> General Science. 2021 July 05;2(7):543-547. doi: 10.37871/jbres1274.
Carcinoma Ex Pleomorphic Adenoma of the Uvula - Case Report
Joana Borges da Costa1*, Ana Isabel Gonçalves1, André Carção1, Joana Santos2, Delfim Duarte1 and Miguel Viana1
2Anatomo-Phatological Department of Hospital Pedro Hispano, Portugal
- Carcinoma ex pleomorphic adenoma
- Pleomorphic adenoma
- Salivary glands
- Uvula
- Oropharyngeal tumor
Abstract
Introduction: Carcinoma Ex Pleomorphic Adenoma (CEPA) results from the malignant transformation of a benign tumor of the Salivary Glands (SG), the Pleomorphic Adenoma (PA). PA is considered the most common salivary tumor with a 5% risk of malignant transformation and its excision is recommended. CEPA is a rare tumor, corresponding to 3.6% of all salivary tumors and 11.6% of all GS carcinomas. About 18% of CEPAs affect minor SG, with the palate being the most common location. The present work serves to describe a case of a CEPA of the Uvula Minor SG (UMSG).
Case Report: We present a case report of a 57-year-old patient, with no relevant medical history, referred to the ENT consultation due to the appearance and progressive growth of a painless uvula lesion. The objective ENT examination showed a 15 mm ulcerative-vegetating lesion with apparent origin on the posterior face of the uvula. The lesion was biopsied and histopathological examination identified the presence of a neoplasm of the minor SG, probably NOS adenocarcinoma. The patient underwent Computed Tomography (CT) scan that showed an irregularity of the uvula, with no signs of invasion of the remaining soft palate, without other significant pharyngo-laryngeal changes. The patient underwent partial pharyngectomy and bilateral selective cervical ganglion dissection, and the histopathology of the surgical specimen confirmed that it was an invasive CEPA, the malignant component of the tumor corresponding to a NOS adenocarcinoma of the UMSG. The patient has been followed up in the ENT consultation, with no signs so far of loco-regional recurrence.
Discussion/Conclusion: In the presented case, the patient probably developed an undiagnosed PA that had become malignant over time. Given that it is a poor prognosis neoplasm, it’s essential that the ENT specialists are aware of this disease, in order to facilitate and anticipate the diagnosis and treatment as much as possible.
Abbreviations
CEPA: Carcinoma Ex Pleomorphic Adenoma; CT: Computed Tomography; ENT: Ear, Nose and Throat Specialist; MSG: Minor Salivary Glands; PA: Pleomorphic Adenoma; SG: Salivary Glands; UMSG: Uvula Minor Salivary Glands
Introduction
Carcinoma Ex Pleomorphic Adenoma (CEPA) is a rare and aggressive epithelial tumor that arises from the malignant transformation of a benign Pleomorphic Adenoma (PA) [1-4], which is the most common Salivary Glands (SG) tumor, representing 45-74% of all salivary tumors and about 40% of Minor SG (MSG) tumors [5,6]. On the other hand, CEPA is more unusual than PA, comprising about 3.6% of all SG tumors, 11.6% of all SG carcinomas and about 18% of the MSG tumors, being the palate the most common location [3].
CEPA has a prevalence rate of 5.6/100.000 cases of malignant neoplasms and it mainly affects patients in their sixth to eighth decades of life [7].
The CEPA pathogenesis is not well understood but, once that it may result from a carcinomatous transformation within a primary or recurrent PA, it seems that the accumulation of mutations on long-standing tumors may be a crucial event in CEPA development [8].
The most common clinical presentation of CEPA is the appearance of a bulky, slow-growing, painless mass, in the majority of cases in the parotid or submandibular glands [9]. CEPA can also be asymptomatic and often have similar clinical presentations as PA, once it’s not commonly invasive [10]. The late onset pain usually results from local invasion of adjacent tissues [10]. In some cases, patients may complaint of skin alterations, lymphadenopathy, dysphagia and dental pain [2,11]. Rarely, some CEPAs can carry a slow grow for over decades of years, making difficult a timely diagnosis [2]. Indeed, the disease often poses a diagnostic challenge to clinicians and pathologists, given that, although the pathological assessment is the gold standard for making the diagnosis, histopathologically the tumor comprises a wide morphological spectrum with variable proportion of the malignant component [12,13].
PA presents a 5% risk of malignant transformation that seems to be proportional to the time that the tumor remains in situ, so surgical excision of the tumor is usually recommended, which may be followed by radiotherapy [6,9]. In general, patients with CEPA have a poor prognosis but an accurate diagnosis and aggressive surgical management of the tumor may increase the survival rates [9,14] Since the CEPA clinical presentation are quite similar to a PA, it is important that clinicians maintain a high level of clinical suspicion, which can be challenging considering the rarity of this cancer.
