> Medicine Group. 2021 July 21;2(7):567-573. doi: 10.37871/jbres1278.
Assessment of Nanosilver Hemocompatibility in Prehypertensive Salt-Induced Animal Model
Ogechukwu K Uche1*, Esiri F Ohiambe1 and Fabian C Amechina2
2Department of Pharmacology, Faculty of Pharmacy, University of Benin, Benin city, Nigeria
- Nanosilver
- Salt-loaded rats
- Hemocompatibility
- Haematological parameters
Abstract
Aim: There are Conflicting reports on safety profile of nanoparticles on biological cells. This study evaluated the impact of nanosilver on hemocompatibility on salt-loaded rats.
Materials and Methods: Sprague-Dawley rats [(inbred) (120-140 g)] randomly divided into of 4 groups, (n = 6) were studied. Group 1(control) received normal rat chow and tap water, Group 2 received rat chow containing 8% NaCl [(salt-loaded rats (SLRs)]. Group 3 received rat chow + Nanosilver Solution (NS) 0.18 mL 10 ppm/kg/day. Group 4 comprised SLRs + NS. After 6 weeks oral gavage treatments, measurements of Blood pressure (Bp) and Heart Rate (HR) were by pressure transducer via cannulation of left common carotid artery following anaesthesia with urethane. HR was computed by the number of arterial pulse per 60 seconds. 5 ml of blood for WBC, PLATELETS, RBC, PCV, HB, MCH, MCHC and MCV analyses using automated haematology analyser and Osmotic fragility reactivity with standard spectrophotometer at 540 nm wavelength.
Results: Exposure of nanosilver to normotensive rats resulted in significantly lower RBC level compared with control, whereas RBC level in Salt-Loaded Co-Treated Nanosilver (SCNS) was comparable with the SLRs. The tenet was the same for HB, PCV, MCH and MCHC. Nanosilver induced leukopenia in normotensive compared with control and prevented WBC elevation in SCNS. Platelets significantly increased in Nanosilver-Treated Normotensive Rats (NTNRs) compared with control and decreased in SCNS. Osmotic burst resistance increased in NTNRs and decreased in cells from treated groups.
Conclusion: Chronic exposure of nanosilver to salt loaded rats alters haematological parameters which may worsen circulatory function and activate risk factors of cardiovascular disorders.
Introduction
The Erythrocytes or Red Blood Cells (RBCs) are the most abundant cellular constituents of blood that play key role in maintaining homeostasis in the internal milieu and provide ideal transport medium for delivery of a host of several organic and inorganic biological substances including drugs and nanoparticles to target sites. This essential function of blood is linked to its unique properties of bioavailability, biocompatibility, and longevity in circulation [1]. However, rheological studies investigating blood-biomaterial interactions have shown that foreign materials and compounds could interact with endogenous substances in blood and body cells including microparticles and platelets to provoke activation of the complement systems, inflammatory responses, thrombosis and endothelial cell dysfunction and tissue damage which may alter and disrupt cellular signalling pathways and physiological functions [1,2].
Empirical epidemiological studies and several experimental animal models, on dietary high sodium salt intake have been associated with the development of high blood pressure, oxidative stress and vascular endothelial dysfunction; although the underlying mechanisms have not been fully elucidated [3]. Multifactorial combination of genetic, environmental, modification in lifestyles and complex web of intravascular interactions of vasoactive substances and dietary factors other than sodium can play a role in the degree and etiology of persistent elevation in blood pressure in rodents and humans [4-6]. Experimental animal models have shown that Sprague-Dawley rats [(SDR, (inbred)] rapidly develop high blood pressure (>170 mm Hg) when fed 8% NaCl diet [7-9]. Hypertension is a major factor in the pathogenesis of several diseases including chronic kidney disease, stroke, and increase incidence of Cardiovascular Disease (CVD) although the effects of high-sodium salt intake on haematological parameters has not been fully characterised.
Functionally, osmotic fragility is widely used to determine erythrocyte integrity and elucidate mechanisms of the influence of different factors on the osmotic properties of RBC membranes, including shear stress and mechanical haemolysis, temperature, ultrasound effects, drugs and irradiation [10,11]. Clinically, osmotic fragility test is also useful for diagnosis of certain haematological diseases like haemolytic anaemia, hereditary spherocytosis, and elliptocytosis, glucose-6-phosphate dehydrogenase deficiency and sickle cell anaemia, as well as for RBC from uremic or diabetic patients [11].
