> General Science. 2021 May 22;2(5):352-357. doi: 10.37871/jbres1242.
Effect of Polyethylene Oxide on the Rheological Behavior of Bentonite Suspensions
Azril N*, Gareche M, Saoudi L and Zeraibi N
- Bentonite
- Polyethylene oxide
- Viscosity
- Yield stress
- Viscoelastic modulus
- Adsorption
Abstract
The effect of Polyethylene Oxide (PEO) with a molecular weight 10000g/mol on the rheological behavior of bentonite suspension was examined in terms of viscosity, yield stress and viscoelastic modulus (G' and G''); characteristic of complex behaviour of montmorillonite in water. A Physica MCR301 rheometer has been used to measure the rheological properties of samples (6% bentonite) as well as bentonite-PEO mixtures at different concentrations of PEO (0.18%, 0.25%, 0.5% and 1%). The polyethylene oxide adsorbs onto clay particles, which changes their basic characteristics depending on the amount of PEO adsorbed.
Introduction
The bentonite clay is widely used in industrial sectors (chemicals; cosmetics; pharmaceuticals; petroleum industry). The Optimization of the process and the quality of the finished product are closely linked to the rheological behavior of suspensions; these rheological properties strongly depend on the interactions that can be established at the microscopic level between anisotropic particles dispersed in aqueous media. The influence of interactions on the rheological properties of bentonite suspensions was widely studied [1-5]; this interaction forces such as the vander Waals forces are responsible for the formation of flocs and aggregates which gives to the structure a rigid appearance (gel formation) which influences the flow properties; since the viscosity of the suspensions will depend on the flocs size and shape.
The PEO put in the suspension presents an affinity for the bentonite particles and affects its rheological properties by changing the flow shear stress; the conservation and loss viscoelastics modules. In fact; this additive can adopt several mechanisms following its nature; its molecular weight and its concentration: adsorb on the bentonite particles; diffuse in the interfoliar space and bridge the clay particles engendering a systematic flocculation [6,7]. Have shown that the addition of PEO-4000 on suspensions of Na + montmorillonite (5%) allow the gradual recovery of the particles of bentonite thus reducing interactions particle-particle and promoting interactions particle-additive which affects the viscosity and the yield stress (decrease) of the bentonite suspensions. Other work on PEO 6000 and PEO200000 has shown that the PEO influences the gelation kinetics of bentonite suspensions it can slow it down or speed it up depending on the concentration and molecular weight of the polymer added [8,9].
Experimental
Materials
The clay used in this study is a sodic bentonite its chemical formula is: Al2O34SiO2H2O; had a pH = 9 and a density of 2.4g/cm3.the chemical composition of this bentonite is given in the (Table 1).
| Table 1: Chemical analysis of bentonite. | ||||
| Chemical Element | SiO2 Al2O3 | Fe2O3 CaO | MgO Na2O | K2O TiO2 |
| Concentration % | 61.55 21.69 | 3.80 1.43 | 2.28 1.82 | 0.35 0.16 |
The polymer used in this work is Polyethylene Oxide (PEO); nonionic polymer of the polyether's family; having the chemical formula: HO-CH2-(CH2-CH2-O) n-CH2-OH with a molecular weight of 104 g/mol.
The ethylene units (CH2 - CH2) constitute the hydrophobic parts of polymer and the oxygen atom forms the hydrophilicpart of PEO. This arrangement hydrophilic- hydrophobic is responsible for the particular properties of the polymer; particularly its solubility in water.
Both of bentonite and PEO are obtained from Sigma Aldrich Company.
Samples Preparation and Rheological measurements
The bentonite-PEO preparations were performed by adopting the following experimental protocol: to the predetermined quantity of distilled water; the mass of bentonite (6%) was intimately added first. After 8 hours of stirring; the polyethylene oxide corresponding to the desired concentrations (0.18%; 0.25%; 0.5% and 1%) is introduced in the basic bentonite suspension. Homogenization is obtained by stirring for 24 hours.
The rheological measurements were performed by using a Physica MCR301 rheometer; equipped with a coaxial cylinders geometry (Ri=13.325; Re=14.4645; with gap length L=39.997) it has a peltier temperature control system which allows us to set the temperature during the tests.
To remedy the problem of water evaporation; a cell was used to keep our samples in a moist atmosphere.
Before performing the rheological measurements; each sample is stirred to obtain a homogeneous suspension. To ensure good reproducibility of the measurements; all samples studied are subject to the same mechanical history.
The steady state test consists to apply a rising ramp of shear rate (0.01 to 1000 s-) for bentonite sample alone and bentonite-PEO suspensions.
