> Medicine Group. 2021 May 27;2(5):412-417. doi: 10.37871/jbres1251.
Profile of Hepatitis B Sero Markers Among Blood Donors Attending Usmanu Danfodiyo University Teaching Hospital, Sokoto-Nigeria
Hussaini Mohammed Alhassan1*, Sa’adetu Haruna Shinkafi2, Ibrahim Yakubu1, Hamisu Abdullahi1, Ahmad Hamidu Marafa3, Abdullahi Isiyaku1 and Mustapha Umar Kalgo1
2Department of Microbiology/IHVN Centre, Usmanu Danfodiyo University, Teaching Hospital (UDUTH), Sokoto-Nigeria
3Department of Haematology, Faculty of Medical Laboratory Sciences, Usmanu Danfodiyo University, Sokoto-Nigeria
Abbreviations
Anti HBc IgG: Anti- Hepatitis B core Immunoglobulin G; Anti-HBc IgM : Anti- Hepatitis B core Immunoglobulin M; DNA: Deoxyribonucleic Acid; HBcAb: Hepatitis B Core Antibodies; HBc IgM: Hepatitis B core Immunoglobulin M; HBeAg: Hepatitis B enveloped Antibodies; HBeAg: Hepatitis B enveloped Antigen; HBIG: Hepatitis B Immune Globulins; HBsAb: Hepatitis B Surface Antibodies; HBsAg: Hepatitis B Surface Antigen; HBV: Hepatitis B virus; HBV DNA: Hepatitis B Virus Deoxyribonucleic Acid; IgG: Immunoglobulins G; IgM: Immunoglobulins M; UDUTH: Usmanu Danfodiyo University Teaching Hospital.
Introduction
Hepatitis B Virus (HBV) is of the Hepadnaviridae family, which has a double-stranded circular DNA genome [1]. The virus has proteins that form significant markers for its diagnosis and treatment. They are five (5) different proteins markers that are found in HBV, they are:
• Hepatitis B surface antigen (HBs Ag); this constituent the outer protein coat of the virus,
• Hepatitis B envelope antigen (HBeAg), this is normally shed during the viral replication
• Hepatitis B core antibodies (HBcAb): this found in the hepatocytes [2]. Others are Anti-Hbe-Aband Anti-HBs Ab.
The replicative and life cycle of this virus can be divided into four stages in the human host, stage one and two are termed the replicative phase, and the two stages are described as the integrative phase [3]. HBsAg, HBeAg and HBV DNA are detected in blood in the first stage, while Anti HBe is found to be negative. This is defined as the active period in the HBV life cycle and is described by immune tolerance where there is the presence of active viral replication occasioned by upregulations of HBV DNA and infectiousness in the host. The second stage in the life cycle is akin to the first except that the level of HBV DNA is drastically reduced and the profile of liver enzymes is found to be elevated [3]. HBV DNA is found to be negative, during the third stage, while Anti-Hbe and is detectable in blood and HBeAg is, negative as well. This is the point where host immune control is achieved and active viral replication stops, this stage is characterised by a complete clearance of the virus in the fourth stage with HBsAg becoming negative or undetectable and infectiousness of the host is drastically reduced [3]. An occult infection could be described as the presence in the liver of replicative-competent HBV DNA, with the absence of HBsAg in the serum [4]. Hepatitis B virus infection is one of the deadly transmissible blood infections, it has chronically infected over 400 million people globally [4]. The prevalence of HBV is much higher in East Asia and sub-Saharan Africa, where 5.5-20.5% of the adult population is infected [5] and 17 to 65% of the general population are found to be positive for one or more of the serological markers of HBV infection. HBV causes a spectrum of infections which often ranges from inactive chronic carrier states to progressive chronic hepatitis which could lead to cirrhosis, followed by primary liver cancer [6]. Regions with the prevalence of HBsAg above 9% are described as HBV endemic region [2]. Despite the availability of vaccines in most countries, like Nigeria, the prevalence of this virus infection is still dangerously high in Nigeria and other countries of the world, and this viral infection is an important cause of morbidity and mortality [7]. HBV is a major cause of liver disease morbidity and mortality worldwide, accounting for over 360 million cases of chronic hepatitis and 620,000 deaths per year [8]. It is hyperendemic (that is >8% of the population infected) in Sub-Sahara Africa and a major cause of chronic liver disease [9]. Estimated that 44% of cirrhotic liver disease and 47% of hepatocellular carcinoma cases in SSA are attributed to HBV [10]. Hepatitis B virus infection is arguably the most significant global public health problem [11]. Of the approximately 2 billion people infected worldwide, 300 million infected patients are chronic carriers of Hepatitis B Virus (HBV) and it is the 10th leading cause of death [12]. HBV is known as one of the first viral infection discovered to be found in the blood and transmitted mainly