In recent times, nanotechnology has attracted much attention among research scientists and has gained wide biomedical applications as therapeutics, antimicrobial agents, transfection vectors, commercial cosmetic products, fluorescent labels, anti-angiogenic, antitumor, biosensors, and bioimaging especially in veterinary and dependent sciences [12]. Nanotechnology is also applied in dental oral care, especially as a major component in dental amalgams used for tooth restoration, dental prostheses, restorative and endodontic dentistry and implantology due to their unique properties [13]. Among other nanomaterials in recent time, Nanosilver has shown huge promises of tremendous scientific and wide biomedical applications due to the novel physico-chemical properties and their potent anti-microbial activity, stability at room temperature, high surface area to mass ratio and bioavailability in both in vitro and in vivo experimental models [14]. Nanoparticles are frequently used as a tool for drug delivery in nanomedicine and are categorised into several different groups such as polymers, inorganic nanoparticles and metallic nanoparticles, depending on their physicochemical properties [15]. Despite the growing application and patronage of nanotechnology, nanosilver particles have been shown to accumulate mainly in the liver, kidney, lung and spleen and can also diffuse through cell membranes easily and cause severe adverse effects on human health [16,17]. Available literature has shown that Silver-Nanoparticles (Ag-NPs) exposure causes toxicity in some fish species and other aquatic animals [18]. Recent studies however have demonstrated a high diffusivity of nanosilver across plasmalemma to influence vascular smooth muscle reactivity and endothelial function on isolated carotid artery and the ability of nanosilver particles to cross the capillary wall causing oxidative stress and genotoxicity [19-21]. 2015, In relation to its high cellular uptake and membrane properties, there are conflicting reports on the potential adverse effects and/or toxicity of nanosilver particles on body organs. And interactions between nanoparticles and blood cells are still poorly understood and vastly understated. The present study examined the impact of nanosilver exposure on hemocompatibility in salt-loaded Sprague-Dawley rats.
Materials and Methods
Animals
Twenty-Four (24) male Sprague-Dawley rats were randomly selected and divided into four groups of six rats each after two weeks of acclimatization. Animals were humanely handled according to animal care standard protocols for the use of laboratory animals, and the College of Basic Medical Sciences Ethical Committee’s guideline on the use of laboratory animals. Animals were fed on normal rat chow and allowed free access to tap water ad libitum.
Group one, Control Rats (CR) were fed with normal rat chow and water ad libitum. Group 2, received high salt diet containing 8% NaCl and water [(Salt-Loaded Rats (SLRs)] as described by Sofola, et al. [7]. Group 3 received normal rat chow and with 0.18 mL /kgbw/day of 10 ppm 50 mg Nanosilver Solution (NS), while group four received high salt diet, but treated with 0.18 mL/kgbw/day of 10 ppm 50 mg nanosilver solution orally for a period of 6 weeks. The blood pressure and heart rate were measured using direct method (blood pressure transducer via cannulation of the left coon carotid artery) following anaesthesia with urethane. 5 ml of blood was collected via left common carotid artery from each animal into Ethylene Diamine Tetra Acetic Acid (EDTA) sample bottles, and analysed for haematological indices using automated haematology analyser and osmotic fragility test within 2 hours of collection.
Osmotic protocols
The Osmotic Fragility (OF) test was done on all blood samples from each group. The samples were graded percentage concentrations of NaCl (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8 and 0.9) as described by Dacie and Lewis [22]. Briefly, blood samples were reacted directly with NaCl salt to access their resistivity to various hypotonic stress. Haemolysis in each tube was expressed as a percentage of the absorbance in distilled water. The optical absorbance was recorded following 30 mins incubation at 37°C with a standard spectrophotometer at 540 nm wavelength. The average values recorded were plotted against the different NaCl concentrations used.
Statistical analysis
Graphs and statistical analyses were by means of OriginPro 8.0 software and Students t-test; followed by one-way Analysis of Variance (ANOVA). Data are presented as means ± SEM. p < 0.05 was considered statistically significant; while n-values denote number of animals in each experimental group.
Results
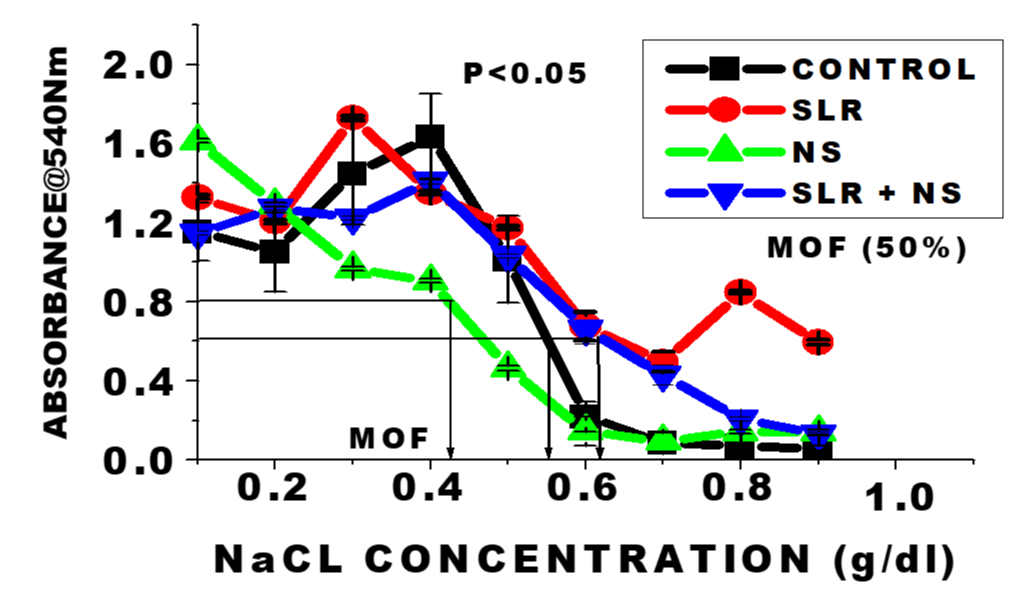
Optical density of haemolysis curves and mean osmotic fragility concentrations for the untreated (Figure 1), Control (CTR), and the test groups at varying concentrations of NaCl (g/dL) at 540 nm wavelength. n = 6 means; ± SEM. There was a leftward-shift of the curve with NS-treated cells in normotensive rats compared with the control; suggesting increase burst of osmotic resistance whereas there was a rightward-shift in the SLRs and the SLRs co-treated NS compared with the control. These indicate increase in osmotic fragility response of the red cells to hypotonic stress. Mean Osmotic Fragility (MOF) (concentration of the solution when 50% of the cells are haemolysed) is a measure of the Osmotic Fragility (OF) parameter. OF magnitude was in the order: SLRs>SLRs+NS>CTR>NS (0.63; 0.62;0.55>0.42) respectively p < 0.05.