To make the connection between the suspension structure and its rheological behavior; we performed tests in shear stress sweep (for ω = 10 1/s) and frequency sweep (for τ = 0.15 Pa).
To see the influence of the concentration of PEO on the gelation kinetics of our blend samples; measures of the temporal variation of the viscoelastic moduli G ‘and G'' for mild conditions were performed (τ = 0.15 Pa; ω = 10 s-1).
Results and Discussion
Flow curve of bentonite –PEO mixtures
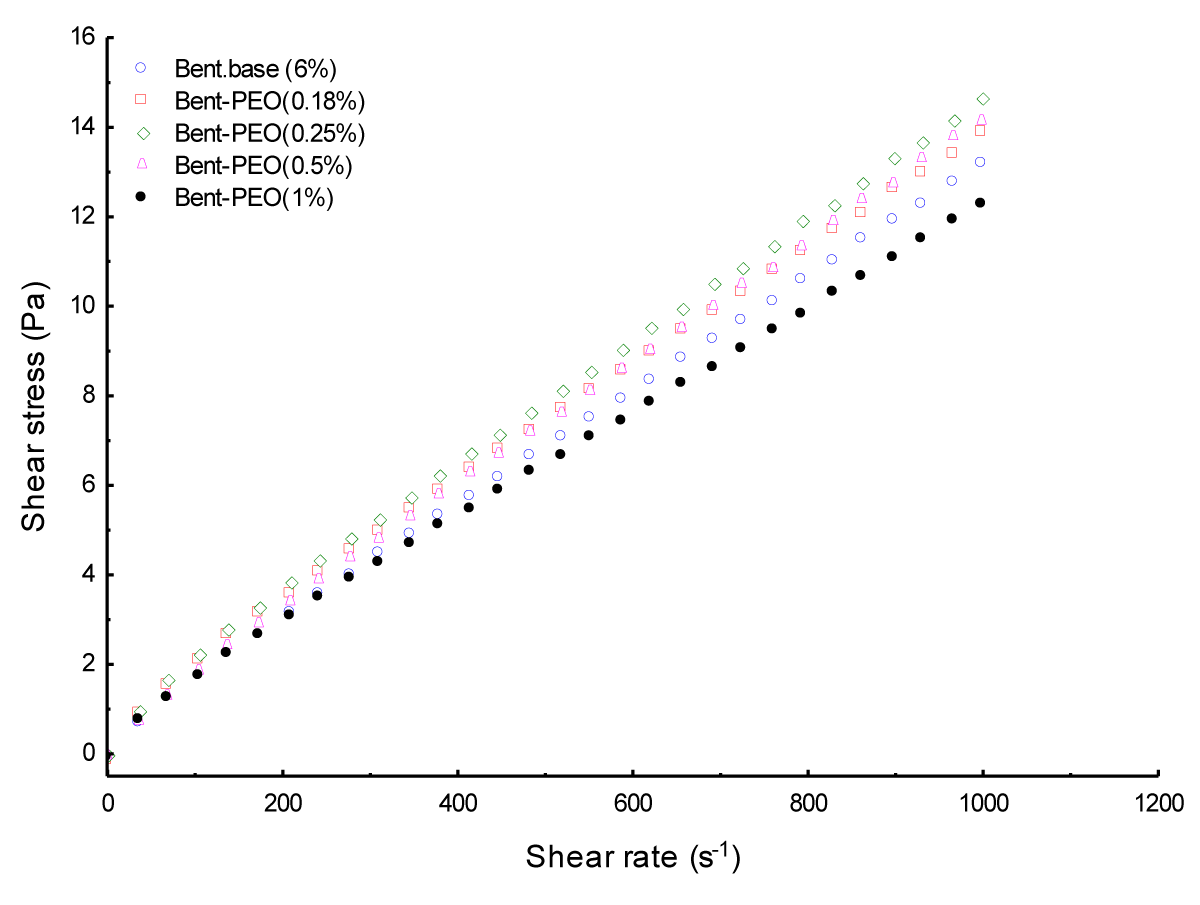