through blood and blood products [13]. In countries with high prevalence, HBV is seen as one of the major complications of transfusion of blood and its products [14], this is due to the facts that, there exist an extended window period between infection with HBV and the actual detection of HBsAg, within which the virus could be transmitted [14]. Researchers have shown that individual who are anti-HBc positive have the possibility of harbouring an occult HBV infection thus maintaining in their liver and blood, HBV-DNA [15]and this serves as a potential source of transmission. The mere absence or undetectable presence of HBsAg in the blood or other bodily fluids of healthy individuals may not be enough to guarantee the absence of circulating HBV, so blood and blood products containing anti-HBc should be treated as infectious, either with or without the detectable presence of HBsAg [16]. Studies from different parts of Nigeria has reported a varying prevalence rate of HBsAg among blood donors, however, information on other markers of HBV is scarce, because DNA testing of all collected units is not feasible because of the cost of running the test in Nigeria [15]. This study was therefore conducted primarily to determine the prevalence of other markers of hepatitis B virus and to evaluate the reliability of infection in screening blood donors in our transfusion centres in Nigeria using HBsAg marker alone in the diagnosis of HBV.
Since an individual with anti-HBc positive may harbour an occult HBV infection and maintain HBV-DNA sequence in their blood and liver. This presents a potential source of HBV transmission, the absence of HBsAg in the blood of apparently healthy individual may not be sufficient to ensure the lack of circulating HBV, this containing anti-HBc with or without the detectable presence of HBsAg might be an infection. This study will provide up-to-date data about the hepatitis B status of the screened donors in the UDUTH blood bank. The data obtained from this research will assist the hospital in deciding transfusion using screened donors and taking preventives measures. It creates awareness among the screened donors to go for regular hepatitis B screening. It also assists in the proper screening of donors to avoid HBV transmissions. This study aims to screen and assess the prevalence of Hepatitis B Virus (HBV) infection among screened donors attending Usmanu Danfodiyo University Teaching Hospital Sokoto using Hepatitis B sero markers cassettes.
Materials and Methods
Study area
The study was carried out in the Faculty of medical laboratory sciences in collaboration with Usmanu Danfodiyo University Teaching Hospital (UDUTH), Sokoto. Sokoto has a total population of 4.2million people [17]. The state has a landed area of 26,64km2 and is located between longitude 11.30 to 13.50 degrees east and latitude 4 to 6-degree north. It is bounded to the North by the Niger Republic, Kebbi state to the South-west and the East by Zamfara state. The state comprised of 23 local governments areas [17].
Study design
This is a descriptive, cross-sectional study design to determine the prevalence markers of HBV among prospective blood donors in Usmanu Danfodiyo University Teaching Hospital (UDUTH) Sokoto, between May 2018 to July 2019.
Determination of sample size: The sample size was calculated using the standard formula for calculating the minimum sample size (18). Sample size (n) is given by the formula below:
where;
n = Minimum sample size
Z = Standard normal deviation at 95% level of confidence = 1.96
Since there was no published work on prevalence of the cement company workers, 50% is considered as the prevalence.
P = Prevalence to be estimated is 14% =
q = Compliment of p i.e. 1-p = 1-0.14 = 0.86
d = Tolerance margin of error = 90% i.e. (100-95%) = 5% = 0.05 therefore then plus 10% alteration ratio = 14.9
149 + 14.9 = 163.9164
Inclusion and exclusion criteria
HBV negative blood donors attending UDUTH were included in the study, donors who found to be positive for the HBV screening test were excluded from the research study.
Study population
The population comprised of 170 blood donors attending UDUTH Sokoto, Sokoto state. The target population are selected at random to include HIV negative blood donors attending UDUTH, Sokoto. Blood donor with positive HBV screening test were all excluded from the research study, this is because those that are confirmed to be positive for HBV cannot serve as Blood donors and thus cannot give blood
Data collection and analysis
The socio-demographic data and other relevant information of each participant were obtained using a self-administered questionnaire, data obtained were entered into SPSS version 22 for analysis. The results obtained were presented using Tables.