Haematological parameters
Figures 2-8 are the results of assays of blood following exposures to nanosilver solution on haematological parameters (total RBCs, HB, PCV, MCH, MCHC, MCV, Platelets, WBC and differential cells in normotensive and salt-loaded sprague-dawley rats.
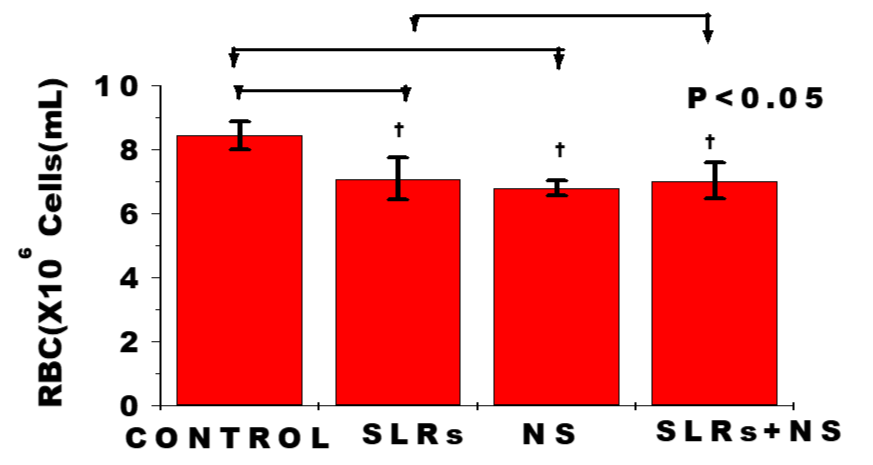
In figure 2, exposure to nanosilver resulted in significantly lower RBC level in cells from normotensive group compared with the control, whereas RBC level from cells of the salt-loaded rats significantly decreased compared with the control but remained fairly constant compared with the salt-loaded-cotreated nanosilver group. The pattern was the same with the PCV measurement; n = 6 mean, ± SEM, p < 0.05.
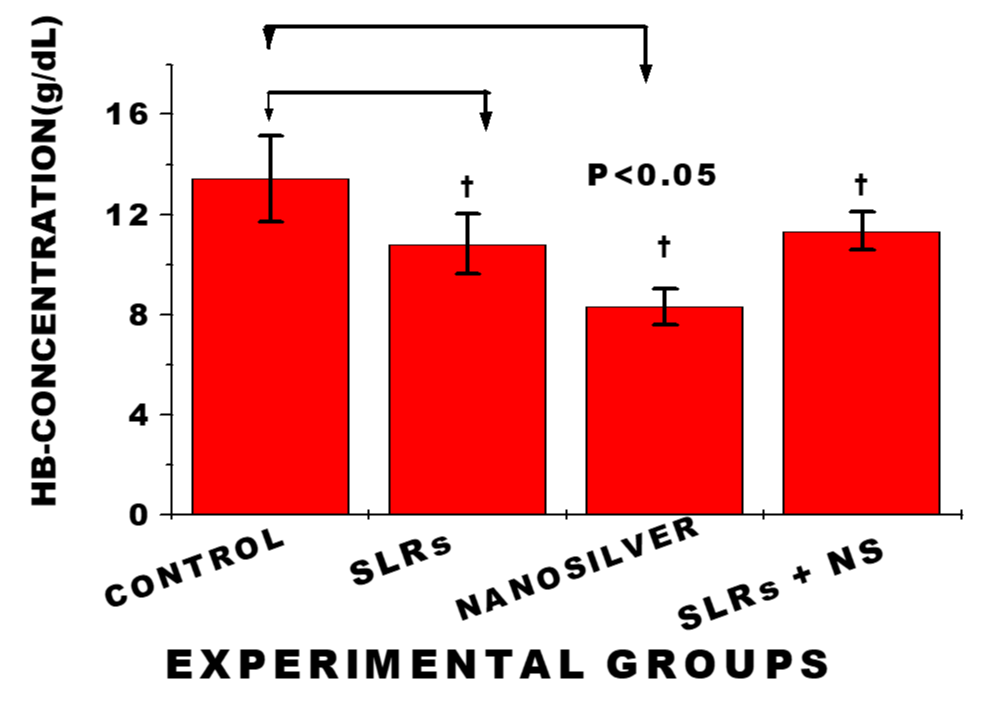
Exposure to nanosilver resulted in significantly lower haemoglobin level in cells from normotensive group compared with the control, whereas HB-level from cells of the salt-loaded rats significantly decreased compared with the control. While HB-level of cells from the salt-loaded cotreated remained fairly constant compared with the salt-loaded group; n = 6 mean, ± SEM, p < 0.05.
