> Medicine Group. 2021 July 27;2(7):618-623. doi: 10.37871/jbres1287.
Research Progress in the Repair of Peripheral Nerve Injury with Adipose-Derived Stem Cell Exosomes
Xin-Yu Ben1#, Hui-Hui Zheng1#, Ya-Ru Wang1#, Fang-Fang Liu1, Qi-Wei Zhu1, Rui Ren2 and Xi-Nan Yi1,2*
2Human Anatomical Department, Hainan Medical University, Haikou, China
1#These authors contributed equally to this work
- Adipose-derived stem cell
- Exosome
- Peripheral nerve injury
Abstract
The repair of peripheral nerve injury has always been a difficult clinical problem. Although a variety of treatment methods are available in clinical practice, their efficacy is limited. In recent years, the components carried by adipose stem cell exosomes and their functions have been increasingly discovered. A large number of experiments conducted around the world have shown that adipose-derived stem cell exosomes have a positive effect on the repair of peripheral nerve injury. This article reviews recent progress toward the use of adipose-derived stem cell exosomes in the repair of injured peripheral nerves and possible future research directions involving adipose-derived stem cell exosomes.
Introduction
Peripheral nerve injury is a common form of neurological injury and accounts for 2.8% of all trauma-related injuries [1]. Although the peripheral nerves have the ability to repair themselves, complete functional recovery is often difficult, especially for longer nerve injuries and proximal nerve defects [2]. At present, various methods are employed to treat peripheral nerve injury in clinical practice [3,4]. Unfortunately, the current methods still do not provide good functional recovery [5] and are associated with disadvantages such as expensive repair materials and high risks of complications of failure [6]. Thus, trauma-related nerve injury imposes a considerable economic burden on societies as while negatively affecting the quality of life of patients and their families [7].
In recent years, the Adipose-Derived Stem Cell Exosomes (ADMSC-Exos) have been widely investigated for their utility in the treatment of many diseases, including in the repair of peripheral nerve injury [8,9]. In the present review, recent research progress achieved toward the application of ADMSC-Exos in the treatment of peripheral nerve injury is reviewed, and relevant application strategies and prospects for ADMSC-Exos in the field of nerve repair are discussed.
Exos are lipid membrane vesicles that contain a variety of non-coding RNA and protein substances that are actively secreted by cells [10]. Research has confirmed that Exos can mediate signal transduction between cells [11] and influence the repair of injured nerves and other pathological processes [12]. Since 1981, when the concept of Exos was first proposed [13], these vesicles have been a hotspot in many research fields. Exos were once considered a redundant component in cellular activities, but more in-depth research revealed that Exos transmit substances between cells, such as RNAs and proteins, and in doing so participate in the occurrence and development of a variety of diseases (such as tumors and genetic diseases) [14,15]. In normal physiological processes, Exos play a role in mediating cell–cell communication [16]. In addition, Exos can also regulate host-pathogen interactions [17]. Studies have also shown that Exos can serve as valuable markers in the clinical diagnosis of various diseases due to the presence of specific proteins [18] or microRNAs (miRNAs) [19] on their surface and can be secreted into the serum, cerebrospinal fluid, urine, saliva and other body fluids [20]. Their specific contents (proteins or miRNAs) enable Exos to play a diagnostic role in the clinic [21].
Research into the clinical potential of Adipose-Derived Mesenchymal Stem Cells (ADMSCs) became a hot topic after Zuk, et al. [22] first extracted these cells from autologous adipose tissue in 2001. Because they can be extracted easily and in abundant numbers from adipose tissue, ADMSCs have been regarded as an ideal source of cells for tissue repair in the treatment of a variety of diseases [23]. A large number of studies have shown that Exos derived from MSCs can regulate cell proliferation and differentiation, limit tissue and cell damage, regulate the immune response, and promote tissue regeneration and cellular repair [24,25]. Owing to these functions, ADMSC-Exos have great potential for clinical application in the repair of damaged nerve tissue.
Advantages of ADMSC-Exos
Adipose-derived stem cells and their Exos
Adipose cells have many advantages such as easy access, rapid growth, high genetic stability, low antigenicity [26,27], and multidirectional differentiation ability. Qian, et al. [28] detected signature proteins unique to neurons after applying a specific inducer to ADMSCs, indicating that ADMSCs have the potential to differentiate into nerve cells. In addition, the cell adhesion ability of ADMSCs is strong, making them easy to culture in vitro, and their nutrient requirements are low, with vigorous growth occurring in the medium [28]. ADMSCs are not only pluripotent and plastic [29], but also abundant within adipose tissue. ADMSCs make up approximately 2% of all adipocytes, which is much higher than the proportion of stem cells in bone marrow (1/25,000–1/100,000) [30,31]. Because of their accessibility and low maintenance requirements, ADMSCs hold great potential for use in many clinical applications. In addition to ADMSCs, Exos are also relatively easy to isolate in vitro at normal or slightly lower oxygen levels [32,33].
