> Medicine Group. 2021 June 08;2(6):466-471. doi: 10.37871/jbres1262.
Clinical Appearance and Diagnosis of Heparin Induced Thrombocytopenia
Tayfun Gunduz*, Murat Cakir, Eftal Murat Bakirci and Husnu DEGIRMENCI
Abstract
Heparin-İnduced Thrombocytopenia (HIT) is a life-threatening complication that occurs in a small percentage of exposed patients (e.g. unfractionated heparin, Low Molecular Weight Heparin [LMWH]) regardless of dose and treatment management.
Heparin-İnduced Thrombocytopenia (HIT) [1] is caused by an autoantibody directed against endogenous Platelet Factor 4 (PF4) in complex with heparin. This antibody activates platelets and can cause arterial and venous thrombosis. Although the mortality rate of untreated HIT is determined as 20 percent; With early diagnosis and treatment, mortality rates have been reported to be less than 2 percent [2,3]. The clinical picture and diagnosis of HIT will be discussed here. The management of HIT and the use of heparin and alternative anticoagulants are discussed separately.
Termınology and variants
There are two forms of HIT, only one of which is clinically significant (Table 1). The distinction between these two forms is made according to the decrease in the platelet count, its timing, clinical parameters, and laboratory testing.
| Table 1: HIT Type 1 & Type 2. | ||
| Type 1 | Type 2 | |
| Frequency | 10-20% | 1-3% |
| Start | 1-4 days | 5-10 days after initiation of heparin |
| Platelet count | 100,000 / microL | Typically> 20,000 microL; median 60,000 / microL |
| Antibody mediated | No | Yes |
| Thromboembolic sequelae | No | 30-80% |
| Hemorrhagic sequelae | No | Rare |
| Administration | observation | Discontinuation of heparin, alternative non-heparin anticoagulation to prevent thrombosis |
HIT Type I (HIT I) is a mild, transient reduction in platelet count that typically occurs within the first two days of heparin exposure. The platelet count typically returns to normal with continued administration of heparin. The mechanism appears to be a direct effect of heparin on platelets and causes non-immune platelet aggregation. Typical platelet count low value is around 100,000/microL. This form of HIT is not considered clinically significant, is not associated with thrombosis, and can be expected without discontinuation of heparin.
HIT type II is a clinically important syndrome due to heparin complexed Platelet Factor 4 (PF4) antibodies, referred to as “HIT antibodies” or “PF4/heparin antibodies”. These antibodies can cause thrombosis with thrombocytopenia, so they are also called Heparin-İnduced Thrombocytopenia and Thrombosis (HITT). The risk of thrombosis, including life-threatening limb gangrene, persists until heparin is eliminated and a non-heparin anticoagulant is initiated.
The HIT stages are defined, including the acute disease stages (Table 2). Subacute HIT refers to the presence of naturally occurring or clinically silent anti-PF4 antibodies (eg, associated thrombocytopenia, thrombosis, recovery of the episode without clinical symptoms, normalization, or onset of platelet count) [2]. Indirect HIT refers to platelet count recovery and negative heparin-PF4 antibodies. Individuals with subacute or indirect HIT are at high risk for HIT recurrence if they are re-exposed to heparin.
| Table 2: HIT stages. |
| Heparin İnduced Thrombocytopenia (HIT) has 5 phases |
| SUSPICIOUS HIT |
| Platelet count - Low Heparin PF4 - Not yet available |
| ACUTE HIT |
| Platelet count - Low Heparin PF4 - Positive |
| SUBACUT HIT A (Platelet count improved but Persistent HIT antibodies) |
| Platelet count - normal or at patient's basal level Heparin PF4 - Positive |
| SUBACUT HIT B (Permanent HIT but functional test no longer positive) |
| Platelet count - normal or at patient's basal level Heparin PF4 - The functional assay has become negative; immunoassay stays positive |
| OLD HIT (HIT resolved but patient at risk of developing acute HIT) |
| Platelet count - Normal or at the patient's basal level Heparin PF4 - negative |
Clinical HIT may be present due to HIT antibodies that activate platelets in the absence of heparin. These syndromes are sometimes called autoimmune HIT (Table 3).
