Biology Group. 2021 November 20;2(11):1101-1110. doi: 10.37871/jbres1354.
Metal-Based Nanomaterials Incorporate with Ultrasound as Acceptable Approach towards Cancer Therapy
Xiaoxiao He, Shiyue Chen and Xiang Mao*
Chongqing Key Laboratory of Biomedical Engineering, College of Biomedical Engineering, Chongqing Medical University, Chongqing, PR China
- Metal Nanomaterials (NMs)
- Approach
- Integration
- Ultrasound
- Cancer therapy
Abstract
Among current biological researches, there have a plenty of works related to cancer therapy issues by using functional or pure-phased composites in non-invasive strategies. Especially in fabricating anticancer candidates, functional composites are divided into different sorts with different characteristics. Additionally, nanotechnology provides various approaches in utilizing composites’ functionality for cancer diagnostics and therapeutics. Compared with previous Photodynamic Therapy (PDT), Photo-Thermal Therapy (PTT), chemotherapy and radiotherapy, ultrasound is used to activate sonosensitizer to produce cytotoxic Reactive Oxygen Species (ROS) toward target cancer cells. In recent years, the form of Sonodynamic Therapy (SDT) has been making much effort to develop highly efficient metal based Nanomaterials (NMs) as sonosensitizers, which can efficiently generate ROS and has the advantage of deeper tissue penetration. However, the traditional sonosensitizers, such as porphyrins, hypericin, and curcumins suffer from complex synthesis, poor water solubility, and low tumor targeting efficacy. For contrasting this limitation, the metal based inorganic NMs show biocompatibility, controllable physicochemical properties, and ease of achieving multifunctional properties, which greatly expanded their application in SDT. In this review, we systematically summarize the metal based inorganic NMs as carrier of molecular sonosensitizers, and produce ROS under ultrasound. Moreover, the prospects of advanced metal based materials application are also discussed.
Introduction
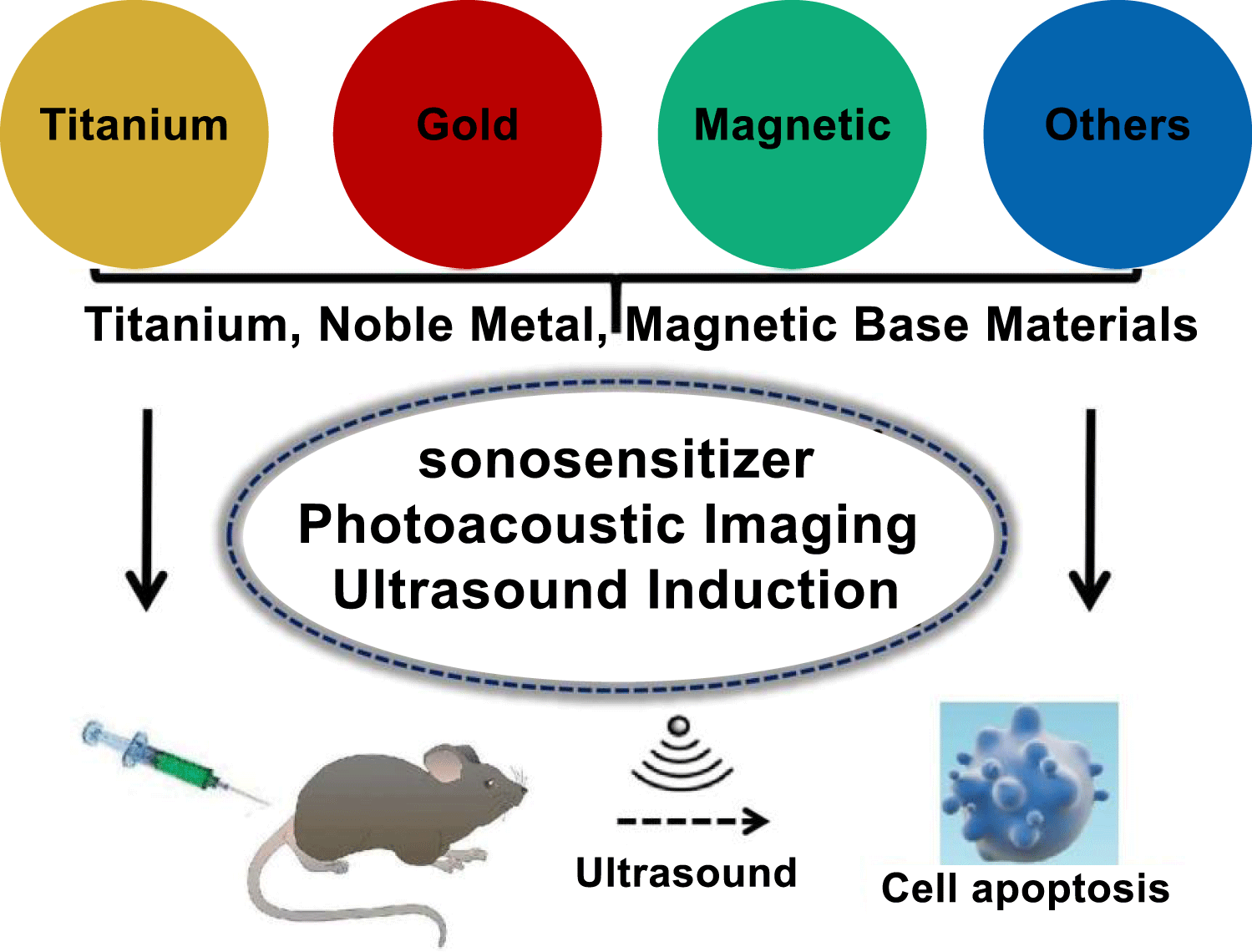
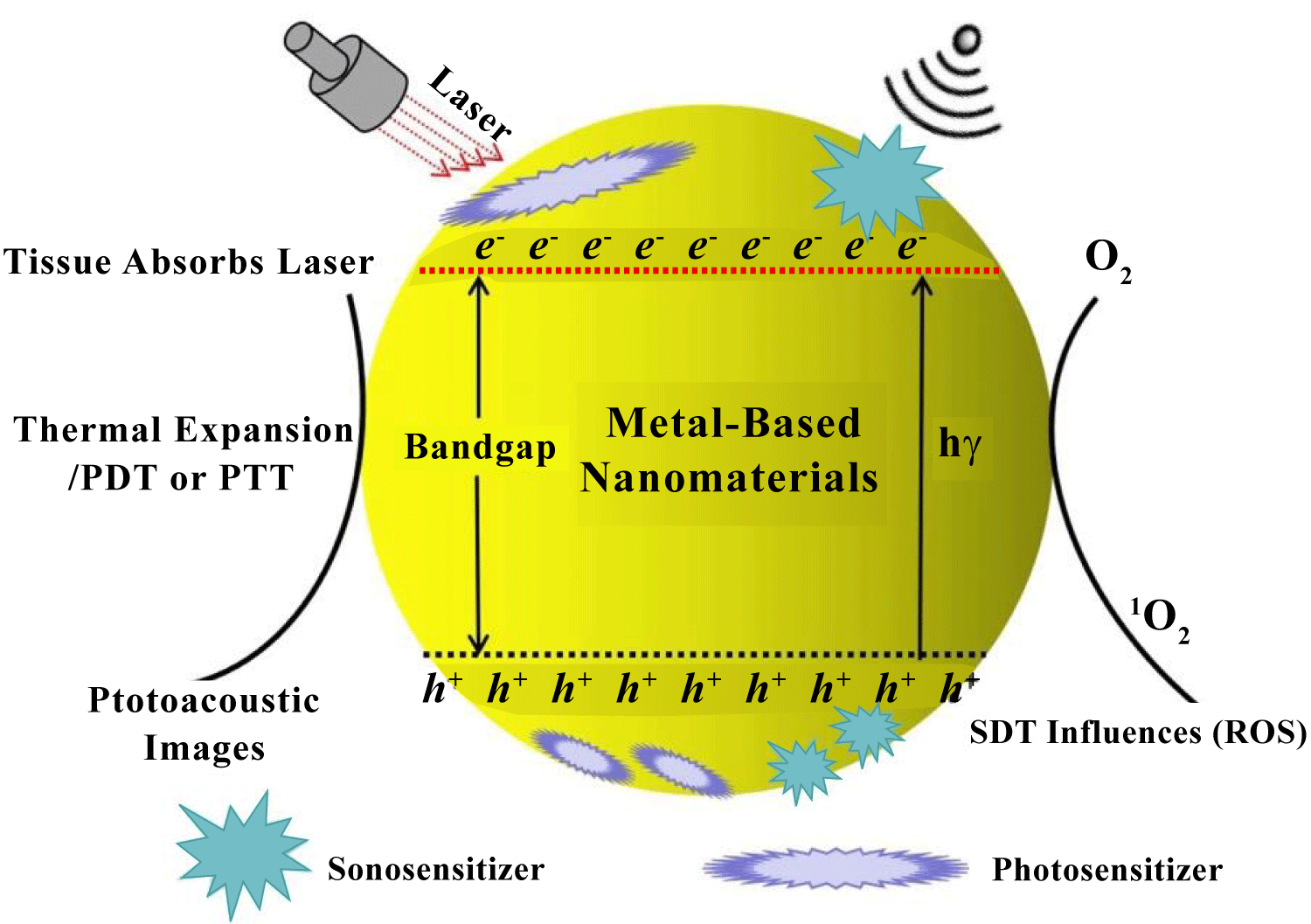