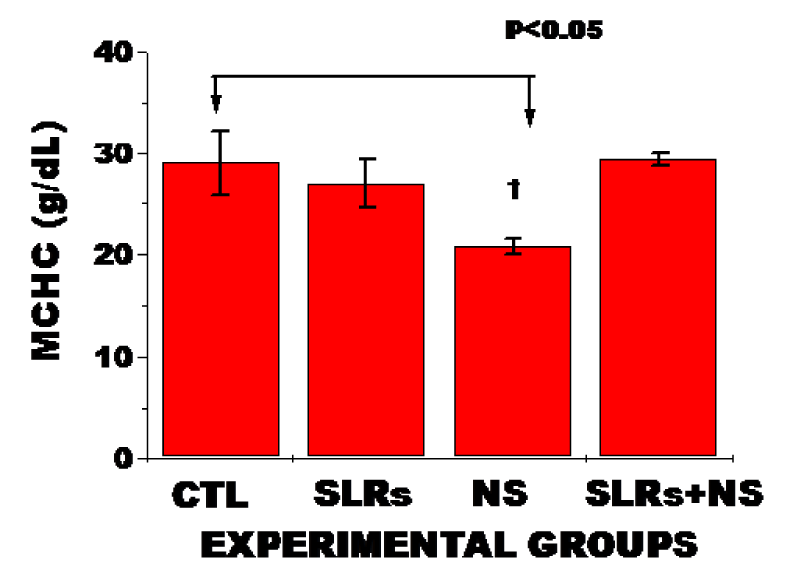
As shown in figure 4, exposure to nanosilver in normotensives animals resulted in significantly decreased MCHC compared with the untreated group. The pattern was the same in MCH; n = 6 mean, ± SEM, p < 0.05.
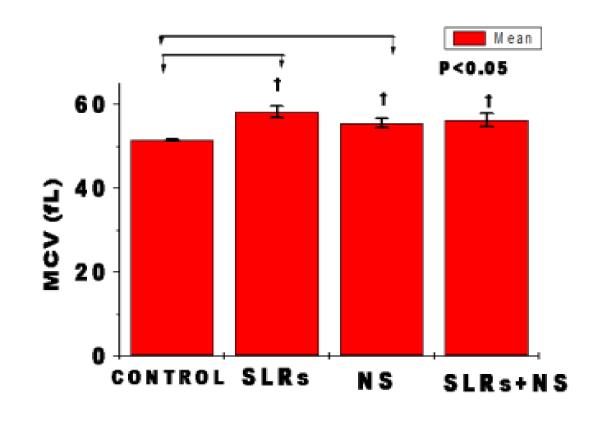
In figure 2F, exposure to nanosilver in normotensive rats resulted in significantly higher MCV level compared with the control. Whereas MCV-level from cells of the salt-loaded significantly increased compared with the control but remained fairly constant compared with the salt-loaded nanosilver-cotreated group. n = 6 mean, ± SEM. p < 0.05.
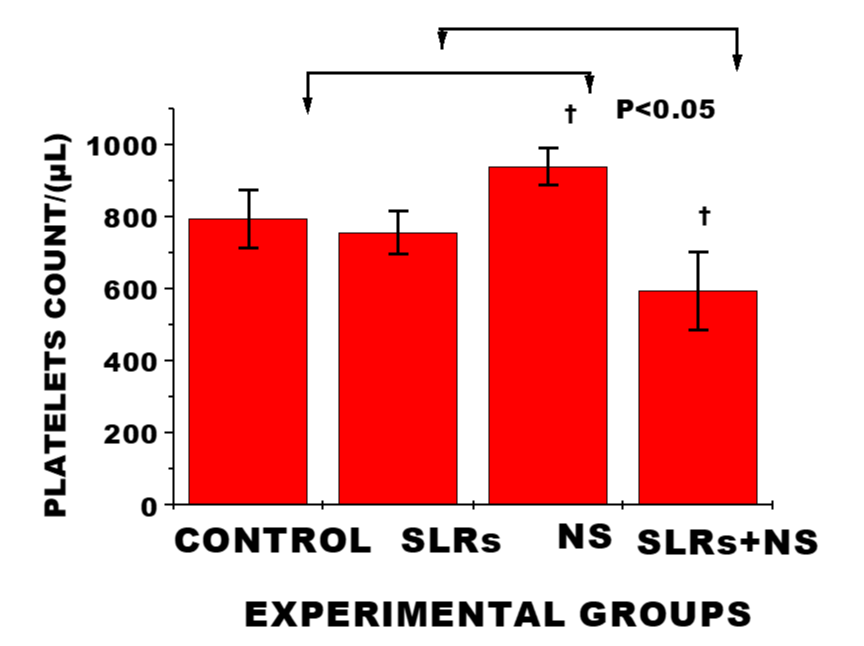
Exposure to Nanosilver in the normotensives resulted in significant increase in platelets level compared with the control, whereas platelets level was significantly lower in salt-loaded rats cotreated with nanosilver compared with the salt-loaded rats; n = 6 mean, ± SEM. This may indicate increased platelet adhesion and impart on coagulation cascade/ procoagulant potential/ increase thrombin activity.