Scope and limitation of the study
The study is limited to a randomized screening of 170 blood donors attending UDUTH Sokoto. The information obtained from this study will be treated with the utmost confidentiality and will only be used for this research. This work is only limited to a screening of blood donors for hepatitis B sero-markers.
Ethical consideration
Ethical clearance for the study was sought and obtained from the ethical committee of Usmanu Danfodiyo University Teaching Hospital (UDUTH), Sokoto, with the reference number, UDUTH/HREC/2017/No.630.
Sample collection
The blood sample was taken from each participant for the serological test. Informed consents were obtained from participants before the collection of the sample. A sample of blood was collected from each participant with a sterile disposable syringe and needle, after disinfection of the selected venipuncture site with 70% alcohol in an expanding circular scrub from the centre to the periphery of the needle insertion. About 5ml of blood was collected by venipuncture and was dropped into an anticoagulated bottle, the plasma was separated and used for serology.
Serological test procedure
Clinically, HBsAg, HBsAb, HBeAg, HBeAb and HBcAb are the important markers in the diagnosis of HBV infections. The onsite HBV-5 Rapid Test can detect HBsAg, HBsAb, HBeAg, HBeAb and HBcAb simultaneously. The plasma specimen and test component were brought from room temperature, thawed and mixed properly. The test component was opened from the notch, and the device was removed. The device was labelled with the specimen Identification number and 2-3drops (60-90l) plasma specimen was added into each of the sample wells in the device. The result was read within 15minute and recorded.
Results
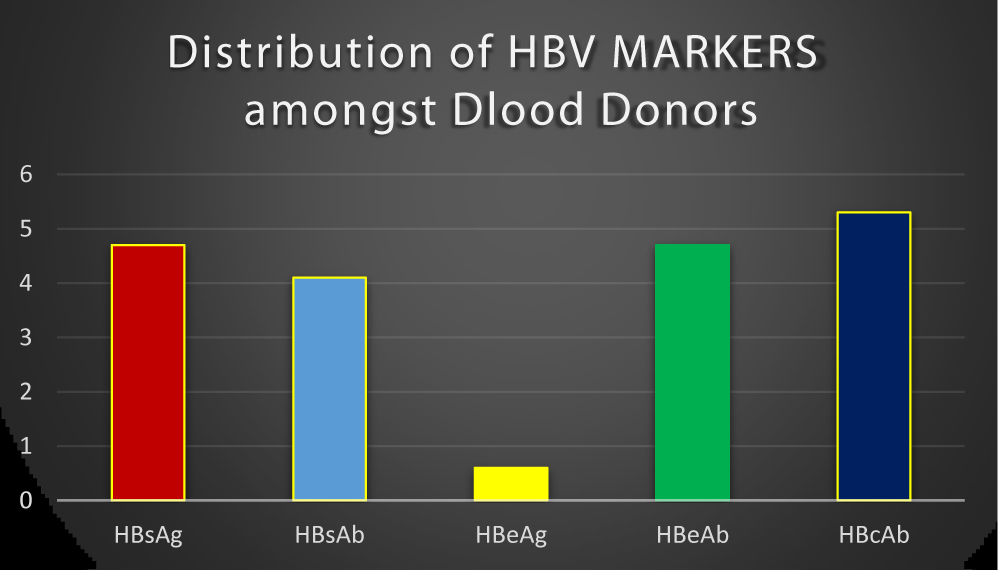
Out of 170 screened blood donors recruited for the study, the following tested positive: 8(4.7%) for HBsAg, 7(4.1%) for HBsAb, 1(0.6%) for HBeAg, 8(4.7%) for HBeAb and 9(5.3%) for HBcAb. This is as shown in figure 1.