| Table 3: Autoımmune HIT Syndromes. | |
| Clinical Feature | Explanation |
| Delayed onset HIT | High titre antibodies to HIT, PF4 / heparin that started or worsened after heparin was withdrawn. |
| Resistive HIT | HIT> 1 week despite discontinuation of heparin |
| Spontaneous HIT | Unexplained thrombocytopenia and / or thrombosis without recent heparin exposure Demonstration of anti-PF4 antibodies of the Immunoglobulin G (IgG) subclass that cause potent in vitro platelet activation in the absence of heparin. |
| Heparin flush HIT | HIT caused by heparin flushing |
| Fondaparinux-associated HIT | HIT triggered by Fondaparinux exposure |
| Severe HIT (Plt <20,000 microL over DIC | HIT associated with DIC, With one or more of the following; Bound / absolute hypofibrinogemia increased INR (other causes should be excluded) Normablastemia |
Pathophysiology
HIT antibodies are directed against PF4 complexed with heparin. Heparin is thought to induce a conformational change in the PF4 protein forming a neoantigen and HIT antibodies bind to this PF4 neoantigen. As a result of this heparin addiction, these antibodies often cause clinical symptoms when only heparin is present. Immune memory does not always occur, but people who have been exposed to heparin recently may develop clinical signs of HIT over a short period of time than those who have not been exposed to heparin.
PF4 protein is stored in platelet alpha granules. CXC is a small cytokine belonging to the chemokine family. Platelet activation causes the release of PF4, which binds to heparin and related endogenous proteoglycan molecules (eg Heparan sulfate, chondroitin sulfate) at endothelial surfaces and forms a tetramer that neutralizes it. Heparin-PF4 complexes also form on the platelet surface. HIT antibodies can bind to heparin-PF4 complexes on the platelet surface (via heparin binding to cell surface proteins) or to PF4, which binds to platelet surface glycosaminoglycans (heparin-like molecules). In an in vitro analysis, HIT antibodies were shown to bind to PF4 complexed with von Willebrand Factor (VWF), high molecular weight multimer sequences released from injured endothelium under flow conditions promoting platelet binding and activation.
When HIT antibodies bind to PF4 on its surface, the visible Fc region is captured by Fc receptors on the surface of the same or adjacent platelets (Fc Gamma Receptor IIA [FcRIIA]) as well as Glycoprotein (GP) Ib/IX. There is a positive feedback loop for further platelet activation, resulting in more antigenic substrates for HIT antibodies and more PF4 release.
• IgG antibodies form rapidly (within days). There is no primary IgM response for clinically important Platelet Factor 4 (PF4)/heparin antibodies [3].
• IgG antibodies disappear over time; However, this does not mean that heparin can be used if antibodies are lost.
• Anti-heparin-PF4 antibodies can be from IgG, IgM and IgA subclasses. IgG is thought to be the only pathogenic antibody because the platelet surface Fc receptor only recognizes IgG.
Naturally occurring antibodies that react with heparin-bound PF4 are present in 3 to 8 percent. It has been suggested that these antibodies are induced when PF4 binds to negatively charged polysaccharides on the bacterial surface and forms an antigen mimicking heparin-bound PF4 in PF4. Prior exposure to PF4 antigen may be responsible for the rapid emergence of HIT antibodies following treatment of some patients with heparin.
The HIT immune response is similar to the T-cell independent B-cell activation seen in immune reactions against viruses with repetitive epitopes. Preliminary evidence suggests that this may be the explanation for the syndrome seen in adenoviral vector COVID-19 vaccines.
Mechanism of thrombocytopenia
Mechanisms of thrombocytopenia in HIT include removal of IgG-coated platelets by macrophages of the reticuloendothelial system (eg spleen, liver, bone marrow) by binding to FcRIIA, similar to types of drug-induced immune thrombocytopenia. Platelet consumption in thrombosis areas; and platelet destruction due to the development of a depleted coagulopathy. A second cause of thrombocytopenia is the depletion of platelets within the thrombus.
Thrombosis mechanism
HIT is associated with arterial and venous thrombosis. The mechanism is probably multifactorial, but the primary mechanism is thought to be due to damage to endothelial cells through activation of platelets and cell surface FcRIIA on platelets and endothelial cells, respectively. Release of procoagulant substances, including microparticles, from active platelets Endothelial cell activation and/or injury induced by the binding of HIT antibodies to heparan sulfate on endothelial cell surfaces leads to increased tissue factor expression and thrombin formation.