In this study, we describe the clinical and histopathological aspects in a case of a CEPA of the uvula MSG.
Case Report
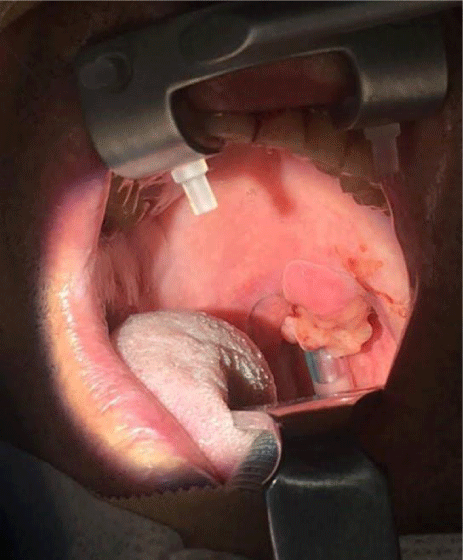
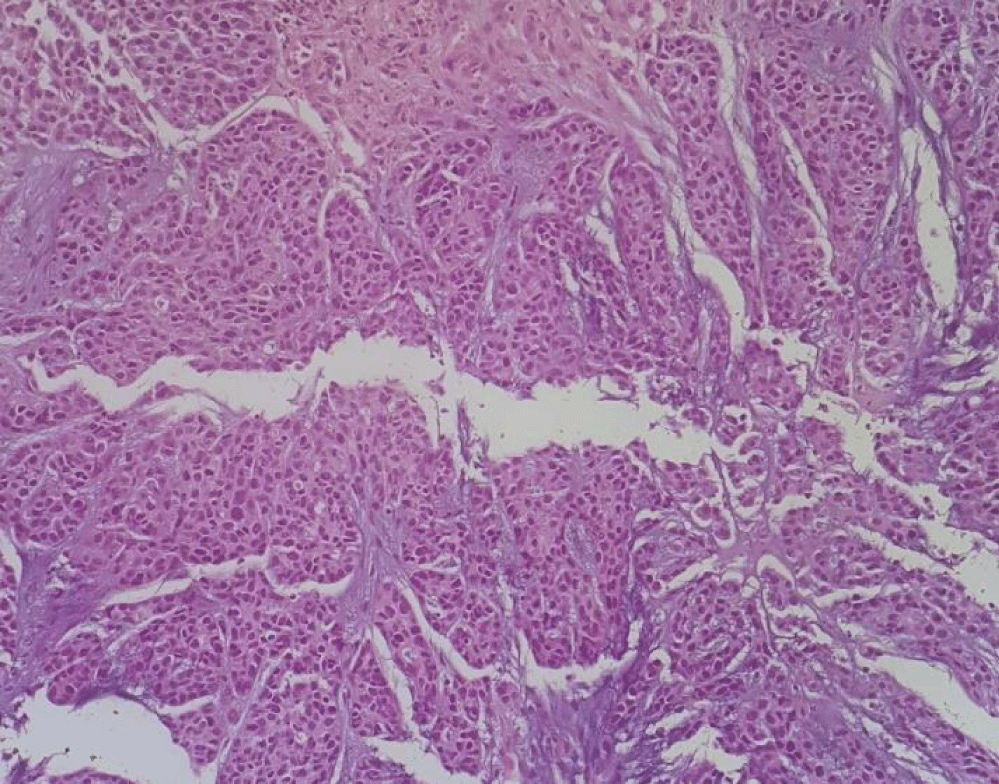
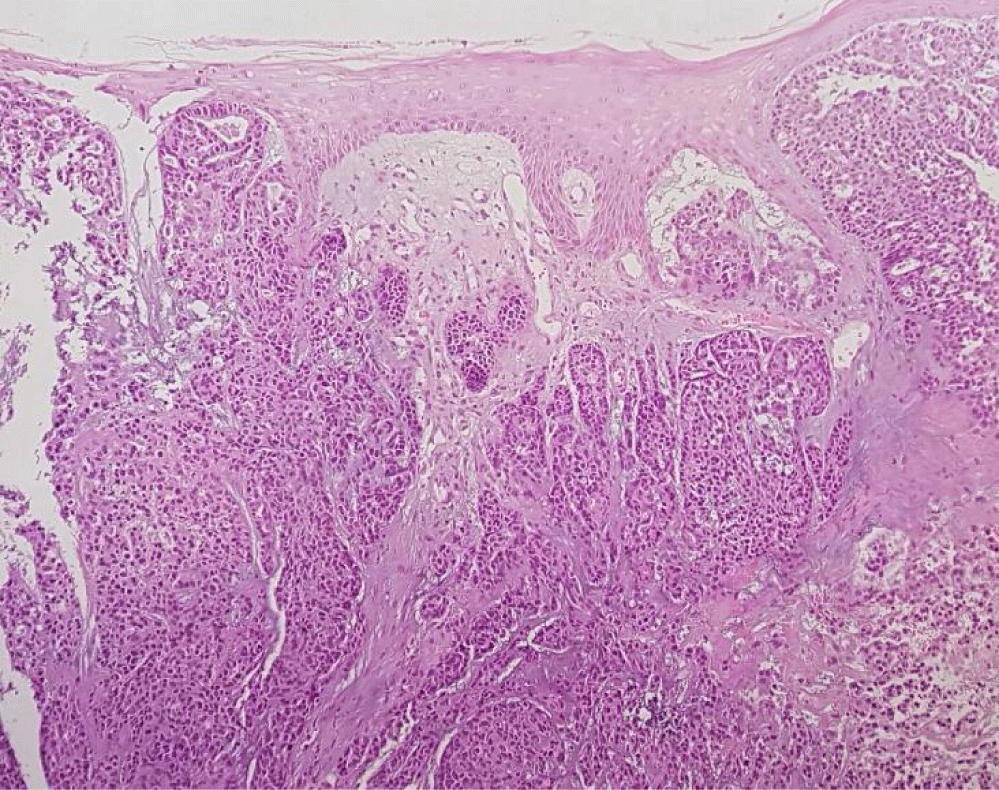
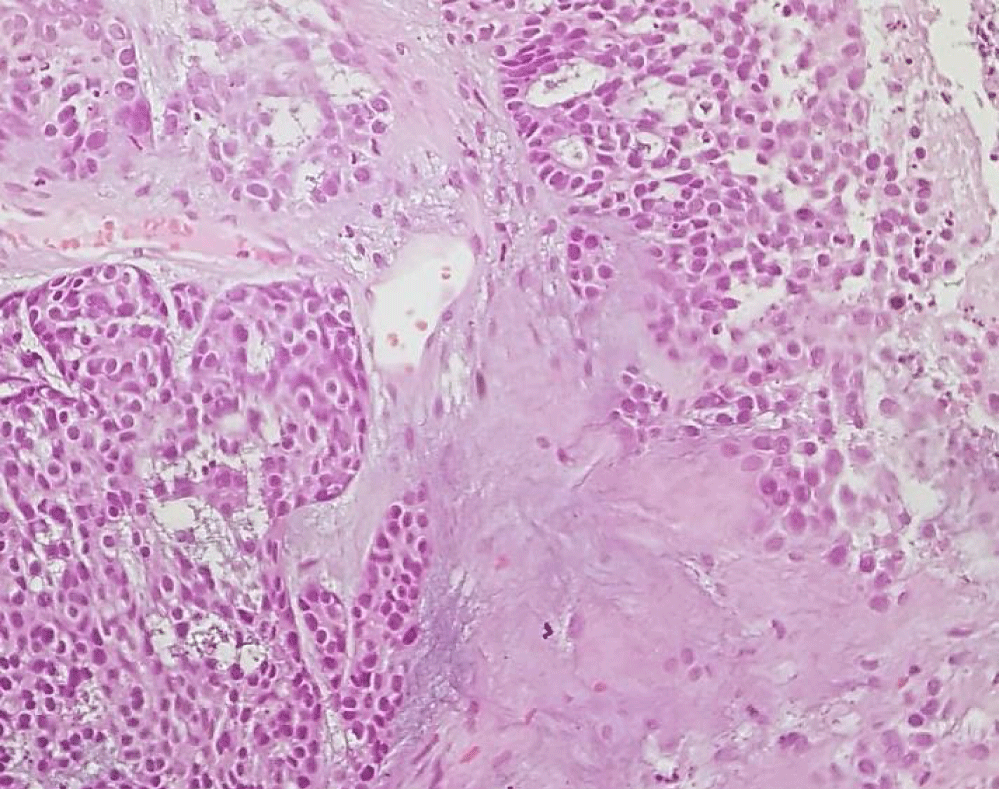
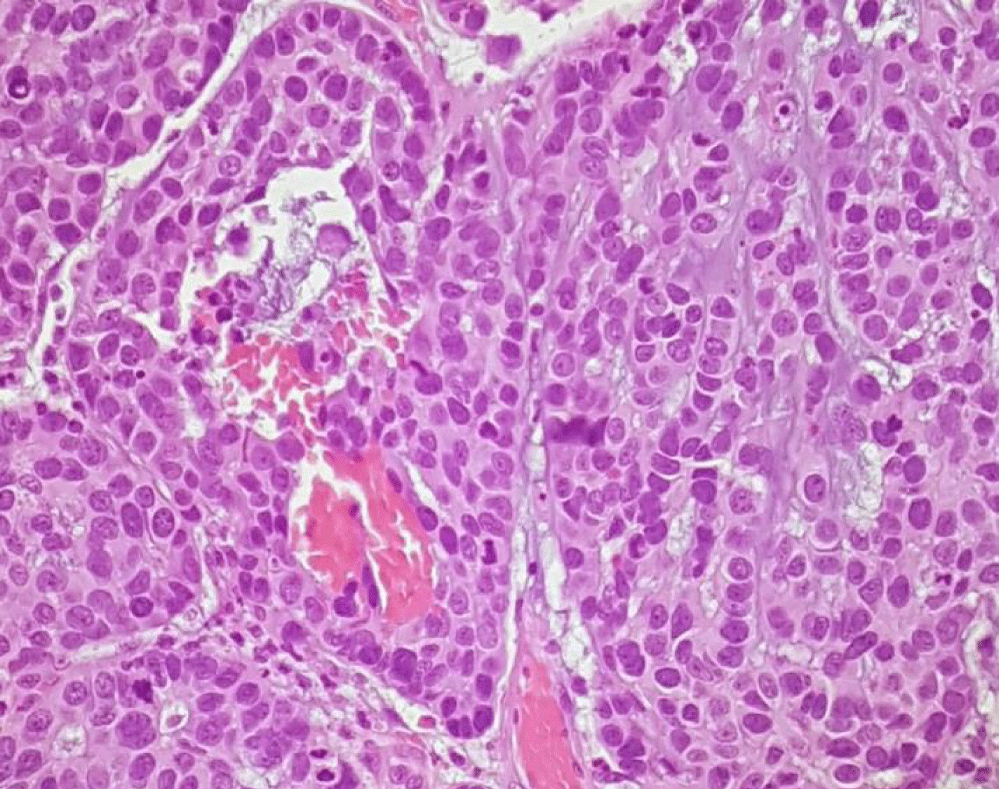
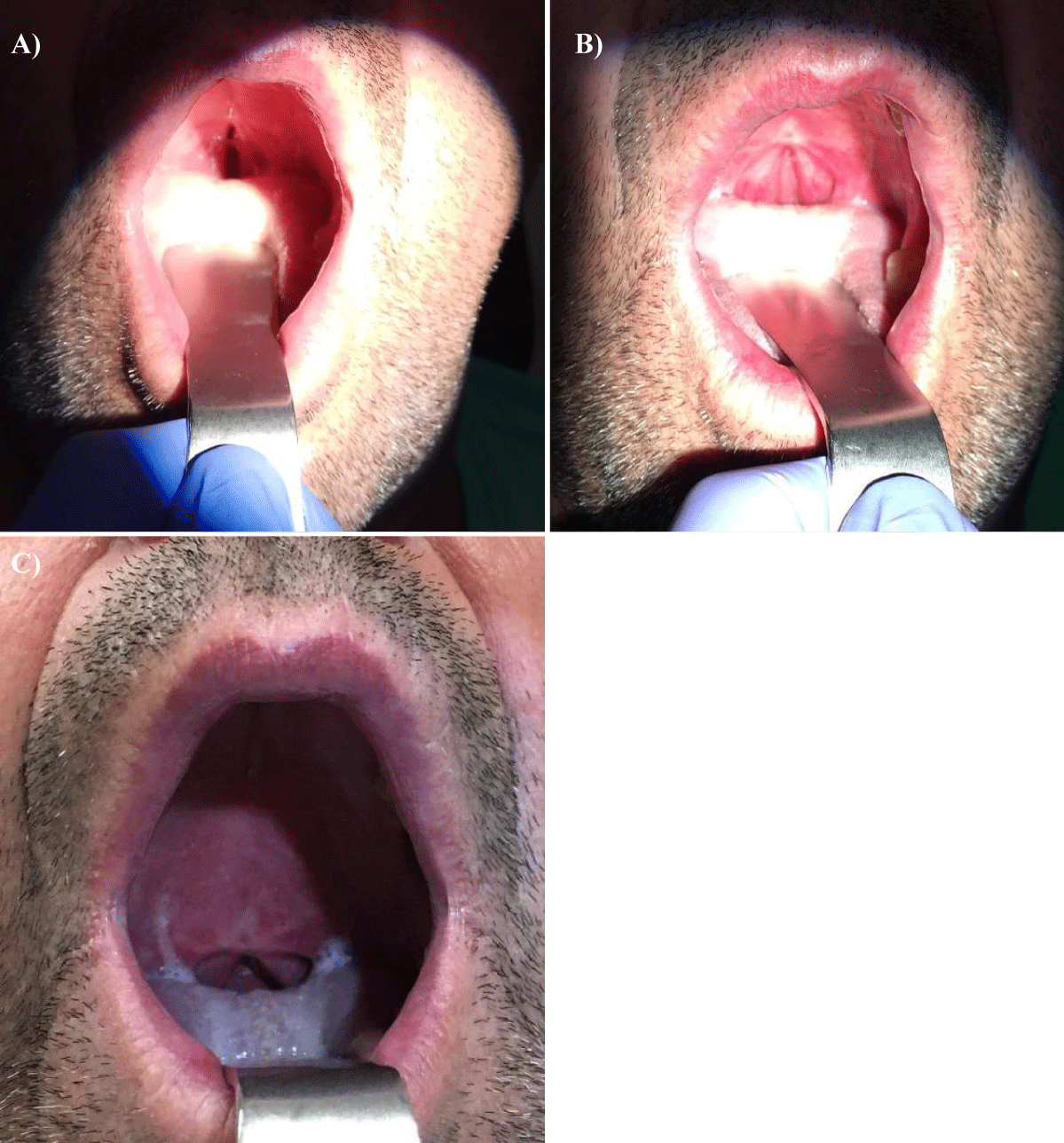
A 57-year-old white man, with no relevant medical history, presented with a progressive growth of a painless uvula lesion. On inspection, there was a 15 mm ulcerative-vegetating lesion with apparent origin on the posterior face of the uvula (Figure 1). The remaining objective examination was normal. A