Molecular characteristics of ADMSC-Exos
With the expansion of Exo research, it became clear that different Exos carry different molecular markers, which can be used for the diagnosis and detection of clinical diseases [34,35]. Sonoda, et al. [36] found in a rat model of acute kidney injury that miRNA-16, miRNA-24 and miRNA-200C levels are increased in urinary exosomes in the state of kidney injury, and their increases were associated with a reduction of target mRNA in the glomerular medulla. Zhang, et al. [37] analyzed the miRNA profile of ADMSC-Exos and found 148 known miRNAs, and proteomic analysis of ADMSC-Exos identified 1466 proteins involved in various cellular functions [38]. Baglio, et al. [24] showed that Exos of MSCs derived from adipose tissue and bone marrow have highly overlapping miRNA expression profiles and identified differences in only a few transporter RNAs between the Exos from these two MSC types.
Effectiveness of ADMSC-Exos for the repair of peripheral nerve injury in vivo
Multiple studies [39-41] have shown that Exos derived from bone marrow MSCs can promote the repair of nerve defects and improve the quantity and quality of regenerated nerve fibers. Therefore, it is speculated that the ADMSC-Exos may have the same effect in the process of nerve injury repair. In recent years, many studies on ADMSCs have found that their Exos play an active role in the repair of peripheral nerves. Ghoreishian, et al. [42] isolated undifferentiated ADMSCs from the adipose tissue of dogs and showed that undifferentiated ADMSCs immersed in alginate hydrogels could be applied to successfully repair a 7-mm nerve defect that caused facial nerve injury. Orbay, et al. [43] cultured ADMSCs in an induction solution containing various growth factors and found that the cells differentiated into cells similar to Schwann cells in morphology. Moreover, compared with treatment with a silicone catheter only, transplantation of ADMSCs and differentiated ADMSCs into a damaged sciatic nerve resulted in greater improvements of nerve function indexes such as the conduction velocity of the sciatic nerve in a rat peripheral nerve gap model. Marconi, et al. [44] injected ADMSCs into rats through the tail vein to study the effect on the injured sciatic nerve, and their results showed that, compared with those in the phosphate-buffered saline–treated control group, the sciatic nerve function index and the number of nerve fibers regeneration were significantly greater. Allbright, et al. [45] placed ADMSCs in hydrogel for their use to repair the damaged sciatic nerve and reported promising results. In the study of stress urinary incontinence, Ni, et al. [38] found that the density of skeletal muscle fibers and peripheral nerve fibers in the urethra of rats treated with ADMSC-Exos was higher than that in the untreated group. In an experimental study of the bilateral cavernous nerve injury rat model, Li, et al. [46] found that ADMSC-Exo treatment could significantly reduce pathological changes, including distortion of normal nerve anatomy, atrophy of smooth muscle and collagen deposition, resulting in improvement of erectile function.
The role of exosomes in protecting neurons
Considerable evidence indicates that many different types of Exos can promote nerve regeneration [47,48]. For example, Schwann cell-derived exosomes were shown to promote neurite outgrowth [49]. However, the isolation of Exos from many sources can require damage to the source tissue, whereas the collection of ADMSCs to obtain ADMSC-Exos is relatively convenient and minimally harmful [50]. In 2018, Lin, et al. [51] applied ADMSC-Exos for the treatment of a sciatic nerve injury model and observed a reduction in the inner injury of the nervous tract, a complete and orderly nervous tract membrane, and promotion of repair of the sciatic nerve injury via reductions in Schwann cell apoptosis and autophagy. In 2019, Ren, et al. [52] found that the ADMSC-Exos modified to carry miRNA-133b significantly promoted the recovery of neural function in animals with spinal cord injury by influencing a signaling pathway related to axonal regeneration and the expression of Neurofilament (NF), Growth Associated Protein 43 (GAP43), Glial Fibrillary Acidic Protein (GFAP) and Myelin Basic Protein (MBP). Wei, et al. [53] identified many insulin-like growth factors and hepatocyte growth factors within human ADMSC-Exos and showed that ADMSC-Exos had a positive effect on the proliferation of PC12 cells after the induction of neuronal injury, reflecting a protective effect of ADMSC-Exos. Di, et al. [54] applied ADMSCs and a fibrin bio-catheter to a sciatic nerve injury model, and this treatment combination achieved an axon regeneration length and proximal stem cell movement distance that were superior to those achieved with only the bio-catheter. Together, these experimental studies confirm that ADMSC-Exos can play an important role in the protection of neurons and the occurrence of neurites.