The rheological behavior of the basic bentonite suspension (6%) and bentonite-PEO mixtures at different concentrations of PEO remain shear-thinning with yield stress (Figure 1). However; the PEO causes a decrease in viscosity and yield stress of mixtures which exceeding 0.25% of PEO
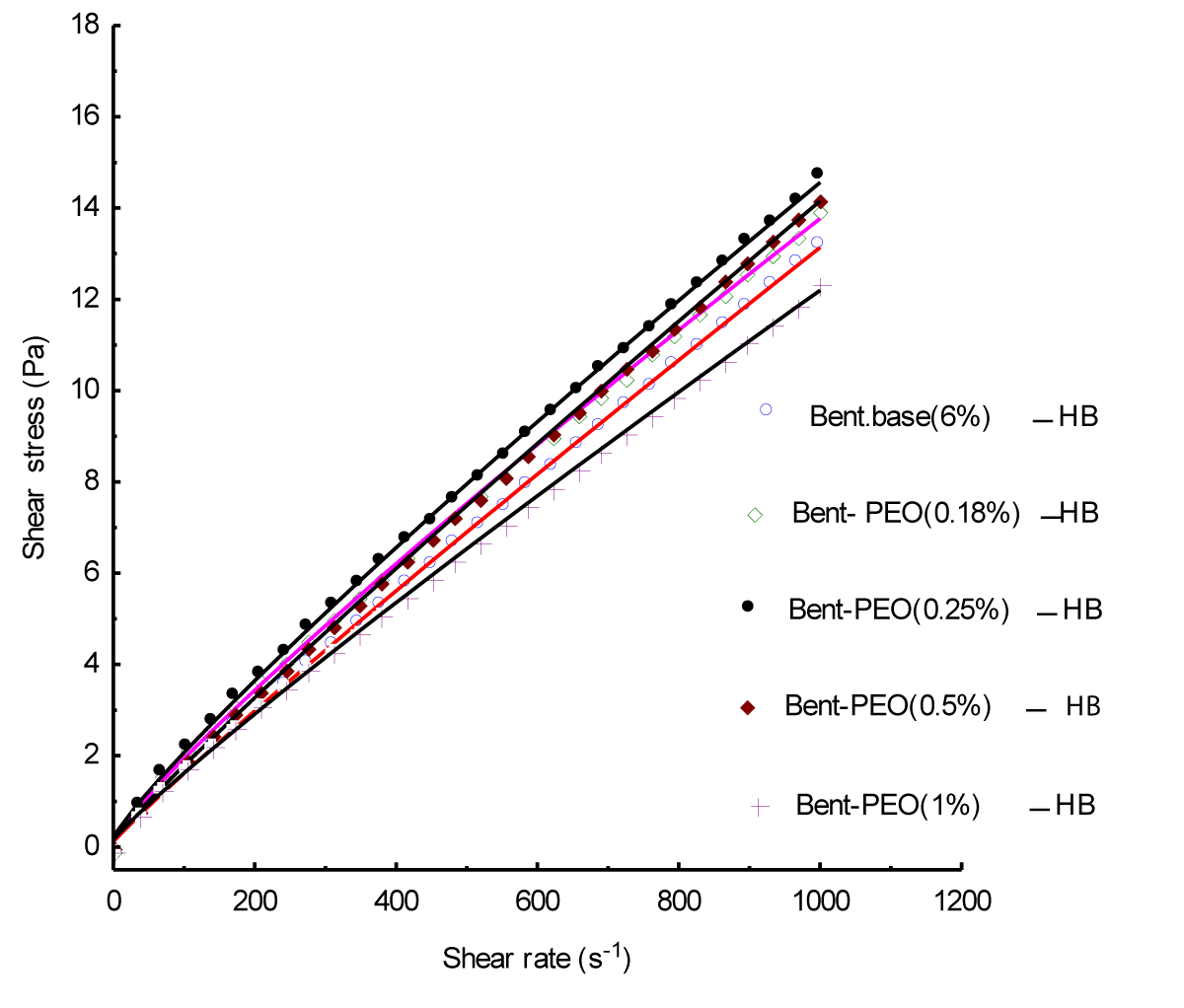
In this study; the Herschel-Bulkley model [5] is used for the correlation of experimental results in simple shear:
where τ is the shear stress (Pa) and g is the shear rate (1/s) and the rheological parameters of the model to identify are: τc yield stress (Pa); the consistency k and n index of flow (Figure 2).
