP = 45 mm Hg), ascending aorta diameter = 44 mm. Electrocardiography was compatible with normal sinus rhythm. Carotid Doppler ultrasonography revealed the following: a sporadically calcified heterogeneous plaque surrounding the lumen at the proximal section of the left Internal Carotid Artery (ICA) (less than 50% obstruction according to flow), and a hypo echoic heterogeneous plaque in the lumen of the right ICA proximal section (again, less than 50% stenosis).
The 24th-hour real-time polymerase chain reaction performed with a nasopharyngeal sample was positive for SARS-CoV-2. After 24 hours, SARS-CoV2 (COVID-19) was positive as a result of PCR, and antiviral therapy and COVID-19 supportive treatment were started on the second day of hospitalization.
On the first day of his hospitalization
Alanine Aminotransferase (ALT): 49IU (normal reference (nr): 13-40), Aspartate Aminotransferase (AST): 77IU (nr: 19-48), C-Reactive- Protein (CRP): 93 (0-5), glucose: 148 mg/dl (83110), creatinine: 1.50 U/L (0.7-1.2), urea: 171.9 mg/dl (nr: 15-50), White Blood Cell (WBC): 12.90 x 103 (nr: 3.1-10), lymphocyte percentage: 11.4 (20-45%), neutrophil percentage: 77.7 (40-75%).
On the second day of his hospitalization
There was a sudden increase in cardiac enzymes and in liver and kidney function tests. The second day laboratory findings of the patient who progressed towards multiple organ failure were as follows:
ALT: 1511 IU, AST: 1391 IU, Creatine Kinase(CK): 1293 U/L, CRP: 163.3, glucose: 231 mg/dl, Troponin I: 0.6 ng/ml, urea: 353 mg/dl, uric acid: 12.0 mg/dl, WBC: 37.76 x 103, lymphocyte percentage: 1.7%, neutrophil percentage: 96.0%, D-dimer > 10,000 ng/ml (nr: 500), fibrinogen > 5.17 g/lt (nr: 2.00-4.50), potassium: 6.79 Meq/L, gamma-glutamyl transferase (GGT): 122 AIU, INR: 1.6, Prothrombin Time (PT): 21.6 second, Platelet Count (PLT): 114 x 103. A significant increase in parameters was observed, supporting the presence of severe systemic inflammatory response (cytokine storm). The patient’s treatment was added to the following: Low Molecular Weight Heparin (LMWH): 2 x 0.6 cc, oseltamivir: 2 x 75 mg/day, Plaquenil: 2 x 200 mg/day, azithromycin: 1 x 500 mg/day, and prednol: 40 mg/day (intravenous).
On the 3rd day of hospitalization
Laboratory and physical examination findings and clinical deterioration continued. Serious impairments were observed in the coagulation system and liver function tests, cardiac enzymes, and kidney function tests. Hypoxic respiratory failure was also observed in the patient whose PO2 pressure in arterial blood gas fell below 60 mm Hg with respiration rate exceeding >35/min, and the patient was not responding sufficiently to high flow nasal cannula oxygen therapy. Endotracheal intubation was performed to preserve the airway. However, the patient’s saturation continued to decrease. The patient, who had a bradycardic and hypotensive course, died on the 3rd day of hospitalization.
Results and Discussion
This case is important due to having a presentation with large vessel occlusion, possibly as a result of cytokine storm triggered by Covid-19. The pathophysiology of the Covıd-19 is not yet fully understood but studies suggest that the virus gains entry into the host through the use of Angiotensin-Converting Enzyme 2 (ACE2) as its cellular receptor. ACE2 plays an important role in the molecular pathways implicated in the development of carotid and coronary atherosclerotic plaques. Based on these pathophysiological mechanisms, it appears possible that subjects infected with SARS-CoV-2 suffer an increased risk of conversion from asymptomatic, subclinical, atherosclerotic disease into an unstable state with vulnerable plaques in the carotid and/or coronary arteries due to the immunopathology associated with the viral infection [6]. Covid-19 not only causes pneumonia, but can also damage other organs such as the heart, liver and kidneys, as well as organ systems including the circulatory and immune system [7].