Mechanism of nerve regeneration by ADMSC-EXOs
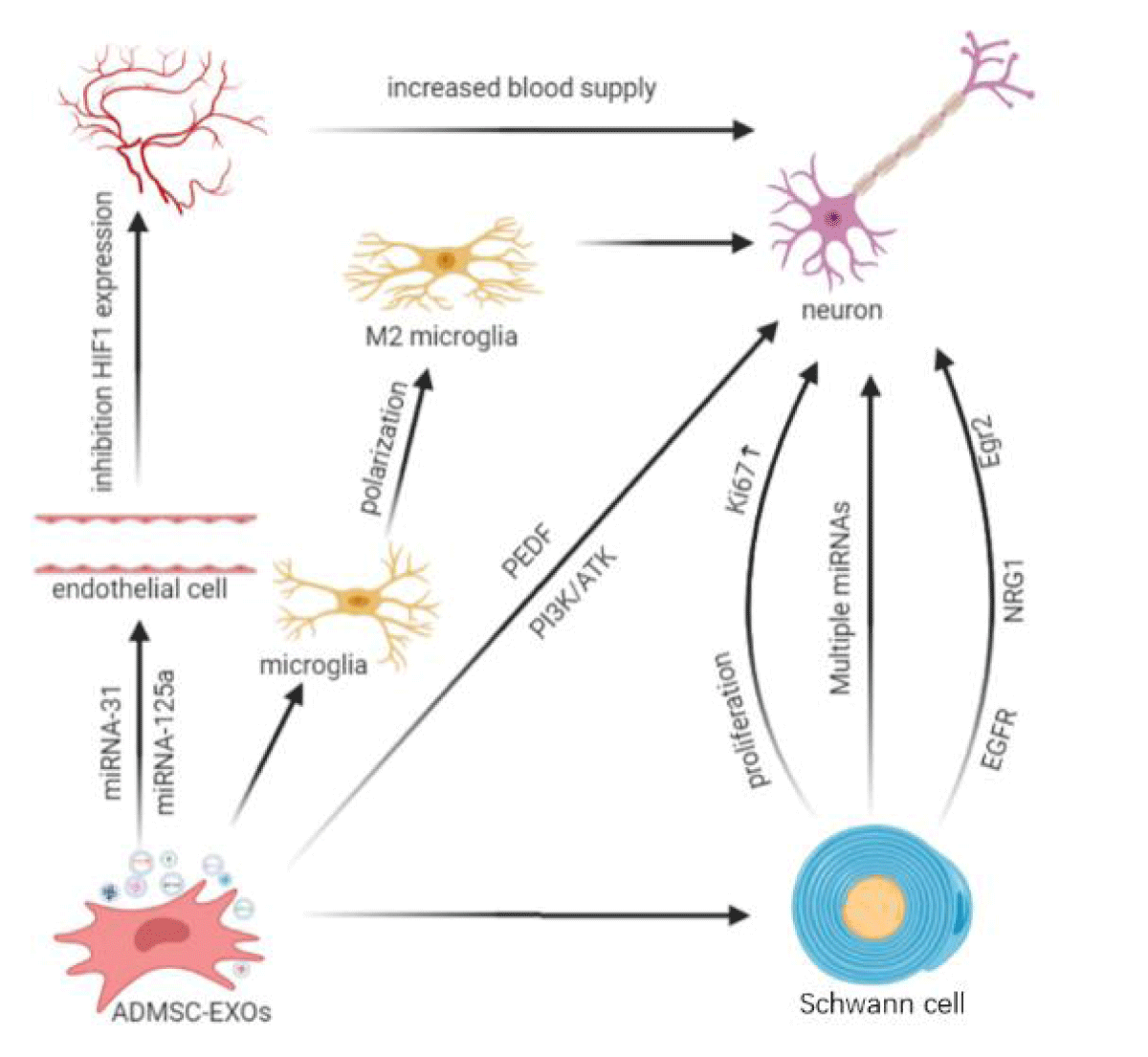
Exosomes affect a variety of nerve regeneration pathways. Some studies have shown that neurotrophic factors combine with exosomes to promote nerve regeneration. For example, ADMSC-Exos modified with Pigment-Epithelial Derivative Factor (PEDF) can activate autophagy and inhibit neuronal apoptosis, thereby reducing cerebral ischemia-reperfusion injury [55]. Recently, it has been confirmed that ADMSC-Exos transmit exosomal miRNA-30d-5p to inhibit autophagy among microglia and ultimately promote the polarization of microglia into an anti-inflammatory phenotype and reverse neuronal damage [56]. In addition, the blood supply to injured nerves is an important factor affecting nerve regeneration. Reconstruction of the vascular network provides a conducive microenvironment for axon growth during peripheral nerve repair [57,58]. Kang, et al. [59] found that ADMSC-Exos induce angiogenesis by affecting the expression of the anti-angiogenic gene Hypoxia Inducible Factor-1 (HIF-1) in endothelial cells through miRNA-31. Liang, et al. [60] found that ADMSC-Exos can deliver miRNA-125a to endothelial cells and reduce the expression of angiogenesis inhibitor Delta-like 4 (DLL4), thereby promoting angiogenesis at damaged sites. Other studies have shown that ADMSC-Exos play a neuroprotective role through the PI3K/AKT signaling pathway [53] (Figure 1).