Discussion
Hepatitis B virus infection is a major blood transmissible infections known to man with about 400 million people chronically infected globally [3]. In 170 blood samples tested, HBsAg was detected in 8(4.7%), HBeAg 1(0.6%), HBsAb 7(4.1%), HBeAb 8(4.7%) and HBcAb 9 (5.3%) respectively (Figure 1). The result of this study showed that 9(5.3%) blood donors had anti-HBc as the serological markers. This finding is in agreement with Geraldine (2006), who reported that testing blood donors for HBsAg alone is not sufficient to eliminate transfusion mediated hepatitis B viral infection. It was observed that the presence of HBV-DNA in one-fifth of anti-HBc only positive donors studied and stated that, reactivity to anti-HBc only can predict HBV infection [19]. It may be possible that some of the blood donors are in their window period and may have HBc IgM antigen at that stage when HBsAg is not detectable in the blood. The only serologic evidence of hepatitis B infection is the presence of IgM antibody to the core antigen [19]. The screening test for detection of HBsAg does not rule out the transmission of Hepatitis B as the donors might be in the window period and detection of the antibody to hepatitis B core antigen (HBc IgM), serves as a useful serological marker during the window period. A prevalence of 5.3% observed for anti-HBc in this study is high compared with 0.56% in the United Kingdom [20], 0.84% in the United State [21], 1.13% in Canadian blood donors [22], 4.85% in Italy [23]. This may due to proper awareness for HBV infection in that area than where this research was conducted. The result from this study showed lower anti-HBc prevalence compared to those obtained in Indian 18.9%, Pakistan 17.28%, Turkey 20.0% [24], 18.1% in Maiduguri, Borno State, Nigeria [15]. IgM class of the anti-HBc is a marker that indicates recent infection, IgG variety of anti-HBc appears later during the infection and points to a past HBV infection. Anti HBc IgG may remain positive for life in an infected individual, although the individual has protective levels of anti-HBs, the blood of such a donor might be free from transmitting HBV [25].
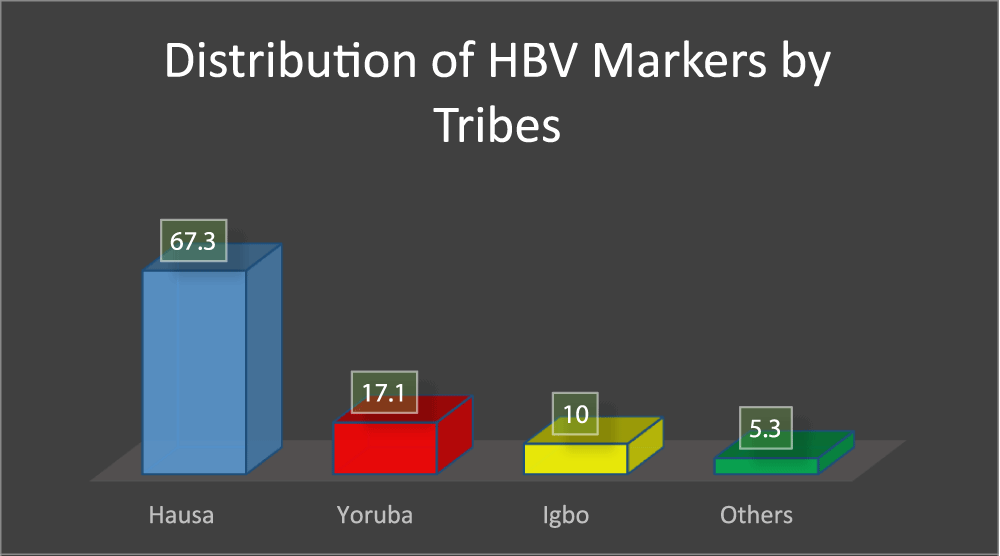
In figure 2, it was indicated that the prevalence of HBV markers among blood donors is tribe dependent, in that Hausa has 67.3% of positive markers, Yoruba has 17.1% of positive markers, Igbo has 10% of positive markers and 5.3% of the positive markers are from the other tribes, but this result could also be attributed to the fact that the research was conducted in a predominantly Hausa dominated state.