Currently, some of contents in ultrasound induced application toward cancer therapy have been well understood in current researches. Although in different applications by using candidate such as nonmetallic composites [1], organic polymers [2,3], and metal based functional materials [4], it has shown these corresponded materials had utilization in Sonodynamic Therapy (SDT) completely. As demonstrated in nanotechnology, it had been used to develop and change current situation as one traditional concept in curing cancer therapy effectively. By using different functional NMs in SDT treatments, it conveyed the relationship of cancer therapy mechanism and the main manufactures in demonstrating effect while producing cytotoxic Reactive Oxygen Species (ROS) [5,6]. Compared to the traditional organic sonosensitizers, inorganic NMs had the advantages of high stability, good hydrophilicity, excellent circulation ability, and high tumor targeting ability (Figure 1). As demonstrated in SDT applications, it seemed metal based functional materials were highly efficient during curing different cancer cell-lines. However, there still were great challenges in metal-based NMs incorporated with ultrasound as acceptable approach toward cancer therapy. As being well considered as one non-invasive approach in cancer therapy, it eliminated the negative influences while utilizing radiotherapy and chemotherapy technologies. It just utilized the physical mechanisms of sound waves and ultrasound energy transferring while integrating with target tissue or cancer cells.
The foundation about ultrasound treatment in organic compounds was mainly referred to ROS response, it firstly reported about this suggestion and considered as SDT influences, was treated as one effective approach toward cancer therapy. As one contrast in light induction, this was utilized in Photodynamic Therapy (PDT). The ultrasound effect showed better penetrability in biological tissues [7,8]. However, the ROS production mechanism in ultrasound treatment had not been well indicated. Conventionally, there had been three different kinds of approaches to produce ROS, the sonosensitizer played key role of being activated by an appropriate intensity of ultrasound, and then reacted with O2 to produce ROS. In other words, the liquid medium formed cavitation of micro-bubbles under ultrasound treatment, which additionally activate the sonosensitizer to generate ROS as much as possible. Here, the ROS could induce a series of biological events including polymers fragmentation, cytoskeletal shrinkage, and chromatin condensation, leading to cell death. So, the ROS production mechanism in SDT should be well documented, such as PDT and PTT utilization and mechanism illustration. Especially