Neuroglial cells in the peripheral nervous system are called Schwann cells and originate from neural crest cells in dorsal tubular nerve cords. Schwann cells gradually differentiate under specific microenvironmental conditions and encapsulate axons to form the nerve fiber myelin sheath. In the process of the growth and development of nerves, Schwann cells are closely related with axons [61]. In addition to providing a protective sheath for axons, Schwann cells secrete nerve growth factors and exosomes to promote the extension of axons [62]. Research has shown that the repair of injured peripheral nerve heavily depends on the proliferation and migration of Schwann cells [63]. Therefore, Schwann cells play a significant role in repairing the peripheral nerve after injury. In 2019, Bucan and colleagues [64] found that ADMSC-Exos can stimulate the proliferation of Schwann cells and increase the expression of cyclin Ki67, which indicates that Exos can enhance the length of neurites of Dorsal Root Ganglia (DRG) neurons. First, they demonstrated that ADMSC-Exos contain a variety of growth factors that facilitate nerve regeneration. Other researchers also demonstrated obvious changes in the expression level of miRNAs in Schwann cells after nerve injury, suggesting that exosomes carrying miRNAs secreted by Schwann cells may play a certain role in nerve regeneration [65,66]. Co-culture of ADMSCs and Schwann cells increased the mRNA expression levels of Epidermal Growth Factor Receptor 3 (EGFR3/ErbB3), Neuregulin 1 (NRG1), early growth response protein 2 (Egr2/Krox20), and MBP, as well as the corresponding protein expression levels of ErbB3, NRG1 and Krox20. In Schwann cells to promote the repair of the injured nerves [67] (Figure 1).
Prospects
With more in-depth research on Exos, evidence has emerged that ADMSC-Exos can avoid problems related to stem cell transplantation and offer advantages such as the ease of collection in large quantities. With more investigators and clinicians in more fields recognizing the potential of ADMSC-Exos, these vesicles are gaining a broad future in the field of neuranagenesis. However, application method, application dosage and safety of exosomes in the human body still need in-depth study in the process of tissue repair after nerve injury. Additionally, any possible long-term or adverse side effects need to be determined. Although the current experimental evidence shows that MSC-derived Exos are safe and effective for the treatment of peripheral nerve injury, the problem of how exosomes are widely used in clinical practice has not been resolved. Safe dosage for use in humans is also unknown [38]. Moreover, combination of ADMSC-Exos with other nerve repair methods needs to be explored. Therefore, continued research to characterize the effects of ADMSCs and their Exos is warranted, and more clinical studies are needed to determine the potential benefit of ADMSC-Exos for nerve repair.
Funding
This study was funded by the National Natural Science Foundation of China [81460196], the Natural Science Foundation of Hainan Province [318QN243], and the Finance Science and Technology Project of Hainan Province.
Data availability statement
The datasets generated and analyzed during the present study are available from the corresponding author upon reasonable request.
References
- Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998 Jul;45(1):116-22. doi: 10.1097/00005373-199807000-00025. PMID: 9680023.
- Sachanandani NF, Pothula A, Tung TH. Nerve gaps. Plast Reconstr Surg. 2014 Feb;133(2):313-319. doi: 10.1097/01.prs.0000436856.55398.0f. PMID: 24150118.
- Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008 Sep;119(9):1951-65. doi: 10.1016/j.clinph.2008.03.018. Epub 2008 May 14. PMID: 18482862.
- Winfree CJ. Peripheral nerve injury evaluation and management. Curr Surg. 2005 Sep-Oct;62(5):469-76. doi: 10.1016/j.cursur.2005.03.008. PMID: 16125601.
- Zheng MX, Hua XY, Feng JT, Li T, Lu YC, Shen YD, Cao XH, Zhao NQ, Lyu JY, Xu JG, Gu YD, Xu WD. Trial of Contralateral Seventh Cervical Nerve Transfer for Spastic Arm Paralysis. N Engl J Med. 2018 Jan 4;378(1):22-34. doi: 10.1056/NEJMoa1615208. Epub 2017 Dec 20. PMID: 29262271.
