> General Science. 2021 June 05;2(6):460-462. doi: 10.37871/jbres1260.
Novel Tailor made Multiblock Polyesters/Polycarbonates Biomaterials via Organometallic Polymerization Synthesis
Anaelle Bolley*
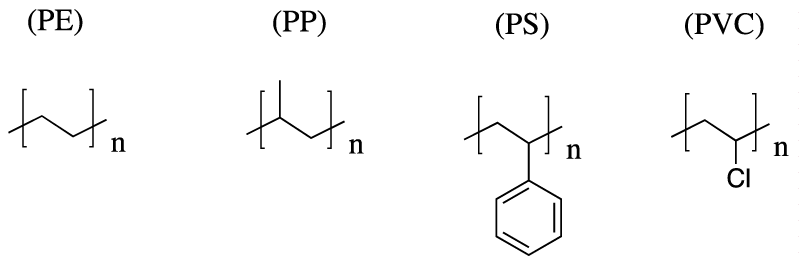
Since 1940, mass production of polyolefin plastics produced annually has increased rapidly [1]. The most common plastics, which account for approximately 80% of the European plastic demand, are: Polyethylene (PE), Polypropylene (PP), Poly (Vinyl Chloride) (PVC) and Polystyrene (PS) (Figure 1) [2]. Therefore, plastic materials have experienced a substantial expansion, and are the most used materials nowadays. These materials have a wide range of application from packaging to electronic devices [3].
However, polyolefin are derived from fossil resources that prove to be non-renewable by petrochemical origin, more scarce and expensive. These materials represent an environmental problem, mainly as regards disposal after use like storage or contamination. It is thus that bio sourced and biodegradable polymers are a viable alternative to plastics of petrochemical origin [4].
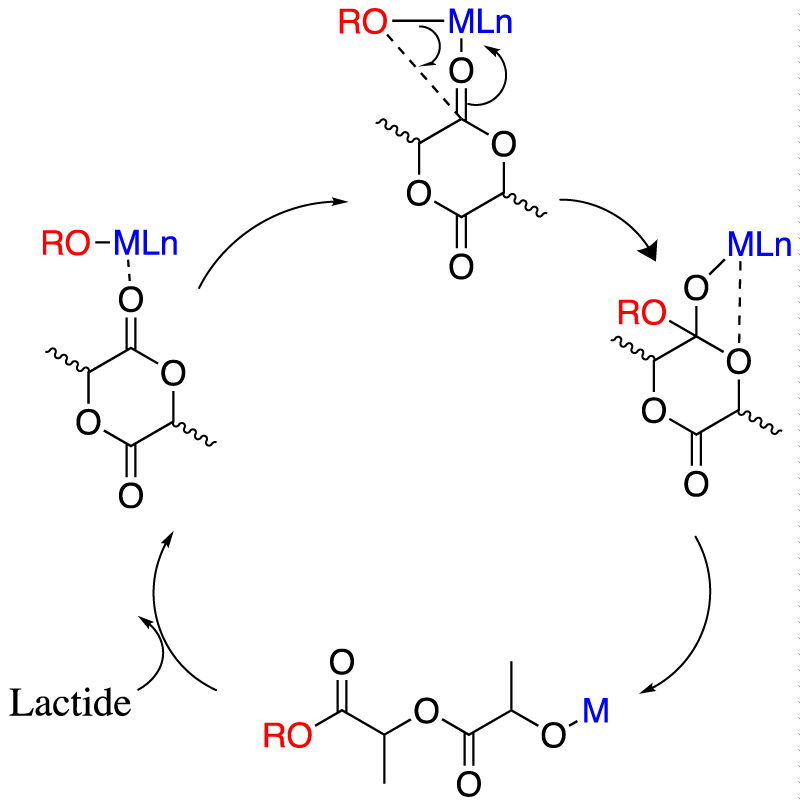
Over the past two decades, the production of biodegradable polyesters has a considerable attention, for example, polylactide derived from Ring Opening Polymerization (ROP) of lactide (dimer of lactic acid, a renewable resource available from starch) and already used for several applications (packaging, biomedical, building and construction, automotive) [5]. This ROP has a common coordination-insertion mechanism (Figure 2) [6].