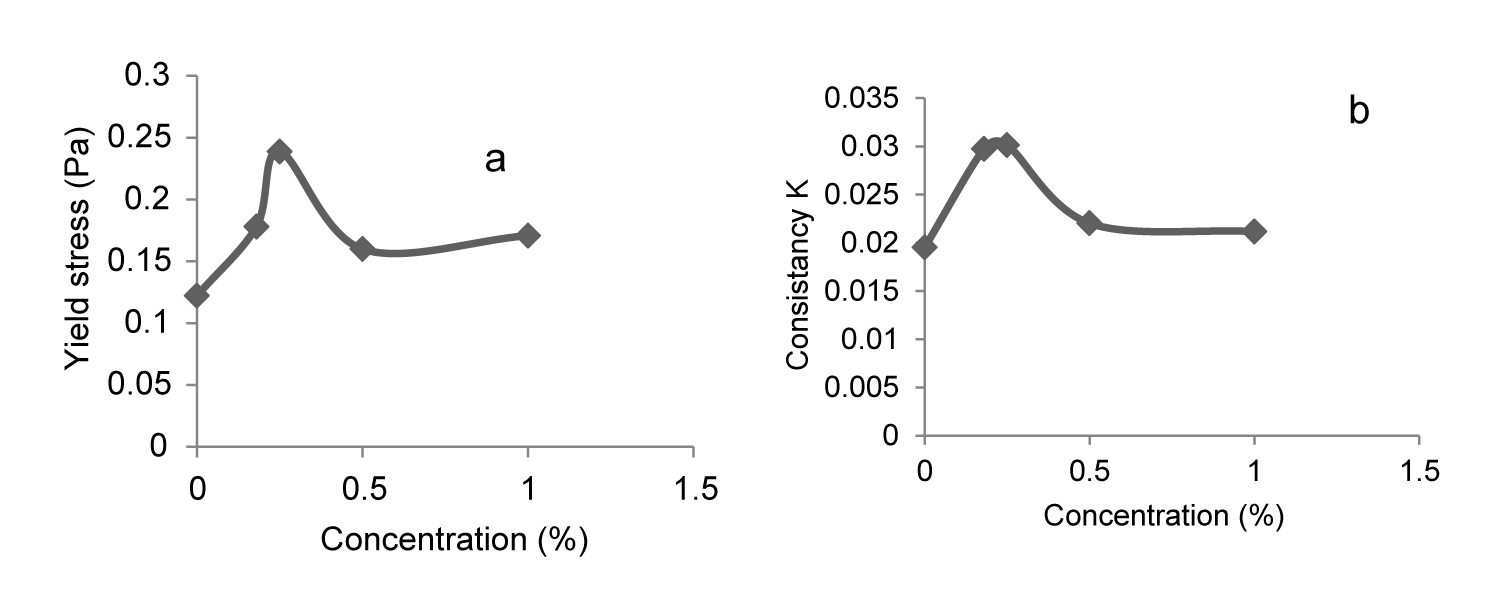
The evolution of rheological parameters (τc and k) as function of PEO concentration; is illustrated in (Figures 3a,3b).
The addition of PEO in the bentonite suspension allowing the progressive recovery of bentonite particles by the polymer there by reducing particle-particle interactions [6,7]. Beyond a critical concentration of 0.25% PEO; the additive-particle interactions (which are less rigid