to integrate SDT with metal based functional materials, it should have several reliable mechanisms such as sonoluminescence, pyrolysis, ROS production by the collapse of cavitation bubbles, and ROS independent cytotoxicity. By undergoing ultrasound treatment, the physical ultrasound interacted with target tissues in the aqueous atmosphere, it caused a phenomenon known as "cavitation". It involved the nucleation, growth, and impulsive collapse of gas bubbles under appropriate ultrasound conditions [9,10]. Basically, this phenomenon can be classified into two types, stable and inertial cavitation. Oscillation of stable cavitation bubbles resulted in the streaming of surrounding media. In contrast, inertial cavitation involved the growth of gas-bubbles to resonance size and then to maximum size before collapsing savagely. As illustrated in initial stage, this phenomenon of ultrasound induced could be used in synthesis of NMs by using different metal elements for fabrication functional materials completely, such as metal oxides, alloyed metallic composition, heterogeneous functional structure, and organic molecular modifications. It was utilized in further works as one efficient candidate well used in biological or medical curing. Similarly, ultrasound function not only can be used in material constructions, but also utilized in bio-application as in clinical treatment due to its physical and chemical effects. So, the combination of above two sides about inorganic metal materials and ultrasound effect could offer one new-approach to overcome original challenge in biological or physical fields as shown in figure 2. Especially in cancer therapy, the inorganic NMs could be treated as one fundamental activator toward cancer cell and enhanced to produce ROS for reducing cancer cell growth. Finally, the introduction of merged nanotechnology might provide a new way of delivering sonosensitizers and showed unique advantages in the field of cancer therapy. Compared to organic compounds, metal based functional NMs had the following advantages: 1) the controllable size distribution and outer morphology, 2) excellent chemical and physiological stability, and 3) high tumor targeting efficacy. In this review, we summarized the recently progress in metal-based NMs incorporated with ultrasound or sonosensitizers as acceptable approach toward cancer therapy, the similar SDT applications were also summarized further.