malignant SG tumor was suspected and a biopsy was made. The histopathological exam showed a mucosa involved by malignant epithelial neoplasia with glandular differentiation, infiltrative growth pattern in nests, cords, and formation of cribiform structures and with an intermediate degree of cytological atypia (Figure 2). The immunohistochemical study was negative for androgen receptors and the proliferative index (ki67) was 70-80%. All these aspects observed favoured the diagnosis of NOS adenocarcinoma.
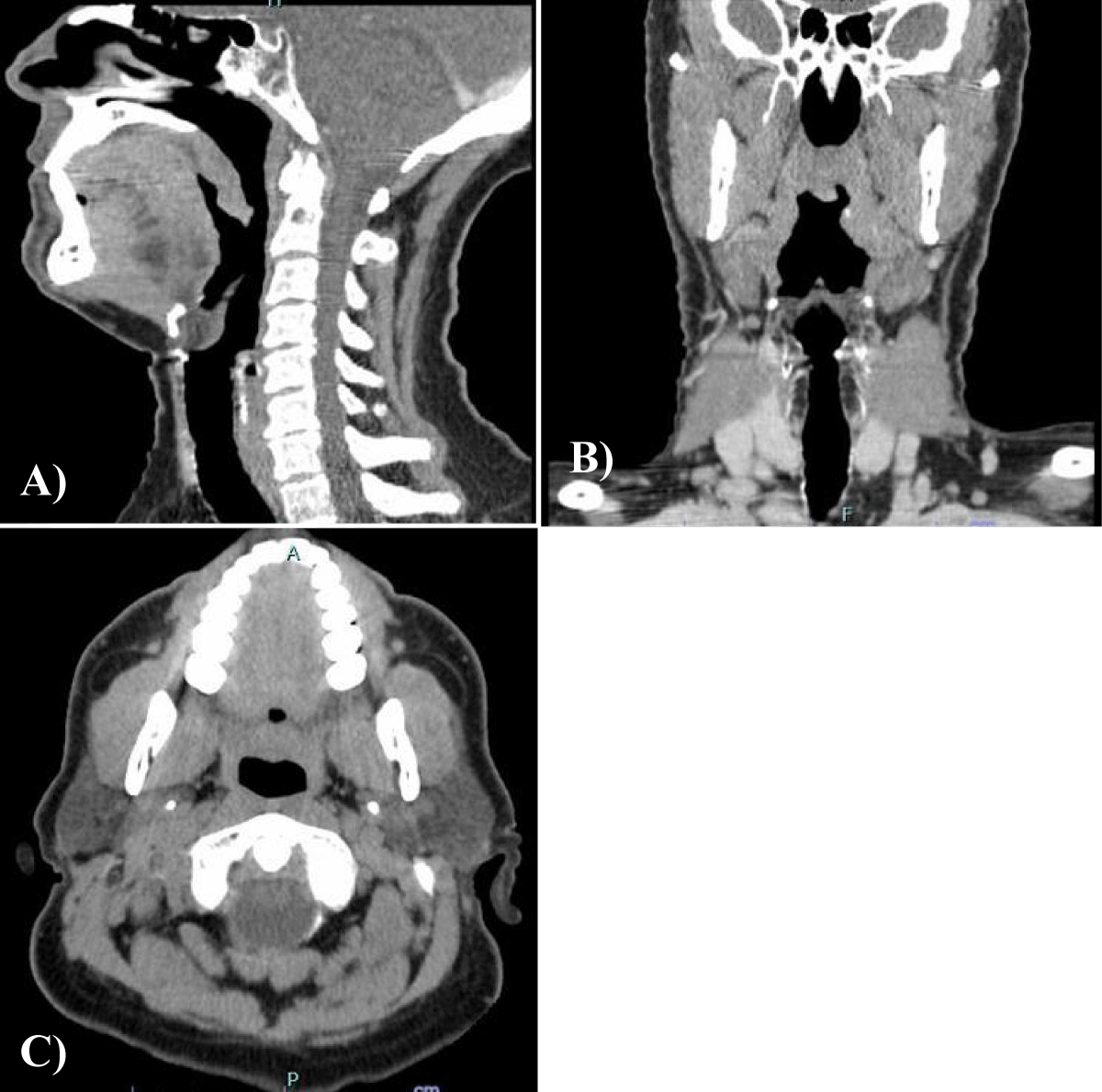
A preoperative contrasted CT scan was taken which showed a prominence and irregularity of the contours of the uvula, without