- Wang X, Ma S, Wu H, Shen X, Xu S, Guo X, Bolick ML, Wu S, Wang F. Macrophage migration inhibitory factor mediates peripheral nerve injury-induced hypersensitivity by curbing dopaminergic descending inhibition. Exp Mol Med. 2018 Feb 16;50(2):e445. doi: 10.1038/emm.2017.271. PMID: 29504609; PMCID: PMC5903823.
- Houdek MT, Shin AY. Management and complications of traumatic peripheral nerve injuries. Hand Clin. 2015 May;31(2):151-63. doi: 10.1016/j.hcl.2015.01.007. Epub 2015 Feb 28. PMID: 25934193.
- Lee M, Liu T, Im W, Kim M. Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington’s disease in vitro model. Eur J Neurosci. 2016 Aug;44(4):2114-9. doi: 10.1111/ejn.13275. Epub 2016 Jun 4. PMID: 27177616.
- Lee M, Ban JJ, Kim KY, Jeon GS, Im W, Sung JJ, Kim M. Adipose-derived stem cell exosomes alleviate pathology of amyotrophic lateral sclerosis in vitro. Biochem Biophys Res Commun. 2016 Oct 21;479(3):434-439. doi: 10.1016/j.bbrc.2016.09.069. Epub 2016 Sep 15. PMID: 27641665.
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002 Aug;2(8):569-79. doi: 10.1038/nri855. PMID: 12154376.
- Yeon JH, Jeong HE, Seo H, Cho S, Kim K, Na D, Chung S, Park J, Choi N, Kang JY. Cancer-derived exosomes trigger endothelial to mesenchymal transition followed by the induction of cancer-associated fibroblasts. Acta Biomater. 2018 Aug;76:146-153. doi: 10.1016/j.actbio.2018.07.001. Epub 2018 Jul 4. PMID: 30078422.
- Qing L, Chen H, Tang J, Jia X. Exosomes and Their MicroRNA Cargo: New Players in Peripheral Nerve Regeneration. Neurorehabil Neural Repair. 2018 Sep;32(9):765-776. doi: 10.1177/1545968318798955. PMID: 30223738; PMCID: PMC6146407.
- Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981 Jul 6;645(1):63-70. doi: 10.1016/0005-2736(81)90512-5. PMID: 6266476.
- Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82-88. doi: 10.1016/j.semcdb.2015.03.001
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007 Jun;9(6):654-9. doi: 10.1038/ncb1596. Epub 2007 May 7. PMID: 17486113.
- van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018 Apr;19(4):213-228. doi: 10.1038/nrm.2017.125. Epub 2018 Jan 17. PMID: 29339798.
- Zhang W, Jiang X, Bao J, Wang Y, Liu H, Tang L. Exosomes in Pathogen Infections: A Bridge to Deliver Molecules and Link Functions. Front Immunol. 2018 Feb 12;9:90. doi: 10.3389/fimmu.2018.00090. PMID: 29483904; PMCID: PMC5816030.
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015 Jul 9;523(7559):177-82. doi: 10.1038/nature14581. Epub 2015 Jun 24. PMID: 26106858; PMCID: PMC4825698.
- Manier S, Liu CJ, Avet-Loiseau H, Park J, Shi J, Campigotto F, Salem KZ, Huynh D, Glavey SV, Rivotto B, Sacco A, Roccaro AM, Bouyssou J, Minvielle S, Moreau P, Facon T, Leleu X, Weller E, Trippa L, Ghobrial IM. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood. 2017 Apr 27;129(17):2429-2436. doi: 10.1182/blood-2016-09-742296. Epub 2017 Feb 17. PMID: 28213378; PMCID: PMC5409448.
- Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009 Aug;21(4):575-81. doi: 10.1016/j.ceb.2009.03.007. Epub 2009 May 11. PMID: 19442504.
- Yang JK, Song J, Huo HR, Zhao YL, Zhang GY, Zhao ZM, Sun GZ, Jiao BH. DNM3, p65 and p53 from exosomes represent potential clinical diagnosis markers for glioblastoma multiforme. Ther Adv Med Oncol. 2017 Dec;9(12):741-754. doi: 10.1177/1758834017737471. Epub 2017 Nov 6. PMID: 29449895; PMCID: PMC5808838.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001 Apr;7(2):211-28. doi: 10.1089/107632701300062859. PMID: 11304456.