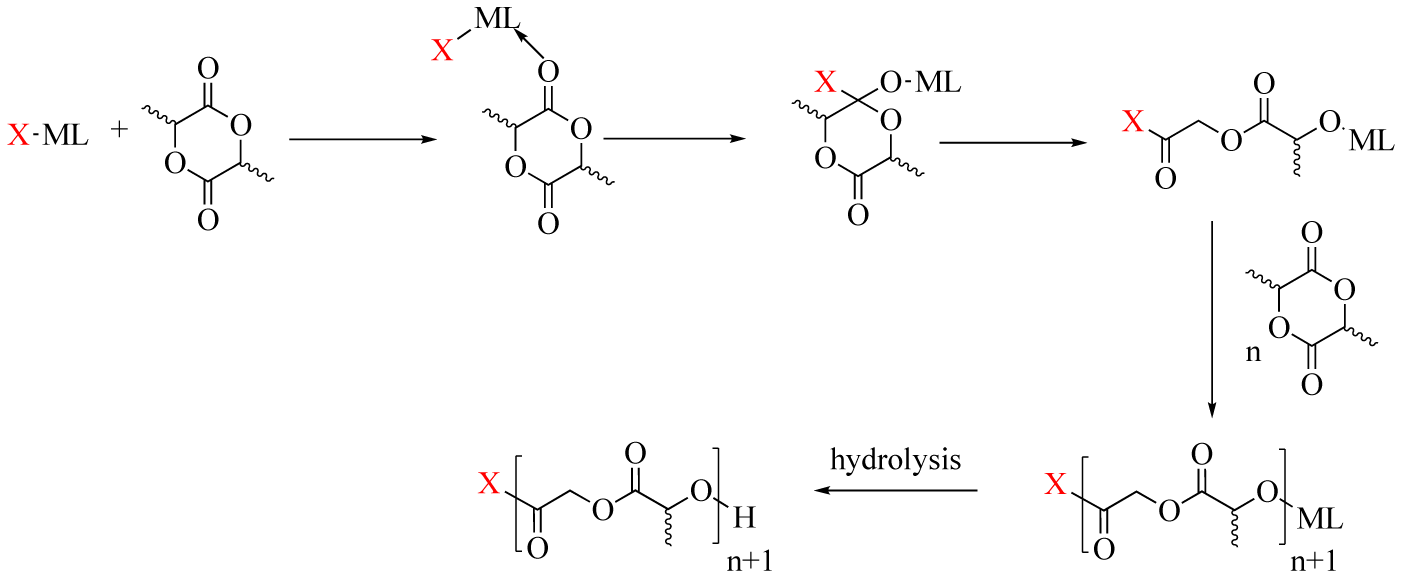
The synthesis of N-Heterocyclic Carbene (NHC), [NHC =1 ,3-bis(2,4,6-trimethylphenyl)imidazole-2-ylidene (IMes), with group XIII trimethylmetals lead to different catalysts of the type (NHC)M’R3 [7] and another reaction between IMesAlMe3 adduct with a stoichiometric amount of B(C6F5)3 in presence of an external base (Et2O) produces a stable cationic alkyl (IMes)AlMe2Et2O [8]. These four compounds act as efficient ROP initiators of rac-lactide and allow an access to linear or cyclic Poly(Lactic Acid) (PLA) depending on the nature of the initiator and the reaction conditions. For all these systems, the addition of a chain transfer agent (an alcohol) improved both, the activity and the control of the ROP. Among the three adducts the better is using indium metal. The time of the reaction is very fast at room temperature and the polymerization is very well controlled. The cation catalyst provides from an ionization of IMesAlMe3 adduct via a Me_ abstraction at the aluminium (III) center with Lewis acid B(C6MF5)3 in the presence of Et2O as external lewis base. This ionization is a well-established method to produce base-stabilized cationic metal alkyl species of group XIII. In presence of alcohol (the chain transfer agent), the polymerization reaction mechanism proceeds via “insertion-coordination” (Figure 3).