than the particle-particle interactions) increase which explain the decrease of the viscosity (same with k) and the yield stress.
Effect of PEO on thixotropic behavior of bentonite
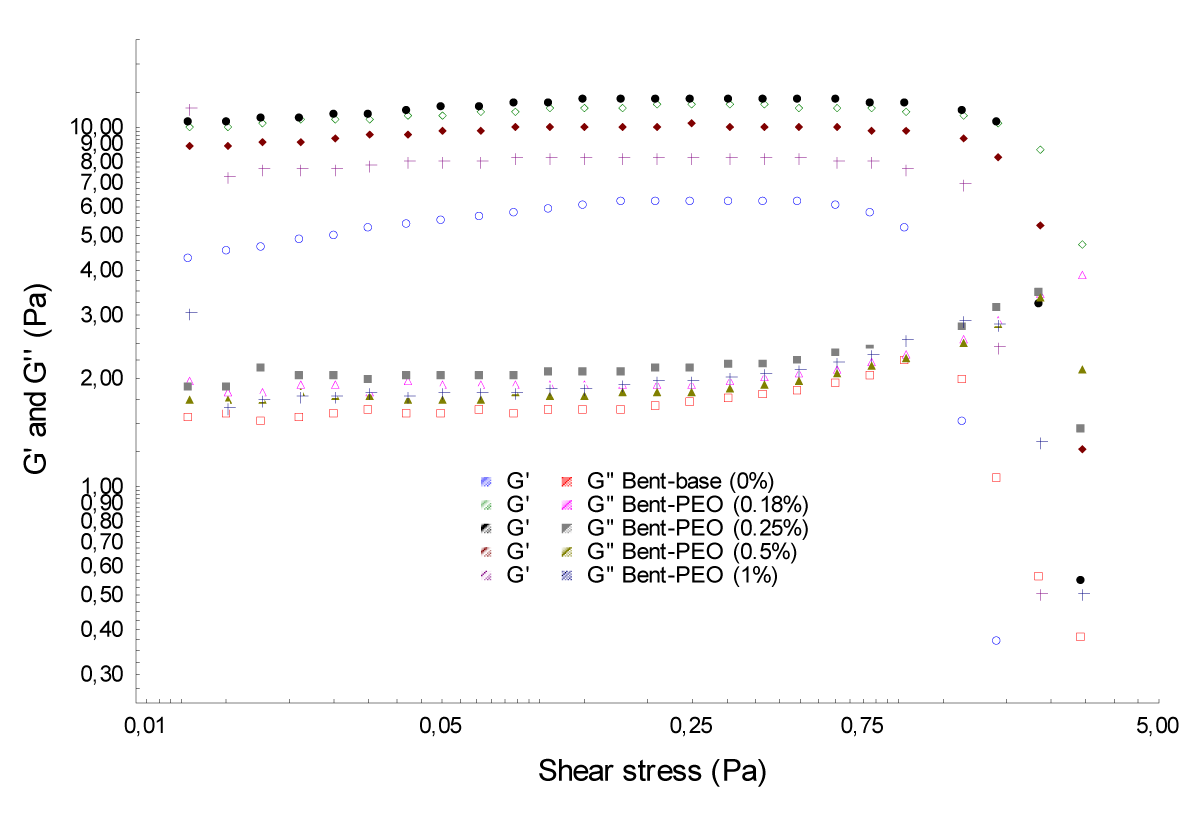
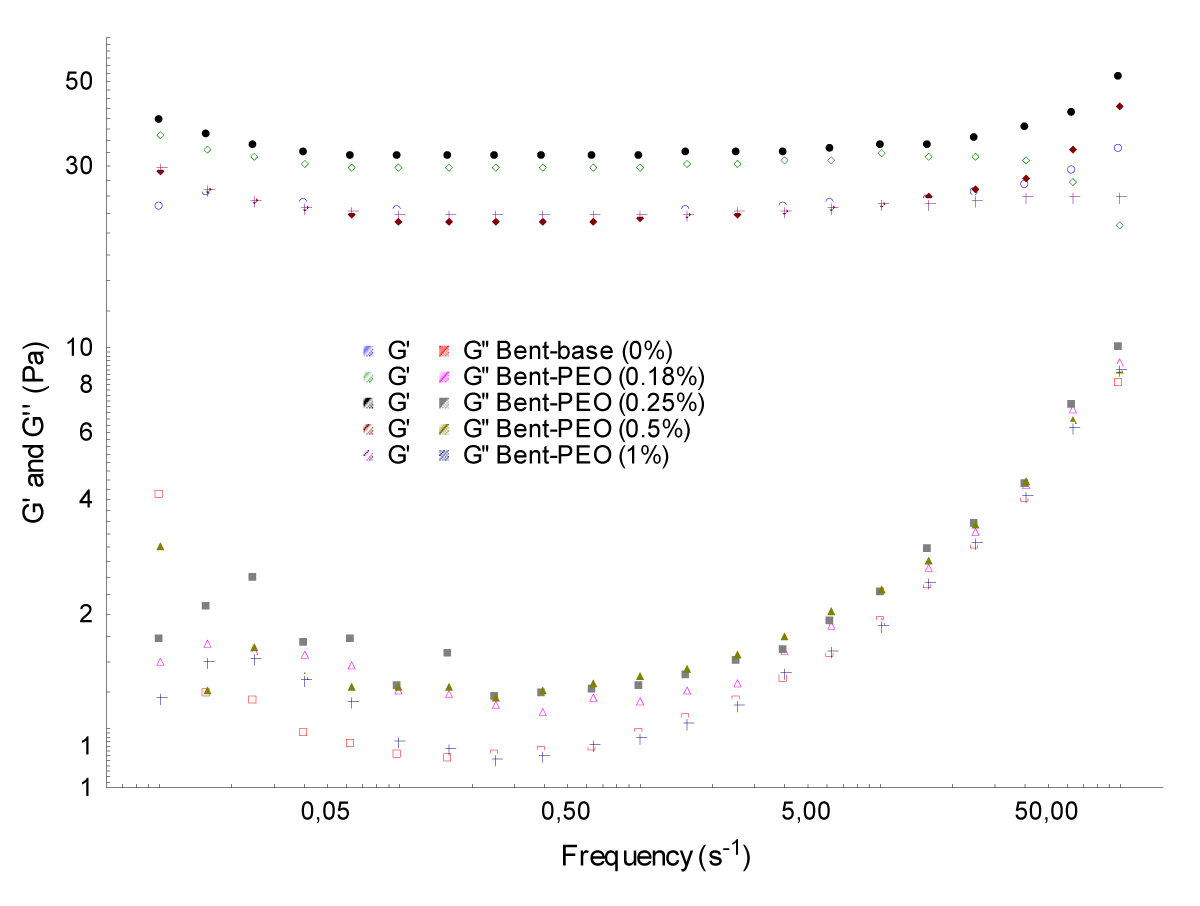
Shear stress and frequency sweep tests: (Figures 4,5) illustrate the evolution of the two moduli G' and G'' of bentonite – PEO samples as a function of shear stress and frequency.