Gold Nanoparticles (NPs) and other typical triggered with ultrasound treatment in further applications
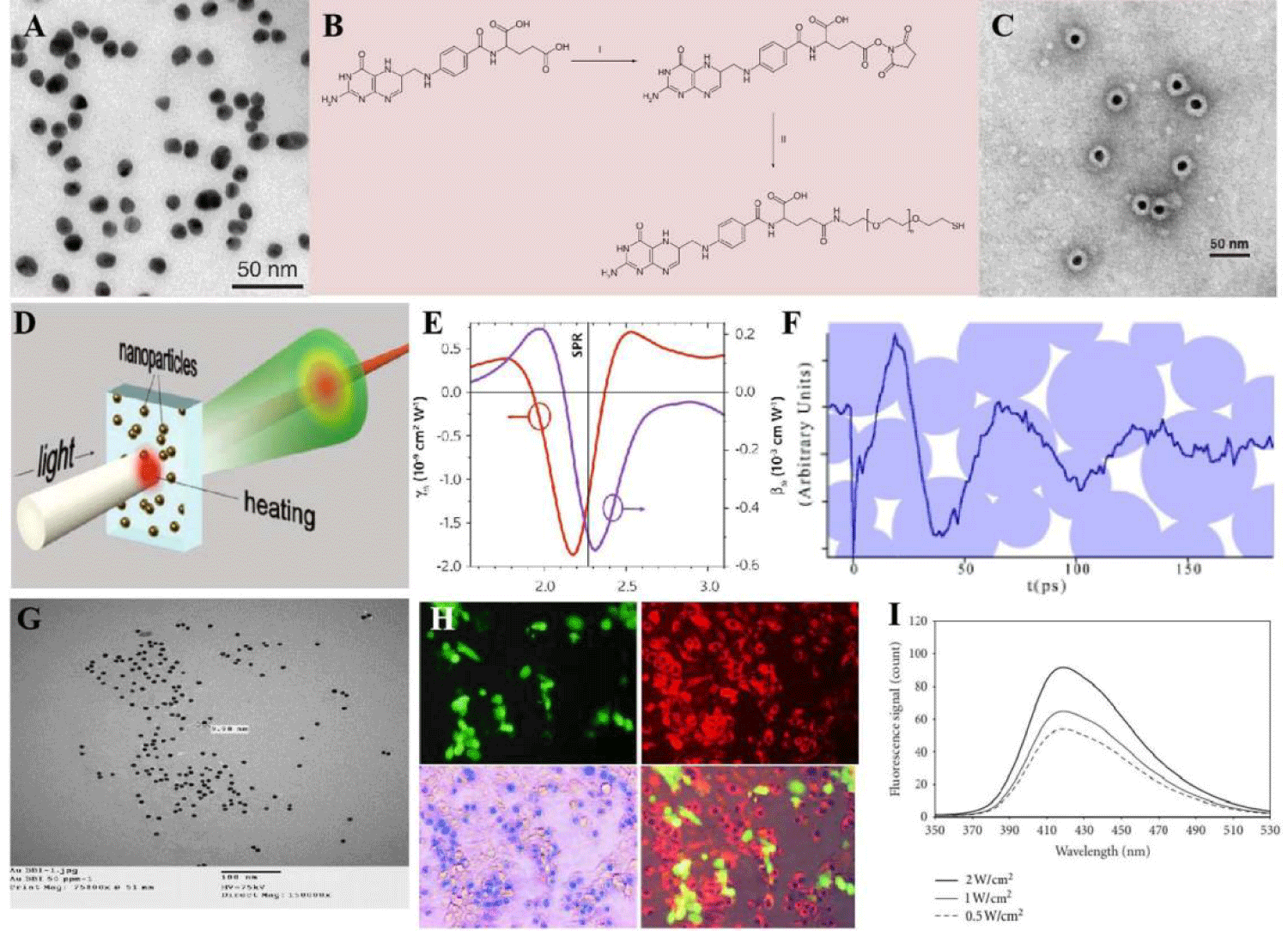
The adjusted optical properties of gold Nanoparticles (NPs) have great potential in sensing and photo-acoustic imaging [11,12]. It had intriguing potential as one of cancer carrier candidates, possesses unique energy absorption (optical), size and stability (physical), and reacted with thiols (chemical) characteristics, which offered them various functional platform for further investigations [13,14]. Preparation of gold NPs by using PEG molecule modified as coating outer, and the folic acid was used as a target moiety toward fabrication (Figures 3A-3C). It has been reported that cellular uptake of the gold NPs was mainly through the receptor-mediated clathrin-dependent endocytosis pathway. It also conveyed that small diameter of nanocarriers could lead to fast uptake and high concentration of gold NPs inside the cells with relative to larger carriers. It found that gold NPs could interact with heparin binding glyco-proteins, which concluded the vascular permeability and basic growth factors that mediate angiogenesis. The Surface Plasmonic Resonance (SPR) was selective in using of optical physical properties while the gold materials induced electron transfer between agent-A and agent B. As demonstrated under ultrasound influenced, gold plays an important role and the morphology determines the SPR (Figures 3D-3F) which comes from electronic relaxation [15,16]. Although gold NPs themselves can be used as therapeutic agents to destroy tumor