apparent signs of invasion of the remaining soft palate. There were no other significant changes in the pharynx and larynx and the submandibular, parotid and thyroid glands had conserved morphology. It was possible to observe that there were enlarged lymph nodes in the right deep cervical chain (Figure 3).
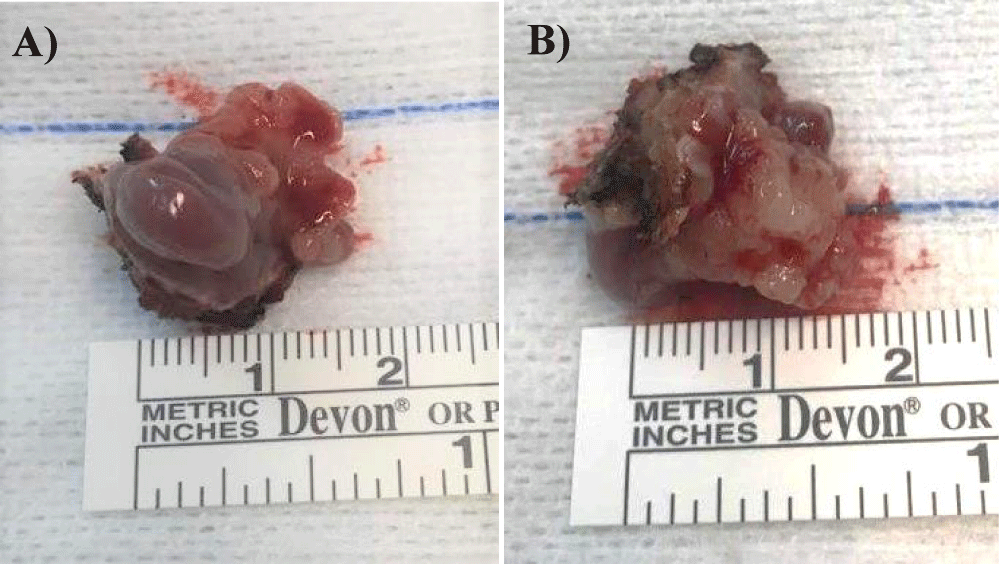
Considering the patient’s clinical symptoms and the results of the complementary diagnostic exams, the patient underwent partial pharyngectomy and bilateral selective cervical ganglionic dissection that took place without complications (Figure 4). Tumor-free margins were confirmed by microscopic examination of intraoperative frozen sections.
The postoperative histopathological report confirmed the presence of an invasive CEPA of the minor salivary glands of the uvula, the malignant component of the tumor corresponding to NOS adenocarcinoma, with 13 mm length and 6 mm invasion depth, without lymphovascular or perineural invasion, positive for BCL2, CKAE1/AE3, CK5/6, vimentin, S100 and P63 and negative for synaptophysin, chromogranin, P16, AML and CEA. There were not any ganglion metastases (Figures 5-7).
After an oncology consulting group, it was decided not to proceed with adjuvant therapy, keeping the patient under clinical surveillance, with monthly consultations in the first postoperative months and then quarterly. To date, the patient remains asymptomatic and with no signs of loco-regional or distant recurrence of the pathology (Figure 8).
Discussion
Pleomorphic adenoma is the most common benign SG tumor and it’s characterized by epithelial-mesenchymal gland tissue proliferation [12]. In the rare cases that PA undergo malignant transformation, a CEPA shows up. CEPA is an uncommon aggressive malignancy and, although most cases affect the major SG, there are reported cases involving oral and oropharyngeal MSG, most of which in the palate, but other sites as the tongue, buccal mucosa and tonsil may also be affected [14].