- De Ugarte DA, Ashjian PH, Elbarbary A, Hedrick MH. Future of fat as raw material for tissue regeneration. Ann Plast Surg. 2003 Feb;50(2):215-9. doi: 10.1097/01.SAP.0000029661.38066.15. PMID: 12567065.
- Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015 Jul 1;6(1):127. doi: 10.1186/s13287-015-0116-z. PMID: 26129847; PMCID: PMC4529699.
- Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016 Apr 21;320:129-39. doi: 10.1016/j.neuroscience.2016.01.061. Epub 2016 Feb 3. PMID: 26851773.
- Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010 May;4(3):214-22. doi: 10.1016/j.scr.2009.12.003. Epub 2010 Jan 4. PMID: 20138817.
- Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014 Jun 1;23(11):1233-44. doi: 10.1089/scd.2013.0479. Epub 2014 Feb 10. PMID: 24367916.
- Qian DX, Zhang HT, Ma X, Jiang XD, Xu RX. Comparison of the efficiencies of three neural induction protocols in human adipose stromal cells. Neurochem Res. 2010 Apr;35(4):572-9. doi: 10.1007/s11064-009-0101-y. Epub 2009 Dec 4. PMID: 19960248.
- Allison DJ, Gabriel DA, Klentrou P, Josse AR, Ditor DS. The Influence of Chronic Inflammation on Peripheral Motor Nerve Conduction Following Spinal Cord Injury: A Randomized Clinical Trial. Top Spinal Cord Inj Rehabil. 2017 Fall;23(4):377-385. doi: 10.1310/sci16-00045. PMID: 29339913; PMCID: PMC5667434.
- Bruin JE, Saber N, Braun N, Fox JK, Mojibian M, Asadi A, Drohan C, O’Dwyer S, Rosman-Balzer DS, Swiss VA, Rezania A, Kieffer TJ. Treating diet-induced diabetes and obesity with human embryonic stem cell-derived pancreatic progenitor cells and antidiabetic drugs. Stem Cell Reports. 2015 Apr 14;4(4):605-20. doi: 10.1016/j.stemcr.2015.02.011. Epub 2015 Mar 19. PMID: 25801507; PMCID: PMC4400611.
- Morigi M, De Coppi P. Cell therapy for kidney injury: different options and mechanisms--mesenchymal and amniotic fluid stem cells. Nephron Exp Nephrol. 2014;126(2):59. doi: 10.1159/000360667. Epub 2014 May 19. PMID: 24854642.
- Han YD, Bai Y, Yan XL, Ren J, Zeng Q, Li XD, Pei XT, Han Y. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018 Feb 26;497(1):305-312. doi: 10.1016/j.bbrc.2018.02.076. Epub 2018 Feb 8. PMID: 29428734.
- Zhang W, Bai X, Zhao B, Li Y, Zhang Y, Li Z, Wang X, Luo L, Han F, Zhang J, Han S, Cai W, Su L, Tao K, Shi J, Hu D. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018 Sep 15;370(2):333-342. doi: 10.1016/j.yexcr.2018.06.035. Epub 2018 Jun 28. PMID: 29964051.
- Alipoor SD, Tabarsi P, Varahram M, Movassaghi M, Dizaji MK, Folkerts G, Garssen J, Adcock IM, Mortaz E. Serum Exosomal miRNAs Are Associated with Active Pulmonary Tuberculosis. Dis Markers. 2019 Feb 11;2019:1907426. doi: 10.1155/2019/1907426. PMID: 30886653; PMCID: PMC6388314.
- Kanaoka R, Iinuma H, Dejima H, Sakai T, Uehara H, Matsutani N, Kawamura M. Usefulness of Plasma Exosomal MicroRNA-451a as a Noninvasive Biomarker for Early Prediction of Recurrence and Prognosis of Non-Small Cell Lung Cancer. Oncology. 2018;94(5):311-323. doi: 10.1159/000487006. Epub 2018 Mar 13. PMID: 29533963.
- Sonoda H, Lee BR, Park KH, Nihalani D, Yoon JH, Ikeda M, Kwon SH. miRNA profiling of urinary exosomes to assess the progression of acute kidney injury. Sci Rep. 2019 Mar 18;9(1):4692. doi: 10.1038/s41598-019-40747-8. PMID: 30886169; PMCID: PMC6423131.
- Zhang Y, Yu M, Dai M, Chen C, Tang Q, Jing W, Wang H, Tian W. miR-450a-5p within rat adipose tissue exosome-like vesicles promotes adipogenic differentiation by targeting WISP2. J Cell Sci. 2017 Mar 15;130(6):1158-1168. doi: 10.1242/jcs.197764. Epub 2017 Feb 6. PMID: 28167681.