cells, they had also been used in the field. Specially, the presence of ultrasound, by combining gold NPs with ultrasound (1 and 3 MHz, 2 W/cm2) and an intense pulse of light was found to significantly improve the therapeutic effect in colon tumors [17]. There were reporting conducted in vivo, it was found that ultrasound (1.1 MHz, 2 W/cm2) applied to gold NPs conjugated to the sonosensitizer PpIX (Au-PpIX) resulted in greater inhibition of the Colon Cancer (CT26) than when using PpIX alone. This was attributed to gold NPs acting as nucleation agents, leading to more collapsing cavities in addition to improving uptake of the Au-PpIX by the tumor cells [18].
Additionally, due to the presence of gold NPs in the liquid medium, it applied nucleation sites for producing bubbles in ultrasound treatment because of its surface roughness. It resulted in decrease in the ultrasonic pressure threshold needed for cavitation to occur, hence enhancing the cavitation processes while adding to cultured media, the initial discovery that gold NPs enhanced inertial cavitation [19]. As incubating of this fabrication with two cancer cell lines (KB and HCT-116), followed by exposure to ultrasound (8×10−6 and 8×10−5 J. cm−2 for 5 min at 1.866 MHz) significantly reduced cancer cells growth and proliferation together with the significant generation of ROS species. Another report as showed in Kosheleva, et al. [20] work, the lung cancer cells (A549) showed higher sensitivity to the treatment with gold NPs combined with 4 MHz high intensity focused ultrasound, than treatment with the gold NPs only (Figures 3G-3I).