The results obtained revealed that the storage modulus G ‘are greater than the loss modulus G'' for every concentration of PEO; it is the viscoelastic solid behavior [8,9]. Any time the modulus G' and G'' of mixtures are still higher than basic bentonite (6%).
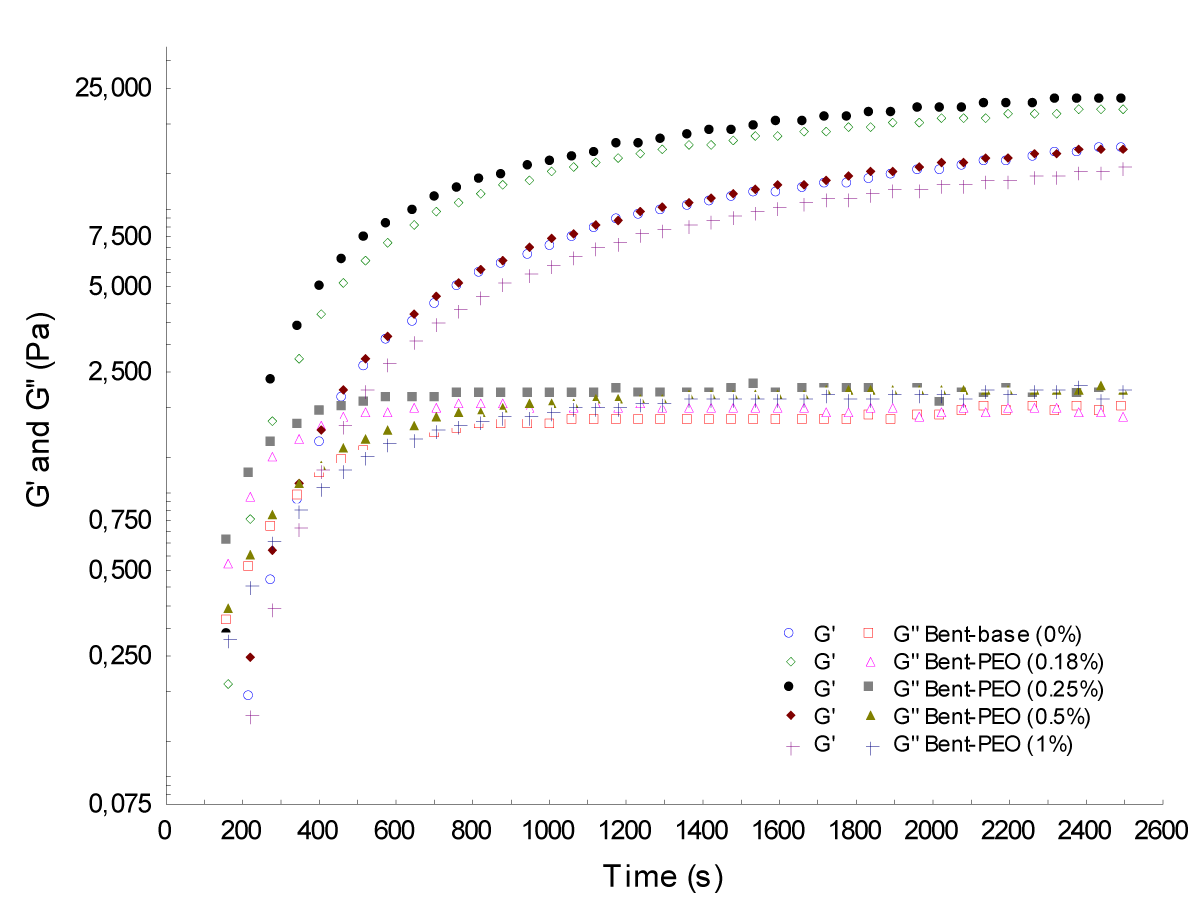
Kinetic of structure: Measurements of the temporal variation of viscoelastic moduli G ‘and G'' for low stress were performed; (Figures 6,7) shows results obtained. An evolution versus time of the viscoelastic modulus mixtures was recorded. It is found that the bentonite-PEO suspensions have two rheological behaviors: the first corresponds to samples with concentrations of PEO less than the critical one (0.25%) where a decrease in gel time has been observed (faster gelation depending on the concentration of PEO). A second concerns mixtures with PEO concentration greater than the critical (0.25%) where a slowing of the kinetics of gelation has been observed (Table 2).