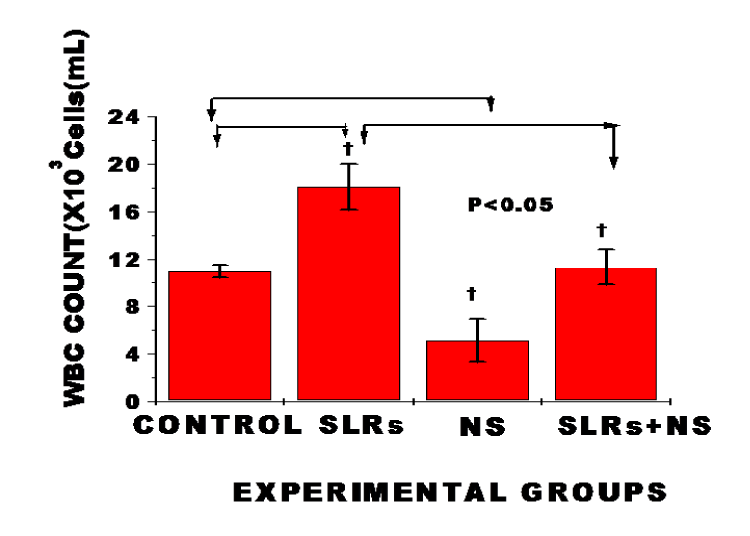
Total WBC count was significantly increased in salt-loaded rats compared to the control, whereas exposure to nanosiliver resulted in significantly lower WBC count in normotensive rats compared with the control and prevented WBC increase in salt-loaded co-treated nanosilver compared to the salt-loaded group; n = 6 means ± SEM. p < 0.05; suggesting reduction in circulating number of WBC probably due to increased utilization and destruction of WBC by nanosilver.
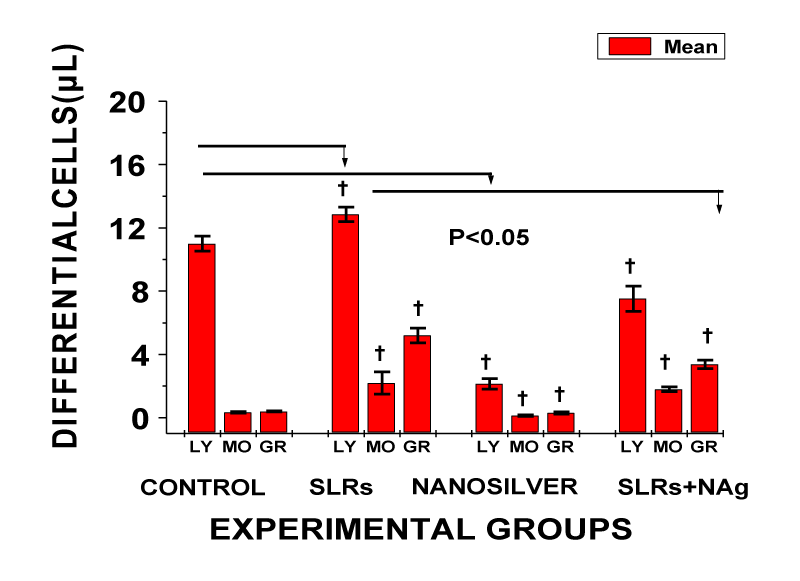
In WBC differential cells, exposure resulted in significantly lower lymphocytes, monocytes and granulocytes levels in the normotensive compared with the control and prevented increase in cells in salt-loaded co-treated with nanosilver cells compared to the salt-loaded group; n = 6 means ± SEM, p < 0.05.
Discussion
Hemocompatibility refers to the evaluation of critical interactions between foreign biomaterials and the different components of blood to determine if any adverse effects and/or toxicity may arise from the exposure of foreign substances to blood. Various experimental modules have been employed in hemocompatibility evaluations. In this study, hemocompatibility was evaluated from data obtained on various haematological parameters including osmotic fragility reactivity responses, total Red Blood Cells (RBCs), Haemoglobin Concentration (HB), Packed Cell Volume (PCV), Mean Cell Haemoglobin (MCH), Mean Cell Haemoglobin Corpuscle (MCHC), Mean Cell Volume (MCV), platelets, total White Blood Cells (WBCs) and differential white cells (lymphocytes, monocytes and granulocytes) by assay of blood collected from the experimental animals (Figures 1-8). The data on fragility showed a decreasing osmotic lysis demonstrated by a leftward-shift of the haemolysate curve of the normotensive rats exposed to 10 ppm nanosilver compared to the untreated control group (Figure 1). This suggests an increase in osmotic burst resistance in the group treated with nanosilver. This result correlates with earlier report of no significance nanosilver-induced lysis after acute exposure on fish [23]. Osmotic Fragility (OF) is defined by shifts in the haemolysis curve, which relates absorbance to varying hypotonic NaCl concentrations, and is often established by determination of 50% of the haemolysis points. The increase in osmotic burst resistance could be attributable to the unique small size and physiochemical properties of nanosilver particles which enhanced rapid direct membrane permeability across cells for even distribution in blood [24]. On the contrary, there was a rightward-shift of fragility curves from cells in the salt-loaded co-treated with nanosilver, suggesting an increase in erythrocyte fragility. The increase in fragility approximated to that from the cells of the salt-loaded group. The magnitude of mean osmotic fragility concentrations (concentration of the solution when 50% of the cells are haemolysed) in this study was in the order: SLRs>SLRs+NS>CTR>NS respectively. Hypernatremia is known to increase erythrocytes fragility [11]. Therefore nanosilver may be acting in synergy with sodium in the salt-loaded co-treated with nanosilver animals to induce the increase haemolytic effect. This observation may have some relevance in nanomedicine application in relation to those with predisposed hypertensive condition in clinical setting since increase release of haemoglobin can lead to adverse health effects; including haemolytic anaemia, pulmonary hypertension, and renal toxicity [10]. Osmotic fragility test is also useful in clinical diagnosis of certain haematological diseases, such as hereditary spherocytosis, chronic liver disease, glucose-6-phosphate dehydrogenase deficiency and sickle cell anaemia [11].