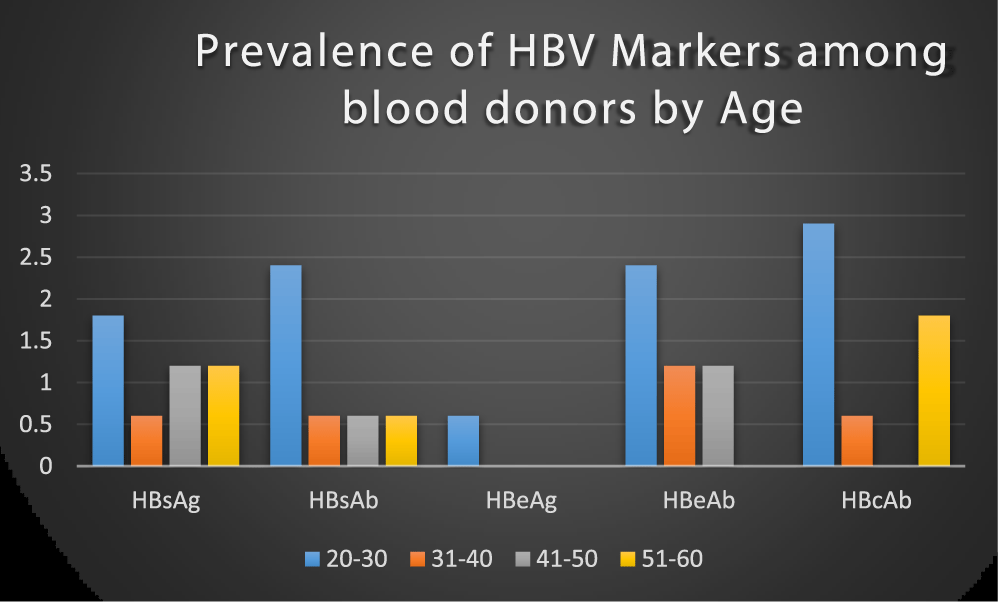
Blood donors within the age group 20-30 years had the highest prevalence of HBV markers than 31-40 years, 41-50 years and 51-60 years as shown in figure 3. This study contradicts the earlier report with a high prevalence of HBV in older subjects 40 years and above than in younger people [26] and Luka [27], in their study, reported higher HBV prevalence among the older age group 30-34 years. The finding in this study supports the work of Buseri (2009), who noted that HBV infection is more prevalent in younger subjects within ages 20-29 years [28], the possible reason for this high prevalence rate in younger people than older people may be attributed to their active sexual activities and drug abuse, however, we observed that HBV is not limited to any particular age group.
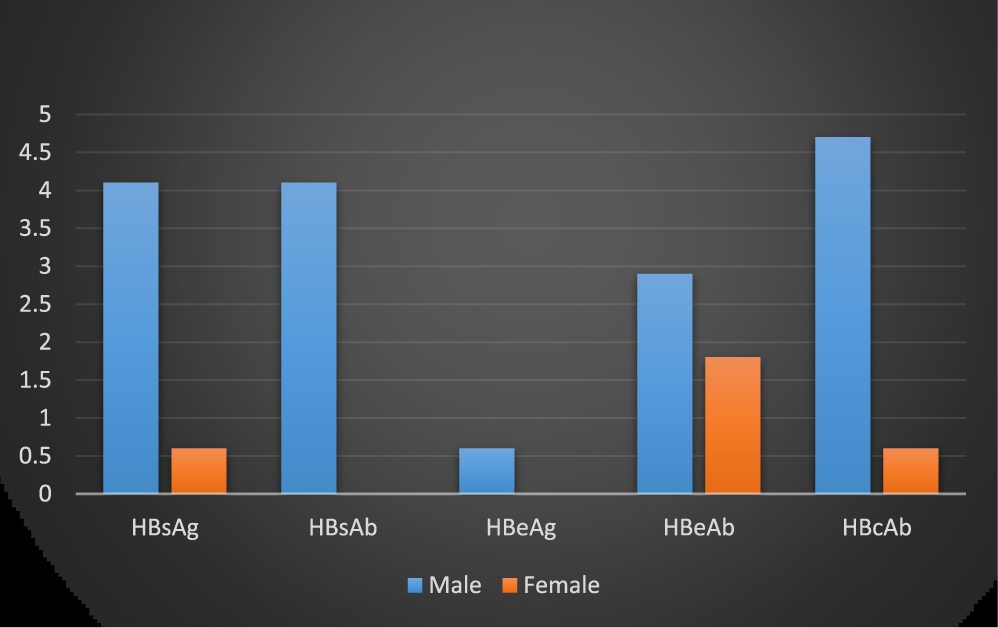
The gender-specific difference shown that male blood donors had higher seropositivity for the five markers than the Female counterpart. This contradicts the report of Uneke, 2005, who stated that females are more infected with HBsAg than the male [29]. The reason for this difference might be due to a large number of male blood donors in this study than the females. However, his observation contradicts what had been previously reported by some authors, Mehmet [30] in his study reported a higher prevalence rate in male than females in both rural and urban, with the observation that male sex is an important factor for HBV positivity (Figure 4). The same high prevalence rate of HBsAg was reported among males than the females in Lagos, Nigeria [31]. A similar study also reported a higher HBsAg prevalence in males than females among patients attending dental Clinic, University College Hospital, Ibadan, Nigeria, this was due to a shorter carrier HBsAg rate in female than the males [24]. The lack of statistical difference in HBV markers suggests that they were equally exposed to HBV in corroboration, with earlier findings [32].