• Endothelial cell release of adhesion molecules (e.g. interleukin-6, von Willebrand factor) [4]
• Activation of monocytes by HIT antibodies
• Alteration of other aspects of coagulation by HIT antibodies (e.g. reduced production of activated protein C)
It has been suggested that a genetic polymorphism at the platelet-associated immunoglobulin Fc receptor enhances platelet activation by increasing the binding of HIT antibodies. In a study of 89 patients with HIT, thrombosis was more common in individuals with arginine rather than a histidine at amino acid position 131 on both alleles of the receptor (FcRIIA 131RR instead of FcRIIA 131HH). The Odds Ratio (OR) for thromboembolic events with the 131RR allele was 5.9 (95% CI 1.7-20) [5,6].
Neutrophil activation and NETosis
Neutrophil Extracellular Traps (NETs) are structures created when neutrophils extract their DNA as non-helical chromatin chains containing cellular contents such as histones, myeloperoxidase, and elastase. NETs play a central role in host defense against infection.Studies show that NETosis also plays an important role in thrombosis in individuals with HIT.
In a study involving 21 people with HIT, NETosis plasma markers, including cell-free DNA, myeloperoxidase, “low density granulocytes” and citrullinated histone H3, were significantly increased compared to control plasma. Plasma and purified antibodies against heparin-PF4 from HIT patients were able to induce DNA release from purified neutrophils. In a mouse model of HIT, depletion of neutrophils or blocking of nuclear decondensation in neutrophils by a NETosis inhibitor prevented thrombosis, while thrombocytopenia was unaffected and supported the important role of NETosis in HIT thrombosis [7].
Ultra high PF4
The optimal 1:1 molar ratio for forming heparin complexes may also explain why those exposed to very high doses of heparin (e.g. during cardiopulmonary bypass) are less likely to develop HIT than those exposed to standard doses. It also highlights the rationale for using high doses of heparin to demonstrate reduced binding of true HIT antibodies in immunoassays for HIT.
The titer of the HIT antibody can also affect the timing of the HIT. High titer antibodies reacting with PF4 bound to non-heparin glycosaminoglycans (e.g. chondroitin sulfate) have been proposed to explain the occurrence of HIT in patients who have never been exposed to heparin or who develop HIT late after heparin withdrawal.
Insidance and risk factors
HIT has been reported in up to 5 percent of patients exposed to heparin for more than four days. Factors that increase the frequency of HIT include;
• Surgical
• (LMWH) versus non-fractionated ones
• Heparin dosage
• Female gender
• Age
Analysis of seven studies comparing non-fragmented and Low Molecular Weight (LMW) heparin found higher incidence of HIT in surgical patients compared to medical patients (Odds Ratio [OR] 3.25; 95% CI 1.98-5.35). There is a very high incidence of anti-PF4/heparin antibody formation (eg 20 to 50 percent) in patients undergoing heart surgery or cardiopulmonary bypass. Patients can develop HIT regardless of whether their previous heparin exposures were fractionated or LMWH, but HIT is more common in surgical patients after exposure to unfractionated heparin than LMWH exposure.
Heparin therapeutic doses may result in a higher incidence of HIT than prophylactic doses, but there are no data describing the relationship between heparin dose and clinical findings. However, there is not a very low dose of heparin to cause HIT. Patients developed HIT after exposure to 250 units of heparin flushing or using a heparin-coated catheter. - Analysis of seven studies undivided by LMWH found that the risk of HIT was approximately twice as high in female patients compared to men (OR 2.37; 95% CI 1.37-4.09). Also, advanced age may be a risk factor for HIT, but there are no data to suggest a relationship between age and HIT. In a study using the National Hospital Discharge Questionnaire database, HIT was rarely found in patients under the age of 40.
Diagnosis and treatment management
Clinical manifestations: HIT typically occurs 5 to 10 days after initiation of heparin. Heparin-dependent antibodies usually develop five to eight days after heparin exposure. Early onset of HIT (i.e. thrombocytopenia within the first 24 hours after exposure) may occur if the patient has been exposed to heparin within the previous one to three months and has circulating HIT antibodies.