- Ni J, Li H, Zhou Y, Gu B, Xu Y, Fu Q, Peng X, Cao N, Fu Q, Jin M, Sun G, Wang J, Jin Y, Liu F. Therapeutic Potential of Human Adipose-Derived Stem Cell Exosomes in Stress Urinary Incontinence - An in Vitro and in Vivo Study. Cell Physiol Biochem. 2018;48(4):1710-1722. doi: 10.1159/000492298. Epub 2018 Aug 3. PMID: 30077997.
- Costa HJ, Bento RF, Salomone R, Azzi-Nogueira D, Zanatta DB, Paulino Costa M, da Silva CF, Strauss BE, Haddad LA. Mesenchymal bone marrow stem cells within polyglycolic acid tube observed in vivo after six weeks enhance facial nerve regeneration. Brain Res. 2013 May 13;1510:10-21. doi: 10.1016/j.brainres.2013.03.025. Epub 2013 Mar 28. PMID: 23542586.
- Zheng L, Cui HF. Use of chitosan conduit combined with bone marrow mesenchymal stem cells for promoting peripheral nerve regeneration. J Mater Sci Mater Med. 2010 May;21(5):1713-20. doi: 10.1007/s10856-010-4003-y. Epub 2010 Jan 26. PMID: 20101439.
- Zheng L, Cui HF. Enhancement of nerve regeneration along a chitosan conduit combined with bone marrow mesenchymal stem cells. J Mater Sci Mater Med. 2012 Sep;23(9):2291-302. doi: 10.1007/s10856-012-4694-3. Epub 2012 Jun 3. PMID: 22661248.
- Ghoreishian M, Rezaei M, Beni BH, Javanmard SH, Attar BM, Zalzali H. Facial nerve repair with Gore-Tex tube and adipose-derived stem cells: an animal study in dogs. J Oral Maxillofac Surg. 2013 Mar;71(3):577-87. doi: 10.1016/j.joms.2012.05.025. Epub 2012 Aug 4. PMID: 22868036.
- Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg. 2012 May;65(5):657-64. doi: 10.1016/j.bjps.2011.11.035. Epub 2011 Dec 3. PMID: 22137687.
- Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S, Farinazzo A, Constantin G, Bedogni G, Bedogni A, Bonetti B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A. 2012 Jun;18(11-12):1264-72. doi: 10.1089/ten.TEA.2011.0491. Epub 2012 Apr 3. PMID: 22332955.
- Allbright KO, Bliley JM, Havis E, Kim DY, Dibernardo GA, Grybowski D, Waldner M, James IB, Sivak WN, Rubin JP, Marra KG. Delivery of adipose-derived stem cells in poloxamer hydrogel improves peripheral nerve regeneration. Muscle Nerve. 2018 Aug;58(2):251-260. doi: 10.1002/mus.26094. Epub 2018 Feb 22. PMID: 29406624.
- Li M, Lei H, Xu Y, Li H, Yang B, Yu C, Yuan Y, Fang D, Xin Z, Guan R. Exosomes derived from mesenchymal stem cells exert therapeutic effect in a rat model of cavernous nerves injury. Andrology. 2018 Nov;6(6):927-935. doi: 10.1111/andr.12519. Epub 2018 Jul 16. PMID: 30009463.
- Jing H, He X, Zheng J. Exosomes and regenerative medicine: state of the art and perspectives. Transl Res. 2018 Jun;196:1-16. doi: 10.1016/j.trsl.2018.01.005. Epub 2018 Jan 31. PMID: 29432720.
- Zhan C, Ma CB, Yuan HM, Cao BY, Zhu JJ. Macrophage-derived microvesicles promote proliferation and migration of Schwann cell on peripheral nerve repair. Biochem Biophys Res Commun. 2015 Dec 4-11;468(1-2):343-8. doi: 10.1016/j.bbrc.2015.10.097. Epub 2015 Oct 22. PMID: 26499078.
- Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013 Nov;61(11):1795-806. doi: 10.1002/glia.22558. Epub 2013 Aug 30. PMID: 24038411.
- Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 2019 Aug 7;10(1):242. doi: 10.1186/s13287-019-1358-y. PMID: 31391108; PMCID: PMC6686455.
- Yin G, Liu C, Lin Y, Xie Z, Hou C, Lin H. [Effect of exosomes from adipose-derived stem cells on peripheral nerve regeneration]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Dec 15;32(12):1592-1596. Chinese. doi: 10.7507/1002-1892.201707051. PMID: 30569689.
- Ren ZW, Zhou JG, Xiong ZK, Zhu FZ, Guo XD. Effect of exosomes derived from MiR-133b-modified ADSCs on the recovery of neurological function after SCI. Eur Rev Med Pharmacol Sci. 2019 Jan;23(1):52-60. doi: 10.26355/eurrev_201901_16747. PMID: 30657546.