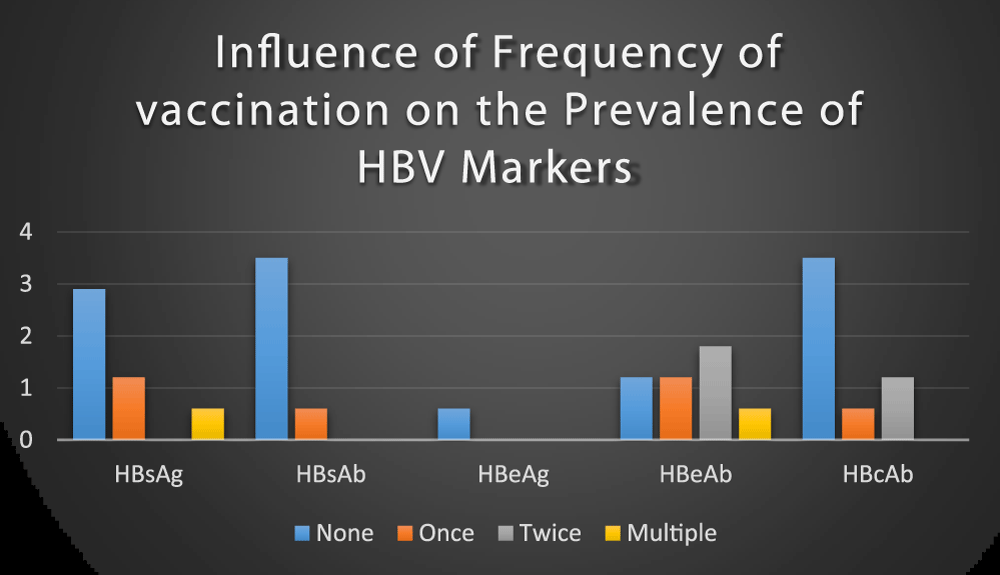
Out of the 170 blood donors, 151(88.8%) samples were first time donors, (not vaccinated) 7(4.12%), were second-time donors received one dose of vaccine, 8(4.71%) were the third time donors received two doses of vaccine and 4(2.35%) were fourth-time donors received three or more dose of vaccine respectively. The prevalence of HBV markers was high among first-time donors (non-vaccinated donors) than the remaining donors (vaccinated donors) and the vaccinated donors are safest than the non-vaccinated donors (Figure 5). This result is in agreement with a previous study conducted among blood donors at Gondar University Teaching Hospital, Northwest Ethiopia [33]. This shows that HBV infection decreases as the frequency of vaccination increases. The possible reason is that the more an individual get vaccinated the more the donor becomes immunized.
Conclusion
This study showed an overall prevalence rate of HBsAg to be 4.7%, HBsAb 4.1%, HBeAg 0.6%, anti-HBc 9(5.3%), among prospective blood donors in Usmanu Danfodiyo University Teaching Hospital Sokoto, Nigeria. This study, however, confirmed the presence of HBV markers among apparently healthy blood donors. The majority of those might have acquired the infection through unsafe sexual practices and blood transfusion during window period or late phase chronic HBV when HBsAg is at an undetectable level. Thus the need for the inclusion of HBc in routine screening of blood donors in Nigeria to reduce the risk of transfusion transmissible HBV infection.
Recommendations
In line with this result fine, it is recommended that all blood donors be routinely screened for HBV profile as part of the routine blood donation process, particularly during their first visit. There should be public awareness campaign for Hepatitis B infection and public vaccination/ immunization. We equally recommended that HBV DNA testing should be included in blood donation screening test. This research should be replicated on a national scale with wider participation of major tertiary health institutions nationwide.