Following discontinuation of heparin and initiation of a non-heparin anticoagulant, thrombocytopenia typically resolves within seven days. In a patient with HIT whose platelet count does not begin to improve within three to four days after heparin discontinuation, the possibility of continued exposure to heparin or an additional cause of thrombocytopenia should be investigated.
• Thrombocytopenia: Thrombocytopenia (platelet count <150,000/microL) is the most common symptom of HIT, seen in 85 to 90 percent of individuals. The average lowest platelet count is about 60,000/microL. Platelet counts below 20,000/microL are rare. About 5 percent of patients with HIT do not have thrombocytopenia as defined by the absolute platelet count, but show a 50 percent reduction in platelet count.
• Bleeding: Bleeding in HIT is rare, and about 6 percent of 6332 hospitalized patients experienced bleeding. The study did not report whether the bleeding was due to thrombocytopenia caused by HIT or anticoagulation to treat HIT. Gastrointestinal bleeding was the most common type in 2.7 percent of patients, and about 1 percent had central nervous system bleeding.
• Thrombosis: Thrombosis occurs in up to 50 percent of individuals with HIT and is more common than venous arterial thrombosis. Thrombosis is the first sign in about 25 percent of patients; This observation led to suggestions that they should have a leg ultrasound to screen for asymptomatic deep vein thrombosis. If clinical signs or symptoms suggest venous or arterial thrombosis at other sites, these should be investigated immediately with appropriate tests.
• Skin necrosis: Skin necrosis where heparin injections are given should immediately suggest HIT. Some patients with HIT have skin necrosis without thrombocytopenia. Skin findings at sites other than the injection site have also been reported.The appearance of erythema is followed by purpura and bleeding, followed by necrosis.
• Limb gangrene: Limb gangrene was associated with venous rather than arterial thrombosis in 8 of 158 HIT patients followed. In one study, patients with venous limb gangrene had a much higher İnternational Normalized Ratio (INR) (median INR: 5.8 versus 3.1) than patients without gangrene, suggesting a possible contribution from acquired protein C deficiency.
How should we know?: In persons with suspected HIT, if there is no high risk of thrombosis, all sources of heparin should be discontinued immediately and a non-heparin anticoagulant administered. Thrombosis should not be expected in HIT because thrombocytopenia usually precedes thrombosis. Early intervention has the potential to prevent thrombotic events that are the main cause of morbidity and mortality in patients with HIT [8].
Any of the following scenarios should increase the likelihood of HIT in patients who are already using heparin or who have received heparin within the previous 5 to 10 days [2].
• New onset of thrombocytopenia (i.e. platelet count <150,000/microL)
• Even if the platelet count exceeds 150,000/microL, a 50 percent or more reduction in the platelet count
• Venous or arterial thrombosis
• Necrotic skin lesions at heparin injection sites
• Acute systemic reactions (e.g. fever/chills, tachycardia, hypertension, dyspnoea, cardiopulmonary arrest) after intravenous administration of heparin.