| Table 2: Variation of yield stress (τc) and time of gelation as a function of PEO concentration. | ||
| % PEO | τc | Gel time |
| 0 | 0.5 | 5.69 |
| 0.18 | 0.59 | 4.19 |
| 0.25 | 0.71 | 3.69 |
| 0.5 | 0.52 | 5.69 |
| 1 | 0.5 | 6.19 |
We can interpret this behavior by the PEO adsorption mechanism on the bentonite particles. Previous work carried out on bentonite -PEO systems; [10-13] studied the influence of several parameters on the adsorption.
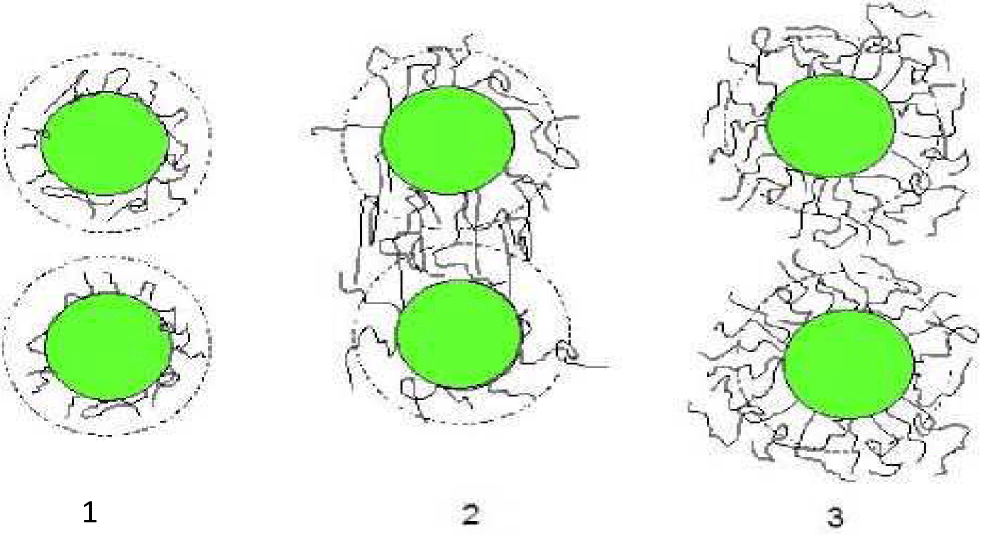
Isci and al [14-19] conducted a study on the adsorption of a nonionic polymer on bentonite particles. The polymer is polyvinyl alcohol (PVA) added at different concentrations. These authors reported that the adsorption of PVA on the surfaces of clay particles changes the load distribution of the electric double layer. Thus the stability and rheological properties of clay suspensions can be adjusted by varying the concentration of PVA added. Somewhat if we increase the polymer concentration; the flattened conformation channels is not possible due to the increase in adsorbed polymer; the thickness of polymer on the surface of the particles becomes larger than the Debye layer. The bridging of two particles by a polymer chain is possible if the surfaces are not saturated in polymer; which causes flocculation of the suspension. In this concentration range; the polymer will have a flocculating effect (in this case <0.25%). If we still increasing the PEO concentration to achieve saturation of the particle surface; such that each particle is surrounded by a polymer layer which is usually repel and in this case we have the phenomenon of steric repulsion.
The electrostatic range is shown by a dotted circle.
(1) Small quantity of adsorbed polymer adopts a flattened conformation; the polymer layer is less than the electrostatic scope; which prevents bridging between particles.
(2) If increasing the concentration; the size of polymer sloops becomes larger than the thickness of the electrostatic layer; then bridging between two particles is achieved which results in flocculation of the system.
(3) The polymer concentration in solution is such that the adsorption on the particles is maximum. Although the polymer layer is greater than the layer of electrostatic repulsion; bridging flocculation and therefore is no longer possible. Particles repel by steric repulsion.
Conclusion
In this study; we have shown the influence of the polymer PEO with low molecular weight (10000g/mol) on the rheological properties of basic bentonite suspension in steady state (viscosity and stress) and in dynamics (G' and G'').