The cellular component of blood principally comprises red blood cells (erythrocytes), white blood cells (leukocytes) and platelets (thrombocytes) with different distinguishing structural properties for their specialised functions. The data from RBCs and haemoglobin indices, showed that chronic exposures to nanosilver on the haematological parameters significantly decreased total RBCs level, HB-concentration, PCV, MCH and MCHC in normotensive rats compared with the control (Figures 2-5). This result corroborates with those of [25,26] Shaluei, et al. [27] and Reddy, et al. [28] who have reported similar trend in their studies. Most nanoparticles have been red-labelled with potentials to induce significant cytotoxicity and oxidative stress in different cell lines and animal models despite the fast-growing increase in the application of nanoparticles in nanomedicine and consumer care products. The tendency for these has been linked to the small size, variations in concentration, period of exposure, shape and structure of nanoparticles [2,15,27]. The mechanism by which Nanosilver interacts with RBCs to induce cell cytotoxicity has been associated with direct action on membrane cell adsorption and uptake by red cells and internalisation into the cytoplasm with rapid biological activity [23]. Nanosilver is also known to aggregate after precipitation with strong affinity for interactions with biological cells, thereby influencing internalisation transduction pathways, uptake efficiency, intercellular localization, and cytotoxicity [24,28]. Ag-NPs can also be taken up by leukocytes and induce immunosupression and leukocytes cytotoxicity intracellularly through the release of highly toxic Ag+ into cells, the so called “Trojan horse mechanism” [29]. In cellular metabolism, RBCs in combination with HB play critical role in oxygen binding and transport of respiratory gases for efficient cell metabolism and energy production. Therefore, the observation in decreased RBCs level and hemoglobin indices could limit this important respiratory cell functions. MCV levels however, were significantly increased in both the normotensive rats and salt-loaded groups co-treated with nanosilver, suggesting moderate increase in the mean cell volume probably due to increase fluid absorption and/ or membrane permeability.
The impact of nanosilver on platelets was not clearly defined in this study. In normotensive rats exposure to nanosilver resulted in significant increase compared with the control, suggesting a procoagulant activity, whereas in the salt-loaded cotreated with nanosilver platelets level was significantly decreased compared with the salt-loaded group; indicating a reversal in trend (Figure 6).
In WBC, chronic exposure to nanosilver resulted in markedly lower levels of WBC (leukopenia) in normotensive rats and prevented an increase in WBC level in the salt-loaded co-treated nanosilver compared with the salt-loaded group (Figure 7). In the differential white blood cells, exposure to salt-loading resulted in significantly increased levels of lymphocytes, monocytes and granulocytes compared with the control. The reverse was the case in nanosilver exposure in normotensive and salt-loaded co-treated with NS (Figure 8). Lymphocytes, monocytes and granulocytes play critical role in inflammation, immunity and host defense system [2]. The mechanisms whereby nanosilver induced leukopenia is not known. However, it has been reported that once nanoparticles gain access into the blood stream, plasma proteins adsorb to the surface of the nanoparticles to form a protein corona that significantly influences their interactions with blood cells and cause the opsonisation of nanoparticles that make them more visible to phagocytic cells and triggers pro-inflammatory responses towards the nanoparticles [2]. In general, however, leukopenia may result from decreased marrow production of leukocytes precursors, peripheral destruction or sequestration of circulating leukocytes, or by autoimmune cellular damage or destruction [30]. Leukopenia may also results from the peripheral destruction of cells within the reticuloendothelial system. Additionally, many antibiotic drugs are known to cause bone marrow suppression. Cellular uptake of nanoparticles after entry into the body is known to occur through two fundamental physiological processes of pinocytosis and phagocytosis. The amount taken up is reported to be particulate size-dependent and was in the order: 20 > 50 > 75 [29]. The nanosilver size used in this study is even much smaller (10 ppm). Additionally, previous studies have established high profile antimicrobial, antifungal and antiviral spectrum of silver nanoparticles even as an alternative to antibiotics and its probiotic properties with increasing immunity in the treatment of cancer and HIV [2,30-32]. Therefore, the present observation in markedly decreased leukocytes level in nanosilver treated cells may not be unconnected with its underlined antibiotic and probiotic properties.