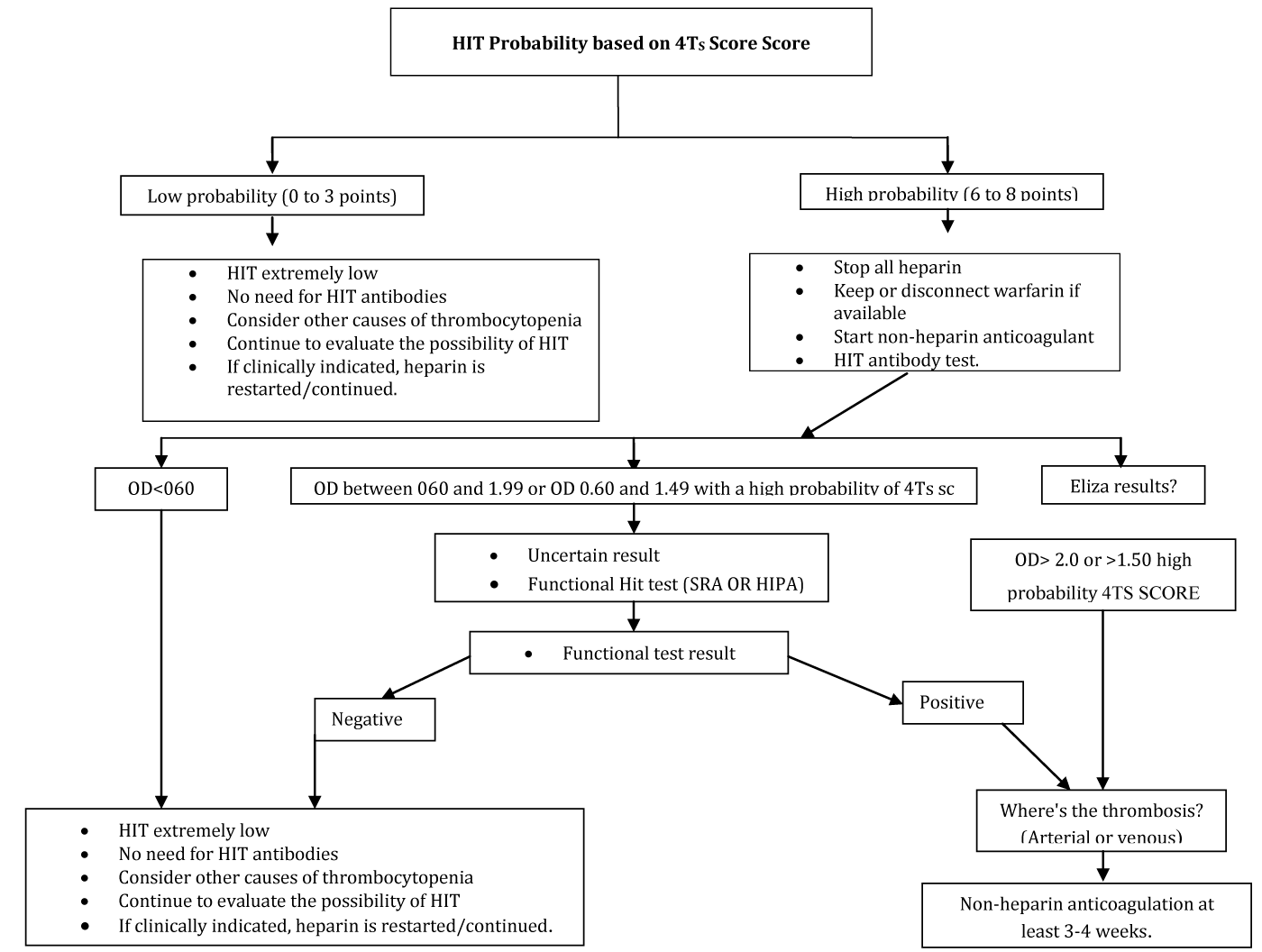
The 4Ts score is used to predict the probability of HIT (pretest probability) based on available clinical features.It is used to make a possible diagnosis of HIT until laboratory data are available and then integrated with laboratory data to make a final diagnosis.
The score evaluates the degree of thrombocytopenia, timing according to heparin exposure, presence of thrombosis, and other causes for thrombocytopenia.
| 4Ts Points Parameters | |
| Thrombocytopenia | |
| Plt decrease> 50% and lowest limit ≥20,000/microL and no surgery in previous 3 days | 2 |
| Plt reduction> 50%, but within 3 days after surgery, or platelet reduction that does not match the 2 or 0 point character | 1 |
| Plt drop <30% or <10,000 | 0 |
| Fırst timing after heparin exposure | |
| 5 to 10 days or 1 day if exposure within the past 5 to 30 days | 2 |
| Possible 5-10 days, or more than 10 days, or if it developed in less than 1 day in the case of exposure within the last 31 to 100 days | 1 |
| Has developed less than 4 days after exposure to the last 100 days | 0 |
| Thrombosis or other clinical sequelae | |
| New diagnosis thrombosis, anaphylactic reaction, skin necrosis, adrenal bleeding | 2 |
| Suspected, progressive or recurrent thrombosis, skin redness | 1 |
| None | 0 |
| Other causes of Thrombocytopenia | |
| No | 2 |
Interpretation: The sum of the point values gives a total from 0 to 8. Pretest possibilities for HIT are as follows;
• 0 to 3 points - Low risk <1%
• 4 to 5 points - medium risk approximately 10%
• 6 to 8 points - high probability approximately 50%
The 4Ts score captures the main clinical features of HIT and the likelihood that these findings are due to heparin rather than any other cause. Patients receiving heparin have an above average risk of initial thrombosis; therefore, the development of isolated Venous Thromboembolism (VTE) [2] or myocardial infarction alone will not produce a medium or high probability 4Ts score. The 4Ts score has not been validated for patients receiving Low Molecular Weight (LMW) heparin, although we use it in this population.