References
- Seeger C, Zoulium F, Mason WS. Hepadnaviruses- 5th Edition. Journal of Virology Philadelphia. 2013;24:321-332.
- WHO. Hepatitis B. 2011. https://bit.ly/3uosTbe
- Lee WM. Hepatitis B virus infection. N Engl J Med. 1997 Dec 11;337(24):1733-45. doi: 10.1056/NEJM199712113372406. PMID: 9392700.
- Bayo P, Ochola E, Oleo C, Mwaka AD. High prevalence of hepatitis B virus infection among pregnant women attending antenatal care: a cross-sectional study in two hospitals in northern Uganda. BMJ Open. 2014 Nov 11;4(11):e005889. doi: 10.1136/bmjopen-2014-005889. PMID: 25387757; PMCID: PMC4244481.
- WHO. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection.
- Tao I, Compaoré TR, Diarra B, Djigma F, Zohoncon TM, Assih M, Ouermi D, Pietra V, Karou SD, Simpore J. Seroepidemiology of hepatitis B and C viruses in the general population of burkina faso. Hepat Res Treat. 2014;2014:781843. doi: 10.1155/2014/781843. Epub 2014 Aug 5. PMID: 25161770; PMCID: PMC4138785.
- Musa BM, Bussell S, Borodo MM, Samaila AA, Femi OL. Prevalence of hepatitis B virus infection in Nigeria, 2000-2013: a systematic review and meta-analysis. Niger J Clin Pract. 2015 Mar-Apr;18(2):163-72. doi: 10.4103/1119-3077.151035. PMID: 25665986.
- WHO. The Global Prevalence of Hepatitis E Virus Infection and Susceptibility: A Systematic Review, World Health Organization, Department of Immunization, Vaccine and Biologicals. 2013.
- Olaso SO, Odaibo GN. Alfa-fetoprotein, HCV and HBV infections in Nigerian patients with primary hepatocellular carcinoma. Nigeria Medical Practices. 2007;51:33-35.
- Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006 Oct;45(4):529-38. doi: 10.1016/j.jhep.2006.05.013. Epub 2006 Jun 23. PMID: 16879891.
- Barker LF, Shulman NR, Murray R, Hirschman RJ, Ratner F, Diefenbach WC, Geller HM. Transmission of serum hepatitis. 1970. JAMA. 1996 Sep 11;276(10):841-4. PMID: 8769597.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004 Mar;11(2):97-107. doi: 10.1046/j.1365-2893.2003.00487.x. PMID: 14996343.
- Energy YE. Overview on Hepatitis B virus. Nature and Science. 2011;9:31-36.
- Durro V, Qyra S. Trends in prevalence of hepatitis B virus infection among Albanian blood donors, 1999-2009. Virol J. 2011 Mar 4;8:96. doi: 10.1186/1743-422X-8-96. PMID: 21375724; PMCID: PMC3060146.
- Jeremiah ZA, Idris H, Ajayi BB, Ezimah AC, Malah MB, Baba MM. Isolated anti-HBc-IgM antibody among blood donors in the semi-arid region of Nigeria. Hum Antibodies. 2011;20(3-4):77-82. doi: 10.3233/HAB-2011-0242. PMID: 22129677.
- Japhet MO, Adesina OA, Donbraye E, Adewumi M. Hepatitis B Core IgM antibody (anti-HBc IgM) among hepatitis B surface (HBsAg) negative blood donors in Nigeria. Virology Journal. 2011;8:513-551.
- National Population Commission. Nigerian Population Census. Abuja-Nigeria. 2009.
- Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013 Apr;35(2):121-6. doi: 10.4103/0253-7176.116232. PMID: 24049221; PMCID: PMC3775042.
- Japhet MO, Adesina OA, Donbraye E, Adewumi MO. Hepatitis B core IgM antibody (anti-HBcIgM) among hepatitis B surface antigen (HBsAg) negative blood donors in Nigeria. Virol J. 2011 Nov 10;8:513. doi: 10.1186/1743-422X-8-513. PMID: 22074048; PMCID: PMC3239416.
- Soldan K, Ramsay M, Collins M. Acute hepatitis B infection associated with blood transfusion in England and Wales, 1991-7: review of database. BMJ. 1999 Jan 9;318(7176):95. doi: 10.1136/bmj.318.7176.95. PMID: 9880282; PMCID: PMC27683.