- Wei JJ, Chen YF, Xue CL, Ma BT, Shen YM, Guan J, Bao XJ, Wu H, Han Q, Wang RZ, Zhao CH. Protection of Nerve Injury with Exosome Extracted from Mesenchymal Stem Cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016 Feb;38(1):33-6. doi: 10.3881/j.issn.1000-503X.2016.01.006. PMID: 26956853.
- di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010 Sep;63(9):1544-52. doi: 10.1016/j.bjps.2009.09.012. Epub 2009 Oct 13. PMID: 19828391.
- Huang X, Ding J, Li Y, Liu W, Ji J, Wang H, Wang X. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp Cell Res. 2018 Oct 1;371(1):269-277. doi: 10.1016/j.yexcr.2018.08.021. Epub 2018 Aug 22. PMID: 30142325.
- Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell Physiol Biochem. 2018;47(2):864-878. doi: 10.1159/000490078. Epub 2018 May 23. PMID: 29807362.
- Wang H, Zhu H, Guo Q, Qian T, Zhang P, Li S, Xue C, Gu X. Overlapping Mechanisms of Peripheral Nerve Regeneration and Angiogenesis Following Sciatic Nerve Transection. Front Cell Neurosci. 2017 Oct 11;11:323. doi: 10.3389/fncel.2017.00323. PMID: 29085283; PMCID: PMC5649188.
- Liu X, Li Q, Niu X, Hu B, Chen S, Song W, Ding J, Zhang C, Wang Y. Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis. Int J Biol Sci. 2017 Feb 6;13(2):232-244. doi: 10.7150/ijbs.16951. PMID: 28255275; PMCID: PMC5332877.
- Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE, Bond VC, Chen YE, Liu D. Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of miRNA-31. Stem Cells Transl Med. 2016 Apr;5(4):440-50. doi: 10.5966/sctm.2015-0177. Epub 2016 Mar 1. PMID: 26933040; PMCID: PMC4798737.
- Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016 Jun 1;129(11):2182-9. doi: 10.1242/jcs.170373. PMID: 27252357.
- Monk KR, Feltri ML, Taveggia C. New insights on Schwann cell development. Glia. 2015 Aug;63(8):1376-93. doi: 10.1002/glia.22852. Epub 2015 Apr 29. PMID: 25921593; PMCID: PMC4470834.
- Lopez-Leal R, Court FA. Schwann Cell Exosomes Mediate Neuron-Glia Communication and Enhance Axonal Regeneration. Cell Mol Neurobiol. 2016 Apr;36(3):429-36. doi: 10.1007/s10571-015-0314-3. Epub 2016 Mar 18. PMID: 26993502.
- Carr MJ, Johnston AP. Schwann cells as drivers of tissue repair and regeneration. Curr Opin Neurobiol. 2017 Dec;47:52-57. doi: 10.1016/j.conb.2017.09.003. Epub 2017 Sep 28. PMID: 28963968.
- Bucan V, Vaslaitis D, Peck CT, Strauß S, Vogt PM, Radtke C. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol Neurobiol. 2019 Mar;56(3):1812-1824. doi: 10.1007/s12035-018-1172-z. Epub 2018 Jun 21. PMID: 29931510; PMCID: PMC6394792.
- Arthur-Farraj PJ, Morgan CC, Adamowicz M, Gomez-Sanchez JA, Fazal SV, Beucher A, Razzaghi B, Mirsky R, Jessen KR, Aitman TJ. Changes in the Coding and Non-coding Transcriptome and DNA Methylome that Define the Schwann Cell Repair Phenotype after Nerve Injury. Cell Rep. 2017 Sep 12;20(11):2719-2734. doi: 10.1016/j.celrep.2017.08.064. PMID: 28903050; PMCID: PMC5608958.
- Chang LW, Viader A, Varghese N, Payton JE, Milbrandt J, Nagarajan R. An integrated approach to characterize transcription factor and microRNA regulatory networks involved in Schwann cell response to peripheral nerve injury. BMC Genomics. 2013 Feb 6;14:84. doi: 10.1186/1471-2164-14-84. PMID: 23387820; PMCID: PMC3599357.
- Yue Y, Yang X, Zhang L, Xiao X, Nabar NR, Lin Y, Hao L, Zhang D, Huo J, Li J, Cai X, Wang M. Low-intensity pulsed ultrasound upregulates pro-myelination indicators of Schwann cells enhanced by co-culture with adipose-derived stem cells. Cell Prolif. 2016 Dec;49(6):720-728. doi: 10.1111/cpr.12298. Epub 2016 Sep 14. PMID: 27625295; PMCID: PMC6496622.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.