The 4Ts score was prospectively validated for patients exposed to unfractionated heparin at two centers. Of the 111 patients with a low probability of HIT, only one had clinically significant HIT antibodies (0.9 percent). In contrast, clinically significant HIT antibodies were present in 11.4 percent and 34 percent of those with medium and high probability scores, respectively.
Lillo-Le louet model: Designed specifically for use in the post-cardiopulmonary bypass environment, this model is based on the timing and duration of thrombocytopenia and cardiopulmonary bypass time. This model has not been evaluated as multicenter prospectively [9].
HIT Expert Probability (HEP) score: This score was developed based on broad expert opinion. Scores are given with resolution better than 4Ts score for the timing and degree of thrombocytopenia, and scores are subtracted for bleeding and other causes of thrombocytopenia. In a comparison study, experts who improved this score found that it had more than 4Ts scores for interobserver agreement. In a prospective cohort of 310 patients with suspected HIT who had a simultaneous calculation of the 4Ts score and the HEP score before the results of the HIT antibody test were known, the HEP score generally provided similar diagnostic accuracy [10].
Conclusion
Heparin-associated thrombocytopenia is a rare syndrome that usually occurs within the first 4 days. Approximately 1-3 percent of patients progress with antibody formation and clinical follow-up gives us confidence in mortality and morbidity. Our aim in this review is to reveal new approaches to heparin-associated thrombocytopenia.
References
- Arepally GM. Heparin-induced thrombocytopenia. Blood. 2017 May 25;129(21):2864-2872. doi: 10.1182/blood-2016-11-709873. Epub 2017 Apr 17. PMID: 28416511; PMCID: PMC5445568.
- Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, Linkins LA, Rodner SB, Selleng S, Warkentin TE, Wex A, Mustafa RA, Morgan RL, Santesso N. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018 Nov 27;2(22):3360-3392. doi: 10.1182/bloodadvances.2018024489. PMID: 30482768; PMCID: PMC6258919.
- Cuker A. Management of the multiple phases of heparin-induced thrombocytopenia. Thromb Haemost. 2016 Oct 28;116(5):835-842. doi: 10.1160/TH16-02-0084. Epub 2016 Apr 14. PMID: 27075525.
- Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017 Nov;15(11):2099-2114. doi: 10.1111/jth.13813. Epub 2017 Sep 28. PMID: 28846826.
- Warkentin TE, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med. 2001 Oct 2;135(7):502-6. doi: 10.7326/0003-4819-135-7-200110020-00009. PMID: 11578153.
- Warkentin TE, Basciano PA, Knopman J, Bernstein RA. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood. 2014 Jun 5;123(23):3651-4. doi: 10.1182/blood-2014-01-549741. Epub 2014 Mar 27. PMID: 24677540.
- Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007 Sep;83(983):575-82. doi: 10.1136/pgmj.2007.059188. PMID: 17823223; PMCID: PMC2600013.
- Christoffersen C, Lethagen S, Gøtze JP. Heparininduceret trombocytopeni [Heparin-induced thrombocytopenia]. Ugeskr Laeger. 2009 Feb 16;171(8):612-5. Danish. PMID: 19284907.
- Chong BH, Isaacs A. Heparin-induced thrombocytopenia: what clinicians need to know. Thromb Haemost. 2009 Feb;101(2):279-83. PMID: 19190810.
- Perdomo J, Leung HHL, Ahmadi Z, Yan F, Chong JJH, Passam FH, Chong BH. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat Commun. 2019 Mar 21;10(1):1322. doi: 10.1038/s41467-019-09160-7. PMID: 30899022; PMCID: PMC6428879.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.