- Kleinman SH, Busch MP. The risks of transfusion-transmitted infection: direct estimation and mathematical modelling. Baillieres Clinical Haematology. 2010;13(4):631-649.
- Gutiérrez-García ML, Fernandez-Rodriguez CM, Lledo-Navarro JL, Buhigas-Garcia I. Prevalence of occult hepatitis B virus infection. World J Gastroenterol. 2011 Mar 28;17(12):1538-42. doi: 10.3748/wjg.v17.i12.1538. PMID: 21472117; PMCID: PMC3070122.
- Manzini P, Girotto M, Borsotti R, Giachino O, Guaschino R, Lanteri M, Testa D, Ghiazza P, Vacchini M, Danielle F, Pizzi A, Valpreda C, Castagno F, Curti F, Magistroni P, Abate ML, Smedile A, Rizzetto M. Italian blood donors with anti-HBc and occult hepatitis B virus infection. Haematologica. 2007 Dec;92(12):1664-70. doi: 10.3324/haematol.11224. PMID: 18055990.
- Lukhwareni A, Burnett RJ, Selabe SG, Mzileni MO, Mphahlele MJ. Increased detection of HBV DNA in HBsAg-positive and HBsAg-negative South African HIV/AIDS patients enrolling for highly active antiretroviral therapy at a Tertiary Hospital. J Med Virol. 2009 Mar;81(3):406-12. doi: 10.1002/jmv.21418. PMID: 19152393.
- Lukhwareni A, Burnett RJ, Selabe SG, Mzileni MO, Mphahlele MJ. Increased detection of HBV DNA in HBsAg-positive and HBsAg-negative South African HIV/AIDS patients enrolling for highly active antiretroviral therapy at a Tertiary Hospital. J Med Virol. 2009 Mar;81(3):406-12. doi: 10.1002/jmv.21418. PMID: 19152393.
- Lawal OA, Bakarey AS, Uche LN, Udeze AO, Okonko IO. HBV infection among intending blood donors who incidentally tested positive for HIV antibodies in two blood banks in Ibadan, Nigeria. World Applied Science Journal. 2009;1(2):97-107.
- Luka SA, Ibrahim MB, IIiya SN. Seroprevalence of hepatitis B surface antigen among pregnant women attending Ahmadu University Teaching Hospital, Zaria, Nigeria. Nigerian Journal of Parasitology. 2008;29(1): 38-41. https://tinyurl.com/b7ez63k
- Buseri FI, Muhibi MA, Jeremiah ZA. Seroepidemiology of transfusion transmissible infectious diseases among blood donors in Osogbo, South-West Nigeria. Blood Transfusion. 2009;7(4):293-299.
- Uneke CJ, Ogbu O, Inyama PU, Anyanwu GI, Njoku MO, Idoko JH. Prevalence of hepatitis-B surface antigen among blood donors and human immunodeficiency virus-infected patients in Jos, Nigeria. Mem Inst Oswaldo Cruz. 2005 Feb;100(1):13-6. doi: 10.1590/s0074-02762005000100002. Epub 2005 Apr 12. PMID: 15867956.
- Mehmet D, Meliksah E, Serif Y, et al. Prevalence of hepatitis B infection in the southeastern region of Turkey: comparison of risk factors for HBV infection in rural and urban areas. Japanese Journal of Infectious Diseases. 2005 Feb;58(1):15-19.
- Balogun TM, Durojaiye IO, Sagoe A, Emmanuel S. Seroepidemiology of hepatitis-B surface antigenaemia in HIV positive patients. West Afr J Med. 2010 May-Jun;29(3):169-73. doi: 10.4314/wajm.v29i3.68215. PMID: 20665460.
- Ugwuja E, Ugwu N. Seroprevalence of Hepatitis B Surface Antigen and Liver Function Tests among Adolescents in Abakaliki, South Eastern Nigeria. The Internet Journal of Tropical Medicine. 2010;6(1):5580-5297.
- Tessema B, Yismaw G, Kassu A, Amsalu A, Mulu A, Emmrich F, Sack U. Seroprevalence of HIV, HBV, HCV and syphilis infections among blood donors at Gondar University Teaching Hospital, Northwest Ethiopia: declining trends over a period of five years. BMC Infect Dis. 2010 May 10;10:111. doi: 10.1186/1471-2334-10-111. PMID: 20459703; PMCID: PMC2881920.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.