The accurate CEPA pathogenesis is still controversial because some authors believe that these tumors are malignant from the onset [15] and others defend that CEPA is the result of a carcinomatous transformation of a benign mixed pre-existing lesion [16]. This suggests that as the mixed tumor grows, the cells collect several transformations that may induce a carcinomatous change, justifying the fact that the risk of CEPA seems to be related with the PA duration [12].
Carcinomas arising from PA may present a wide morphological spectrum but, in most cases, CEPA is usually a high-grade adenocarcinoma or undifferentiated carcinoma that characteristically shows as histological features a capsule invasion, haemorrhage and necrosis alternating with areas with PA benign classical features [17]. Sometimes, the malignant component may be adenoid cystic carcinoma, mucoepidermoid carcinoma or salivary duct carcinoma or may also be a mixture of subtypes [10]. Based on the presence and extent of invasion of the fibrous capsule, CEPA can be subdivided into non-invasive, minimally invasive and invasive sybtipes, with prognosis implications [18]. The non-invasive subtype has its malignant component confined within the PA fibrous capsule, it typically marks the beginning of the PA carcinomatous transformation and it tends to behaviour like a PA [13]. The minimally invasive cases present a malignant component that penetrates <1.5 mm into extracapsular tissue and invasive CEPA is defined as an extracapsular invasion >1.5 mm by the malignant portion [1]. The importance of this classification is due to the fact that, according to LiVolsi [13], the extent of tumor infiltration beyond the capsule is the most reliable prognostic marker, in a way that patients with non-invasive or minimally invasive tumors have a low recurrence rate and usually no metastasis, whereas patients with invasive tumors have a poor prognosis, with a 23-50% recurrence rate and up to 70% distant metastases [1].
Treatment for CEPA often involves an ablative surgical procedure which may or may not be combined with concomitant neck ganglionic dissection and followed by adjuvant therapy [9]. The surgical approach varies according to the CEPA localization and grade and post-operative radiotherapy is commonly used for high grade disease, in cases of questionable resection adequacy and for lymph node and peri-neural invasion [19]. A combination of post-op radio and chemotherapy may also be an option for some patients, despite there is limited literature demonstrating the effectiveness of chemotherapy in the management of CEPA [4,19].
Features associated with an unfavourable prognosis include a high tumor grade, large size, soft tissue invasion, perineural invasion and lymph node metastases [12]. CEPA metastasizes exclusively as a carcinoma and distant metastases, with particularly affinity to lung and bone, are more common than regional ones [20].
The presented case report probably represents a case of an undiagnosed PA that may had become malignant over time. It was decided to perform a bilateral selective cervical ganglionic dissection due to the identification of cervical adenopathies on CT. Even though it was an invasive tumor, it is considered that the prognosis is good given the absence of many of the features associated with an unfavourable prognosis. Despite that, the patient must be followed up for a large period of time.
Conclusion
In summary, we report an unusual case of uvula minor salivary gland CEPA composed of adenocarcinoma without nodal involvement based on histopathological and immunohistochemical findings.
This type of tumor is difficult to diagnose, as the mixed tumor component is often small and overlooked, and the malignant component may be difficult to classify. As the presenting symptoms are quite similar to those presenting with benign PA, it is important that clinicians maintain a high level of clinical suspicion, which can be challenging, considering the rarity of this cancer. Improved understanding of the pathobiology of this tumor and other salivary gland tumors may lead to rationally designed targeted therapies that could improve the outcome of patients with CEPA.
References
- Barnes L, Eveson JW, Reichart P, Sidransky D. Pathology and Genetics of Head and Neck Tumours. In: WHO Classification of Tumours (Third edition), WHO Press, Geneva, Switzerland. 2005;9.
- Olsen KD, Lewis JE. Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck. 2001 Sep;23(9):705-12. doi: 10.1002/hed.1100. PMID: 11505478.
- Gnepp DR. Malignant mixed tumors of the salivary glands: a review. Pathol Annu. 1993;28 Pt 1:279-328. PMID: 8380049.
- Nouraei SA, Hope KL, Kelly CG, McLean NR, Soames JV. Carcinoma ex benign pleomorphic adenoma of the parotid gland. Plast Reconstr Surg. 2005 Oct;116(5):1206-13. doi: 10.1097/01.prs.0000181654.68120.0f. PMID: 16217459.
- Weidner N, Cote R, Suster S, Weiss L. Modern Surgical Pathology, Volume 1 (2nd Edition), Saunders Elsevier, Philadelphia, United States of America. 2009.