Conclusion
In conclusion, chronic exposure of nanosilver to salt-loaded animal model alters haematological parameters which may worsen circulatory function and activate risk factors of cardiovascular disorders in sprague-dawley rats.
References
- Pan D, Vargas-Morales O, Zern B, Anselmo AC, Gupta V, Zakrewsky M, Mitragotri S, Muzykantov V. The Effect of Polymeric Nanoparticles on Biocompatibility of Carrier Red Blood Cells. PLoS One. 2016 Mar 22;11(3):e0152074. doi: 10.1371/journal.pone.0152074. PMID: 27003833; PMCID: PMC4803339.
- de la Harpe KM, Kondiah PPD, Choonara YE, Marimuthu T, du Toit LC, Pillay V. The Hemocompatibility of Nanoparticles: A Review of Cell-Nanoparticle Interactions and Hemostasis. Cells. 2019 Oct 7;8(10):1209. doi: 10.3390/cells8101209. PMID: 31591302; PMCID: PMC6829615.
- Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, Dominiczak AF, Grassi G, Jordan J, Poulter NR, Rodgers A, Whelton PK. Hypertension. Nat Rev Dis Primers. 2018 Mar 22;4:18014. doi: 10.1038/nrdp.2018.14. PMID: 29565029; PMCID: PMC6477925.
- Chen AX, Haas AV, Williams GH, Vaidya A. Dietary sodium intake and cortisol measurements. Clin Endocrinol (Oxf). 2020 Nov;93(5):539-545. doi: 10.1111/cen.14262. Epub 2020 Jun 24. PMID: 32511774; PMCID: PMC7859973.
- Dong OM. Excessive dietary sodium intake and elevated blood pressure: a review of current prevention and management strategies and the emerging role of pharmaconutrigenetics. BMJ Nutr Prev Health. 2018 Sep 19;1(1):7-16. doi: 10.1136/bmjnph-2018-000004. PMID: 33235949; PMCID: PMC7678480.
- Grillo A, Salvi L, Coruzzi P, Salvi P, Parati G. Sodium Intake and Hypertension. Nutrients. 2019 Aug 21;11(9):1970. doi: 10.3390/nu11091970. PMID: 31438636; PMCID: PMC6770596.
- Shenouda N, Ramick MG, Lennon SL, Farquhar WB, Edwards DG. High dietary sodium augments vascular tone and attenuates low-flow mediated constriction in salt-resistant adults. Eur J Appl Physiol. 2020 Jun;120(6):1383-1389. doi: 10.1007/s00421-020-04370-0. Epub 2020 Apr 18. PMID: 32306153; PMCID: PMC7315828.
- Smiljanec K, Mbakwe A, Ramos Gonzalez M, Farquhar WB, Lennon SL. Dietary Potassium Attenuates the Effects of Dietary Sodium on Vascular Function in Salt-Resistant Adults. Nutrients. 2020 Apr 25;12(5):1206. doi: 10.3390/nu12051206. PMID: 32344796; PMCID: PMC7281996.
- Adejare AA, Sofola OA. Amlodipine Corrects Changes in Blood Pressure and Baroreceptor Reflex Sensitivity in Sprague Dawley Rats Fed a High Salt Diet. Niger J Physiol Sci. 2017 Jun 30;32(1):63-67. PMID: 29134979.
- Laloy J, Minet V, Alpan L, Mullier F, Beken S, Toussaint O, Lucas S, Dogné JM. Impact of Silver Nanoparticles on Haemolysis, Platelet Function and Coagulation. Nanobiomedicine (Rij). 2014 Jan 1;1:4. doi: 10.5772/59346. PMID: 30023015; PMCID: PMC6029236.
- Walski T, Chludzińska L, Komorowska M, Witkiewicz W. Individual osmotic fragility distribution: a new parameter for determination of the osmotic properties of human red blood cells. Biomed Res Int. 2014;2014:162102. doi: 10.1155/2014/162102. Epub 2014 Jan 2. PMID: 24527436; PMCID: PMC3909971.
- Pieszka M, Bederska-Łojewska D, Szczurek P, Pieszka M. The Membrane Interactions of Nano-Silica and Its Potential Application in Animal Nutrition. Animals (Basel). 2019 Nov 28;9(12):1041. doi: 10.3390/ani9121041. PMID: 31795229; PMCID: PMC6940791.
- Burdușel AC, Gherasim O, Grumezescu AM, Mogoantă L, Ficai A, Andronescu E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials (Basel). 2018 Aug 31;8(9):681. doi: 10.3390/nano8090681. PMID: 30200373; PMCID: PMC6163202.
- Rodriguez-Garraus A, Azqueta A, Vettorazzi A, López de Cerain A. Genotoxicity of Silver Nanoparticles. Nanomaterials (Basel). 2020 Jan 31;10(2):251. doi: 10.3390/nano10020251. PMID: 32023837; PMCID: PMC7075128.
- Khanna P, Ong C, Bay BH, Baeg GH. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials (Basel). 2015 Jun 30;5(3):1163-1180. doi: 10.3390/nano5031163. PMID: 28347058; PMCID: PMC5304638.