The results obtained allow us to conclude that the PEO has two different behaviors depending on the concentration added: for c <0.25%; the addition of PEO increases the rheological parameters of the suspension (yield stress; viscosity and viscoelastic modulus) and beyond the critical concentration (0.25%) PEO has a reverse effect. The gel time of bentonite-PEO mixtures is also influenced by the concentration of PEO added (acceleration for c <0.25% and slowing for c> 0.25%). This behavior can be explained by the modification of the charge distribution of the electric double layer according to the adsorption mechanism of PEO on the clay particles.
There is a very good agreement between the results of tests in steady state and those under dynamic conditions.
References
- Rossi S, Luckham PF, Zhu S, Briscoe BJ, Tadros TF. Influence of low molecular weight polymers on the rheology of bentonite suspensions. Revue de l'institut Français du Pétrole. 1997;52(2):199-206.
- Benna M, Ariguib N, Magnin A, Bergaya F. Effect of pH on Rheological Properties of Purified Sodium Bentonite Suspensions. J Colloid Interface Sci. 1999 Oct 15;218(2):442-455. doi: 10.1006/jcis.1999.6420. PMID: 10502376.
- Almedar A, Gungor N. The rheological properties and characterization of bentonite dispersion in the presence of non-ionic polymer PEG. J of Materials Science. 2005;40:171-177.
- Ebagninin KW, Benchabane A, Bekkour K. L'empreinte pont-polymère sur le comportement rhéologique des mélanges bentonite-PEO. 41ème colloque du GFR. 2006 Oct;18-20:271-274.
- Herschel WH, Bulkley R. Konsistenzmessungvon Gummi-Benzollösungen, Kolloid Zeitschrift. 1926;39:219-300.
- Rossi S, Luckham PF, Tadros TF. Influence of non-ionic polymers on the rheological behaviour of Na+ montmorillonite clay suspensions. Part II. Homopolymer ethylene oxide and polypropylene oxide-polyethylene oxide ABA copolymers. Colloid and susfaces A: physicochem Eng Aspects. 2003a;215:1-10.
- Rossi S, Luckham PF, Green N, Cosgrove T. NMR solvent relaxation studies of Na+ montmorillonite clay suspensions containing nonionic polymers. Colloids and surf. 2003b;215:11-24.
- Gareche M, Zeraibi N,Allal A, Dupin JC, Roby F. Study of rheological properties of the system bentonite- polyethylene oxide (PEO). 6th international symposium on hydrocarbons and chemistry. Zeralda/Algérie. 2012a:14-15.
- Gareche M, Zeraibi N, Allal A, Amoura M. The influence of low molecular weight polymer on the rheological behavior of bentonite suspensions. Petroleum Science and Technology. 2012(b);30(19):1981-1989.
- Rubio J, Kitchener JA. The mechanism of adsorption of poly (ethylene oxide) flocculant on silica. Journal of Colloid and interface Science. 1976;57(1):132-142.
- Zhao X, Urano K, Ogasawara S. Adsorptions of polyethylene glycol from aqueous solution on montmorillonite clays. Colloid and Polymer Science. 1989;267(10):899-906.
- Bujdak J, Hackett E, Giannelis EP. Effect of layer charge on the interaction of poly(ethylene oxide) in layered silicates: Implications on nanocomposite polymer electrolyte. Chemistry of materials. 2000;12(8):2168-2174.
- Mongondry P. Structure et comportement rhéologique des suspensions aqueuses de Laponite en présence de plusieurs additifs. Université du Maine- Le mans. 2003.
- Isci S, Gunister E, Ece OI, GungorN. The modification of rheologic properties of clays with PVA effect. Mater Lett. 2004;58:1975-1978.
- Kauthold S, Dohtmann R. Detachment of colloidal particles from bentonites in water .Applied Clay Science. 2008;39:50-59.
- Pusino A, Gennari M, Premoli A,Gessa C. Formation of polyethylene glycol on monmorillonite by strelisation with ethyleoxide. Clays and Clay Minerals.1990;38(2):213-215.
- Finocchio E, Baccini I, Cristiani C, Dotelli G, Gallo Stampino P, Zampori L. Hybrid organo-inorganic clay with nonionic interlayers. mid- and near-IR spectroscopic studies. J Phys Chem A. 2011 Jul 7;115(26):7484-93. doi: 10.1021/jp200845e. Epub 2011 Jun 15. PMID: 21630683.
- Moreno M, Quijada A. Electrical and mechanical properties of poly (ethylene oxide)/ intercalated clay polymer electrolyte. Electrochimica Acta. 2011;58:112-118.
- Aranda P, Ruiz-Hitzky E. Poly(ethylene-oxide)-Silicate intercalation materials. Chem Mater. 1992;4:1395-1403.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.