- Jorge L, Gouveia M, Rodrigues J, Luís A. Carcinoma ex-adenoma pleomórfico - a propósito de um caso clínico. Rev Port Estomatol Med Dentária e Cir Maxilofac. 2013 Oct 1;54:e44.
- Cardesa A, Slootweg PJ, Gale A, Franchi A. Pathology of the Head and Neck (2nd Edition), Springer-Verlag Berlin Heidelberg, Heidelberg, Germany. 2016.
- Matsubayashi S, Yoshihara T. Carcinoma ex pleomorphic adenoma of the salivary gland: an immunohistochemical study. Eur Arch Otorhinolaryngol. 2007 Jul;264(7):789-95. doi: 10.1007/s00405-007-0256-6. Epub 2007 Feb 20. PMID: 17310348.
- Antony J, Gopalan V, Smith RA, Lam AK. Carcinoma ex pleomorphic adenoma: a comprehensive review of clinical, pathological and molecular data. Head Neck Pathol. 2012 Mar;6(1):1-9. doi: 10.1007/s12105-011-0281-z. Epub 2011 Jul 9. PMID: 21744105; PMCID: PMC3311945.
- Zbären P, Zbären S, Caversaccio MD, Stauffer E. Carcinoma ex pleomorphic adenoma: diagnostic difficulty and outcome. Otolaryngol Head Neck Surg. 2008 May;138(5):601-5. doi: 10.1016/j.otohns.2008.01.013. PMID: 18439465.
- Dewan K, Owens J, Silvester K. Maintaining a high level of suspicion for recurrent malignant disease: report of a case with periapical involvement. Int Endod J. 2007 Nov;40(11):900-7. doi: 10.1111/j.1365-2591.2007.01281.x. Epub 2007 Aug 30. PMID: 17764459.
- Keerthi R, Raut RP, Vaibhav N, Ghosh A. Carcinoma ex pleomorphic adenoma: Diagnostic dilemma and treatment protocol. Indian J Dent. 2014 Jul;5(3):157-60. doi: 10.4103/0975-962X.140840. PMID: 25565746; PMCID: PMC4213878.
- LiVolsi VA, Perzin KH. Malignant mixed tumors arising in salivary glands. I. Carcinomas arising in benign mixed tumors: a clinicopathologic study. Cancer. 1977 May;39(5):2209-30. doi: 10.1002/1097-0142(197705)39:5<2209::aid-cncr2820390540>3.0.co;2-8. PMID: 192443.
- Sedassari BT, da Silva Lascane NA, Tobouti PL, Pigatti FM, Franco MIF, de Sousa SCOM. Carcinoma ex pleomorphic adenoma of the palate composed of invasive micropapillary salivary duct carcinoma and adenoid cystic carcinoma components: an unusual case with immunohistochemical approach. Medicine (Baltimore). 2014 Dec;93(27):e146. doi: 10.1097/MD.0000000000000146. PMID: 25501054; PMCID: PMC4602770.
- Gerughty RM, Scofield HH, Brown FM, Hennigar GR. Malignant mixed tumors of salivary gland origin. Cancer. 1969 Sep;24(3):471-86. doi: 10.1002/1097-0142(196909)24:3<471::aid-cncr2820240309>3.0.co;2-0. PMID: 4310021.
- BEAHRS OH, WOOLNER LB, KIRKLIN JW, DEVINE KD. Carcinomatous transformation of mixed tumors of the parotid gland. AMA Arch Surg. 1957 Oct;75(4):605-13; discussion 613-4. doi: 10.1001/archsurg.1957.01280160115015. PMID: 13457639.
- Myers EN, Ferris RL. Salivary gland disorders. Ed. Springer (1st Edition). Springer-Verlag Berlin Heidelberg, Heidelberg, Germany. 2007;3:65-67.
- Altemani A, Martins MT, Freitas L, Soares F, Araújo NS, Araújo VC. Carcinoma ex pleomorphic adenoma (CXPA): immunoprofile of the cells involved in carcinomatous progression. Histopathology. 2005 Jun;46(6):635-41. doi: 10.1111/j.1365-2559.2005.02157.x. PMID: 15910594.
- Lüers JC, Wittekindt C, Streppel M, Guntinas-Lichius O. Carcinoma ex pleomorphic adenoma of the parotid gland. Study and implications for diagnostics and therapy. Acta Oncol. 2009;48(1):132-6. doi: 10.1080/02841860802183604. PMID: 18607845.
- Mitchell DA, Eveson JW, Ord RA. Polymorphous low-grade adenocarcinoma of minor salivary glands--a report of three cases. Br J Oral Maxillofac Surg. 1989 Dec;27(6):494-500. doi: 10.1016/s0266-4356(89)80008-7. PMID: 2688740.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.