Pure-phased titanium dioxide as effective candidate in ultrasound type therapy
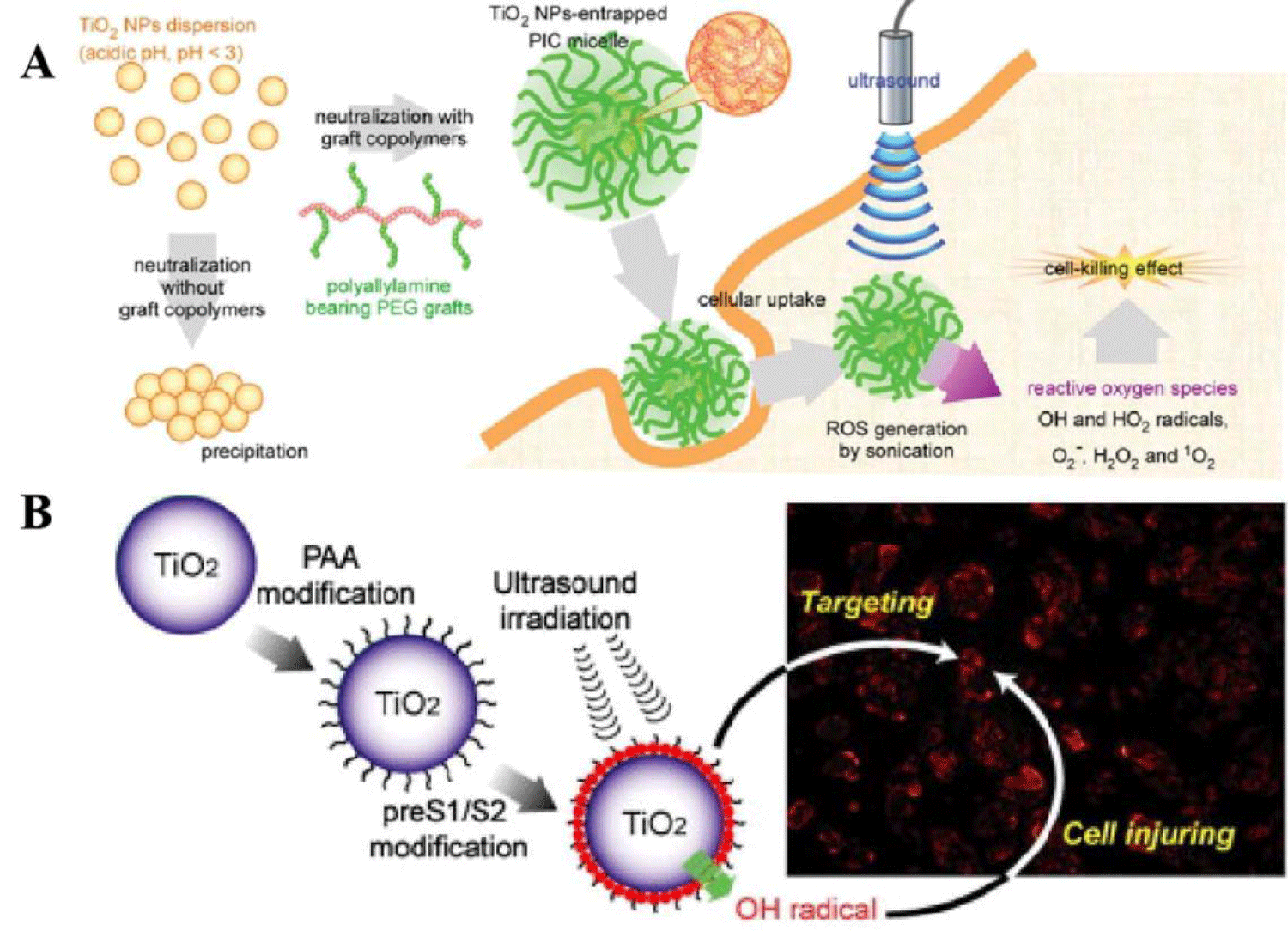
The development in this area about pure phased inorganic materials as candidate in ultrasound were well focused on the metal based materials and composites, which was modified and the surface could be linked strongly through adjusting the solution atmosphere (pH and solvent components). Some of the phase in different conditions, it gave the corresponded phenomenon while the candidate was integrated with target tissues. In order to do the determination about full systems in cancer therapy, the efficiency of SDT candidates, it influenced the cancer cell completely in cell applications, during the mechanism process, such as the sensitive functional material during in electron transfer process, Titanium Dioxide (TiO2) NMs as sonosensitizers in generating ROS under ultraviolet light irradiation [21,22]. It conveyed the demonstration about TiO2 materials that can explore the ROS under ultrasound and provided a new sonosensitizer for SDT application. Therefore, it was necessary to fabricate one effective candidate by using TiO2 as hard template. There were some requirements as follows: the resulted candidate should overcome its low hydrophilic stability in materials’ integration, because pure TiO2 has poor water dispersion and low ROS efficacy [23]. To overcome these issues, TiO2 were modified with some macromolecular polymer materials (e.g., proteins, dextran, and polyion complexes), which can improve the biocompatibility and water solubility [24]. For example, You, et al. synthesized Carboxymethyl Dextran (CMD)-modified TiO2 had superior in vivo stability and excellent inhibitory effect on subcutaneous tumors under ultrasound irradiation. Similarly, it had proved that grafted TiO2 with polyallylamine to form one coreshell structure, this composite micelle which can improve the dispersion stability of hard-template under physiological conditions. In addition, it was one of useful approach of making heterogeneous structure by using TiO2 integrated with fluorescent materials. This fluorescent labeling results conveyed that the cellular uptake rate of TiO2 was very fast. TiO2 itself could generate ROS by using ultrasound treatment. There was another one example such as the green fluorescence produced by the reaction between oxygen pieces, it was sensitive toward green and could be observed in the cytoplasm, but almost no fluorescence signal was observed in ultrasound-blank areas.