The nervous system damage caused by viral infection may be mediated by the immune system. The pathology of severe viral infections is closely linked with the development of Systemic Inflammatory Response Syndrome (SIRS) [5]. Numerous fatalities are encountered due to Multiple Organ Failure (MOF) caused by SARS-CoV or SARS-CoV-2-induced Systemic Inflammatory Response Syndrome (SIRS) or SIRS-like immune disorders [8]. It has been widely reported that infections by coronaviruses, especially SARS-CoV-2, causes cytokine storm syndrome, which may be one of the factors that cause acute cerebrovascular disease [5]. In addition, patients with severe SARS-CoV-2 infection and critically ill individuals often have high levels of D-dimer and severe platelet depletion, which may predispose these patients to acute cerebrovascular events [9]. Thus, activation of the neuro-immune system can create a cytokine storm that ultimately results in inflammation and necrosis [10]. The presentation of Covid-19 infection has been associated with a dominant prothrombotic state that affects fibrinolysis and is regulated by a variety of pro-inflammatory cytokines. In a study by Mao et al., Covid-19 cases showing Central Nervous System (CNS) manifestations had significantly lower lymphocyte and platelet counts with elevated blood urea nitrogen. Also, in severe cases of Covid-19, a significantly higher C - reactive protein (CRP) and D-dimer level is seen, indicating the activation of a pro-inflammatory cascade due to cytokine (IL-1, IL-6, etc.) release [3]. The secretion of these cytokines, also termed Cytokine Release Syndrome (CRS), is closely related to development of clinical symptoms [6]. Higher levels of such pro-inflammatory markers in Covid-19 patients have been associated with more severe symptoms, worse outcomes, and more frequent neurological and cardiac symptoms [3]. Based on the present pathogenesis, we assume that SARS-CoV-2 has the potential to trigger cellular and molecular processes in coronary and carotid atherosclerotic lesions promote an increased vulnerability with subsequent increased risk of cerebral ischemic stroke or myocardial infarction.
As revealed by our case, in addition to mortality in relation with large vessel occlusion (possibly due to potential to trigger cellular and molecular processes of Covid-19 infection), patients may die due to the development of cytokine storm and “Kounis Syndrome” which can swiftly lead to multiple organ failure, shock, acute respiratory distress syndrome, heart failure, arrhythmia and renal failure.
Results and Discussion
The authors have no conflict of interest and also declare that no funding was received for the conduct of this study.
References
- Wang HY, Li XL, Yan ZR, Sun XP, Han J, Zhang BW. Potential neurological symptoms of COVID-19. Ther Adv Neurol Disord. 2020 Mar 28;13:1756286420917830. doi: 10.1177/1756286420917830. PMID: 32284735; PMCID: PMC7119227.
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020 Jun 1;77(6):683-690. doi: 10.1001/jamaneurol.2020.1127. PMID: 32275288; PMCID: PMC7149362.
- D’Anna L, Kwan J, Brown Z, Halse O, Jamil S, Kalladka D, Venter M, Banerjee S. Characteristics and clinical course of Covid-19 patients admitted with acute stroke. J Neurol. 2020 Nov;267(11):3161-3165. doi: 10.1007/s00415-020-10012-4. Epub 2020 Jun 24. PMID: 32583054; PMCID: PMC7313245.
- Mahboob S, Boppana SH, Rose NB, Beutler BD, Tabaac BJ. Large vessel stroke and COVID-19: Case report and literature review. eNeurologicalSci. 2020 Jun 16;20:100250. doi: 10.1016/j.ensci.2020.100250. PMID: 32632380; PMCID: PMC7297179.
- Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020 Jul;87:18-22. doi: 10.1016/j.bbi.2020.03.031. Epub 2020 Mar 30. PMID: 32240762; PMCID: PMC7146689.
- Saba L, Gerosa C, Wintermark M, Hedin U, Fanni D, Suri JS, Balestrieri A, Faa G. Can COVID19 trigger the plaque vulnerability-a Kounis syndrome warning for “asymptomatic subjects”. Cardiovasc Diagn Ther. 2020 Oct;10(5):1352-1355. doi: 10.21037/cdt-20-561. PMID: 33224760; PMCID: PMC7666923.
- Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, Jiang B. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020 Mar 21;395(10228):e52. doi: 10.1016/S0140-6736(20)30558-4. Epub 2020 Mar 11. PMID: 32171074; PMCID: PMC7270177.
- Yin CH, Wang C, Tang Z, Wen Y, Zhang SW, Wang BE. [Clinical analysis of multiple organ dysfunction syndrome in patients suffering from SARS]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2004 Nov;16(11):646-50. Chinese. PMID: 15535894.
- Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020 Jun;92(6):568-576. doi: 10.1002/jmv.25748. Epub 2020 Mar 29. PMID: 32134116; PMCID: PMC7228347.
- Nagel MA, Mahalingam R, Cohrs RJ, Gilden D. Virus vasculopathy and stroke: an under-recognized cause and treatment target. Infect Disord Drug Targets. 2010 Apr;10(2):105-11. doi: 10.2174/187152610790963537. PMID: 20166970; PMCID: PMC2909030.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.