- Ash GI, Kim D, Choudhury M. Promises of Nanotherapeutics in Obesity. Trends Endocrinol Metab. 2019 Jun;30(6):369-383. doi: 10.1016/j.tem.2019.04.004. Epub 2019 May 21. PMID: 31126754; PMCID: PMC6716370.
- McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014 Mar;22(1):116-127. doi: 10.1016/j.jfda.2014.01.010. Epub 2014 Feb 7. PMID: 24673909; PMCID: PMC4281024.
- Vali S, Mohammadi G, Tavabe KR, Moghadas F, Naserabad SS. The effects of silver nanoparticles (Ag-NPs) sublethal concentrations on common carp (Cyprinus carpio): Bioaccumulation, hematology, serum biochemistry and immunology, antioxidant enzymes, and skin mucosal responses. Ecotoxicol Environ Saf. 2020 May;194:110353. doi: 10.1016/j.ecoenv.2020.110353. Epub 2020 Mar 5. PMID: 32146193.
- Bachler G, von Goetz N, Hungerbühler K. A physiologically based pharmacokinetic model for ionic silver and silver nanoparticles. Int J Nanomedicine. 2013;8:3365-82. doi: 10.2147/IJN.S46624. Epub 2013 Sep 2. PMID: 24039420; PMCID: PMC3771750.
- Holland NA, Becak DP, Shannahan JH, Brown JM, Carratt SA, Winkle L, Pinkerton KE, Wang CM, Munusamy P, Baer DR, Sumner SJ, Fennell TR, Lust RM, Wingard CJ. Cardiac Ischemia Reperfusion Injury Following Instillation of 20 nm Citrate-capped Nanosilver. J Nanomed Nanotechnol. 2015 Nov;6(Suppl 6):006. doi: 10.4172/2157-7439.S6-006. Epub 2015 Oct 1. PMID: 26966636; PMCID: PMC4780684.
- Uche OK, Ehanire VO. Influence of Nanosilver on Endothelial Function and Vascular Reactivity of Isolated Rabbit Carotid Artery. Niger J Physiol Sci. 2018 Dec 30;33(2):139-144. PMID: 30837766.
- Walkowska A, Kuczeriszka M, Sadowski J, Olszyñski KH, Dobrowolski L, Červenka L, Hammock BD, Kompanowska-Jezierska E. High salt intake increases blood pressure in normal rats: putative role of 20-HETE and no evidence on changes in renal vascular reactivity. Kidney Blood Press Res. 2015;40(3):323-34. doi: 10.1159/000368508. Epub 2015 May 31. PMID: 26067851; PMCID: PMC4583220.
- Mojiminiyi FB, Audu Z, Etuk EU, Ajagbonna OP. Attenuation of salt-induced hypertension by aqueous calyx extract of Hibiscus sabdariffa. Niger J Physiol Sci. 2012 Dec 18;27(2):195-200. PMID: 23652235.
- Dacie JV, Lewis SM. Practical Haematology. 6th Edition. London: Churchill Livingstone; 1984. P. 7-49. https://bit.ly/3x1W8Cl
- Chen LQ, Fang L, Ling J, Ding CZ, Kang B, Huang CZ. Nanotoxicity of silver nanoparticles to red blood cells: size dependent adsorption, uptake, and hemolytic activity. Chem Res Toxicol. 2015 Mar 16;28(3):501-9. doi: 10.1021/tx500479m. Epub 2015 Feb 2. PMID: 25602487.
- Pan DC, Myerson JW, Brenner JS, Patel PN, Anselmo AC, Mitragotri S, Muzykantov V. Nanoparticle Properties Modulate Their Attachment and Effect on Carrier Red Blood Cells. Sci Rep. 2018 Jan 25;8(1):1615. doi: 10.1038/s41598-018-19897-8. PMID: 29371620; PMCID: PMC5785499.
- Shaluei F, Hedayati A, Jahanbakhshi A, Kolangi H, Fotovat M. Effect of subacute exposure to silver nanoparticle on some hematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix). Hum Exp Toxicol. 2013 Dec;32(12):1270-7. doi: 10.1177/0960327113485258. Epub 2013 Apr 30. PMID: 23632006.
- Reddy SJ and Vineela D. Effects of silver nanoparticles on biochemical indices of the liver of catla. Journal of international academic research for multidisciplinary. 2016;4(1):140-152.
- Weber M, Steinle H, Golombek S, Hann L, Schlensak C, Wendel HP, Avci-Adali M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front Bioeng Biotechnol. 2018 Jul 16;6:99. doi: 10.3389/fbioe.2018.00099. PMID: 30062094; PMCID: PMC6054932.
- Kettler K, Giannakou C, de Jong WH, Hendriks AJ, Krystek P. Uptake of silver nanoparticles by monocytic THP-1 cells depends on particle size and presence of serum proteins. J Nanopart Res. 2016;18(9):286. doi: 10.1007/s11051-016-3595-7. Epub 2016 Sep 22. PMID: 27774037; PMCID: PMC5034003.
- Cater CM, Charlene AMQ. Alteration in blood components Comprehensive toxicology. 2018;10:249-293.
- Frazer RA. Use of Silver nanoparticles in HIV treatment protocols: A research proposal. J Nanomedic Nanotechnol. 2012;3(1):1000127-132.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.