Due to the obtained correspondence, it had reported one approach such as protein modified TiO2 which had much higher accumulation efficiency in bicompatibility test while compared with unmodified metal candidate. Also, it showed optimized SDT effect about TiO2 by controlling the ultrasound dose in further investigation [25,26]. It was found that the pre-S1/S2 protein modified metal candidate combined with dual ultrasound treatment caused the amount of damage to biological tests. The avidin modified TiO2 could effectively target MCF-7 cells, and its phagocytosis rate could reach to 80%, while the phagocytosis rat of normal cells was only 30%. With longtime treatment by using ultrasound, the survival rate of tumor cells was significantly reduced (Figure 4). Similarly, due to the higher surface area of structural characterizations, it could load some of drugs or chemicals toward the combination of therapeutic effects. The surface of MTN was modified with ROS responsive chemical bonds and assembled with β-cyclodextrin (β-CD), which further broken the ROS response chemical bond and released DTX. MTN@DTX-CD composite had an excellent tumor inhibition efficacy in MCF-7 tumor-bearing mice model due to the combination of SDT and chemotherapy. However, the rapid electron–hole recombination of TiO2 resulted in a low ROS generation yield, which limits its SDT efficacy. Much effort had been devoted to improve the ROS generation ability of TiO2.
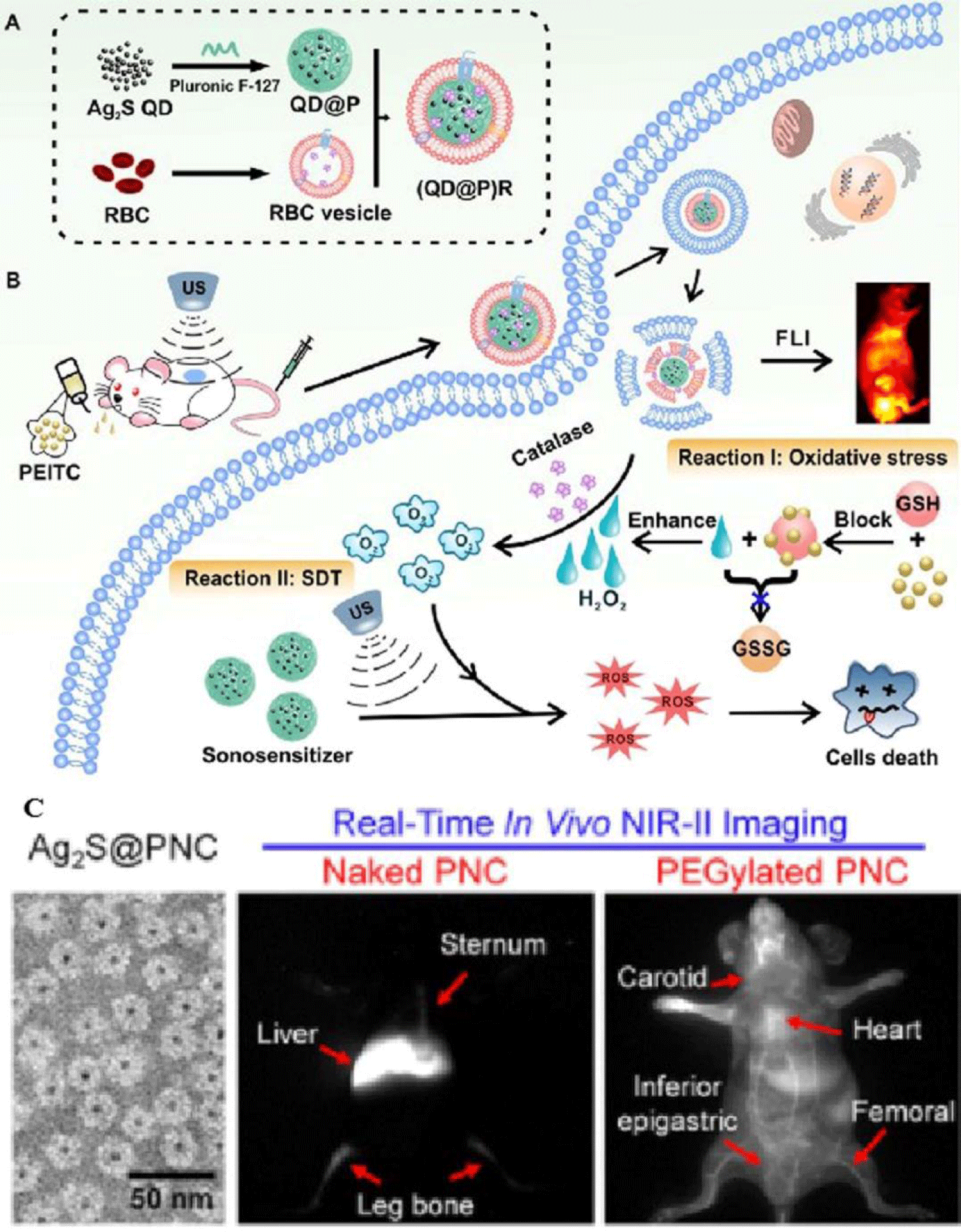
In recent progress, Ag2S QDs were treated as one good fluorescence imaging agent in vivo and also had ideal PTT and PDT therapeutic effects under laser irradiation, indicating that they could be an excellent multifunctional NMs for tumor therapy [27,28]. To date, application of Ag2S QDs as a sonosensitizer had not been investigated. In this study, it successfully constructed biomimetic candidate by wrapping Pluronic F-127-modified Ag2S QDs toward enzyme-augmented SDT. These particles were employed in vivo for fluorescence image-guided non-invasive treatment of tumors, in which, Ag2S was used as a sonosensitizer to generate ROS under ultrasonic stimulation immediately. Encapsulation of the QDs in RBC membranes, it not only prolonged the circulation time of the probe but also catalyzed endogenous hydrogen peroxide by the catalase in RBCs to ameliorate tumor hypoxia. Besides, ultrasound effect could also promote tumor blood flow, relieved the hypoxic condition, and enhanced the SDT effect of the probe. This novel nanoplatform provided an elegant approach for improving tumor hypoxia for the treatment of deep-seated tumors (Figure 5).