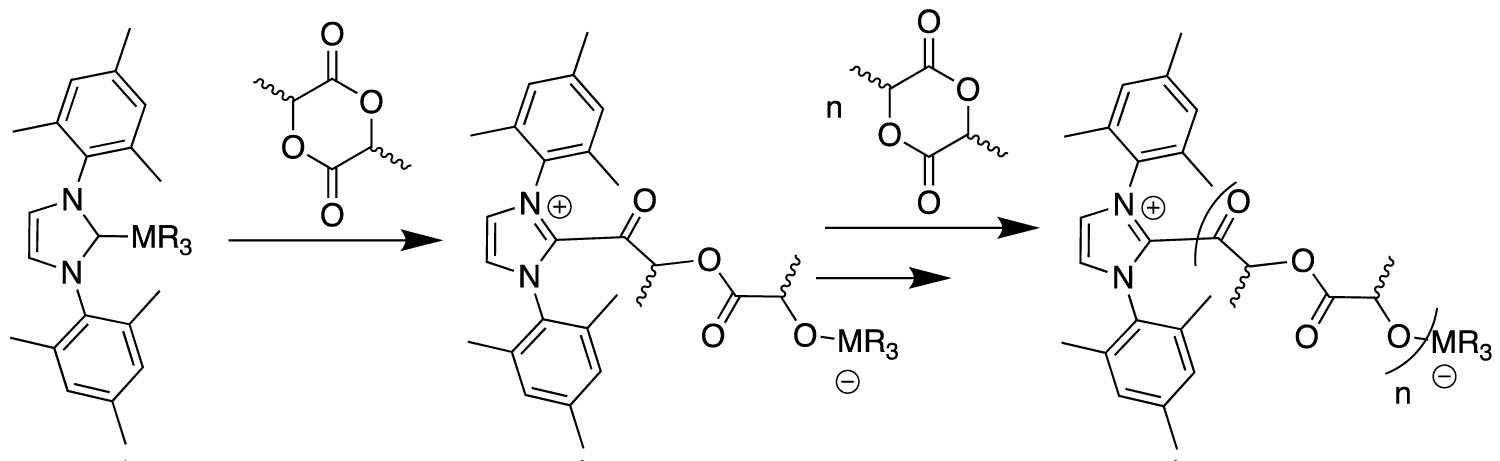
The polar metal-NHC bond in the synthesized complexes may potentially be reactive in the presence of lactide, with the MMe3 fragment acting as Lewis acid for monomer activation and the NHC moiety as a nucleophile able to ring-open the lactide monomer. Following propagation steps (successive insertions into the formed M-O alkoxide bond) may allow PLA chain growth from the metal center (Figure 4).
In summary, different adducts were found to promote the ROP of rac-lactide under a controlled ROP reaction with full conversion to PLA. The addition of an external alcohol source, like BnOH, acting as an effective chain transfer agent, was shown to improve the ROP control and activity. The mechanism first step is probably a ROP proceeding via activation/dissociation of the M-Ccarbene (Figure 4) followed by a transesterification reaction with BnOH acting as a nucleophile and yielding in the release of the NHC, and finally the classical «coordination-insertion» mechanism. Without any chain transfer agent, the ROP occurs probably via an activated monomer mechanism where the NHC acts as the nucleophile and promotes the ROP while the metal derivatives M(Me)3 operate as electrophiles.
References
- WD Sauter, M Taoufik, C Boisson. Polyolefins, a success story. Polymers 2017;9:1-13. https://tinyurl.com/3mcjmww5
- Andrady AL, Neal MA. Applications and societal benefits of plastics. Philos Trans R Soc Lond B Biol Sci. 2009 Jul 27;364(1526):1977-1984. doi: 10.1098/rstb.2008.0304. PMID: 19528050; PMCID: PMC2873019.
- S Slomkowski, S Penczek, A Duda. Polylactides-An overview. Polym Adv Technol. 2014;25:436. https://tinyurl.com/sdkfbua
- A Steinbüchel. Non-biodegradable biopolymers from renewable resources: perspectives and impacts. Curr Opin Biotechnol. 2005;16:607-613. https://tinyurl.com/2cpw5rcx
- Nomura N, Akita A, Ishii R, Mizuno M. Random copolymerization of epsilon-caprolactone with lactide using a homosalen-Al complex. J Am Chem Soc. 2010 Feb 17;132(6):1750-1751. doi: 10.1021/ja9089395. PMID: 20099817.
- K. Nakano, N Kosaka, T Hiyama, K Nozaki. Metal-catalyzed synthesis of stereoregular polyketones, polyesters, and polycarbonates. Dalton Trans. 2003;4039-4050. https://tinyurl.com/n9bd4err
- G Schnee, A Bolley, F Hild, D Specklin, S Dagorne. Sterically bulky NHC adducts of GaMe3 and InMe3 for H2 activation and lactide polymerization catal today. 2016;289:204-210. https://tinyurl.com/vfx4c4tt
- G Schnee, A Bolley, C Gourlaouen, R Welter, S Dagorne. Synthesis and structural characterization of NHC-stabilized Al(III) and Ga(III) alkyl cations and use in the ring-opening polymerization of lactide J Organomet Chem. 2016;820:8-13. https://tinyurl.com/ua6fce86
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.