Gold based heterogeneous structure as effective candidate in cancer therapy under ultrasound treatment
Toward the treatment effect, there is much effort being done in order to devote to improve the ROS generation ability of metal candidate. As used Au based heterogeneous structure, this structure could maximize the efficient production of ROS. As mentioned in previous works, it had successfully synthesized Au@ TiO2 with the adjustable shell as an anti-cancer therapeutic agent. Under the ultrasound irradiation, the heterogeneous structure provided a charge transfer channel at the metal–semiconductor interface [29,30]. Au crystalline had electron traps that could effectively inhibit the recombination of pairs of electrons (e−) and holes (h+) that were generated by TiO2 under ultrasound treatment. Thus, the ROS production of Au@TiO2 was enhanced. It was worth noting that Au had a strong absorbance at 1064 nm, and the photothermal conversion efficiency was 42.05%. According to in vitro experiments, the cell viability was decreased to 20.3% after the combination of PTT and SDT [31,32]. By changing the size of gold NPs, it illustrated that the SDT effect of TiO2 co-loaded with small and large sized gold was stronger than that of smaller gold NPs modified TiO2 surface. It could be attributed to the density of gold NPs being too low to prevent (e-) and (h+) recombination, while gold NPs with large size in heterogeneous structure, it resulted plasma-induced electron pump effect only in the visible light region (beyond 500 nm). The surface of gold NPs were modified by TiO2 and showed higher ROS generation efficiency and better tumor suppression rate than those pure gold NPs or TiO2, respectively.
Recently, the prepared PEG modified ultrafine Ti nanorods, which had greatly improved sonosensitization and Fenton-like catalytic activity [33]. In this system, the oxygen deficient structure in TiO1+x Nanorods (NRs) could be used as the charge trap to inhibit the recombination of (e−) and (h+) pairs, resulted a higher ROS generation yield. The PEG-TiO1+x NRs also exhibit horseradish peroxidase activity, which could catalyze endogenous hydrogen peroxide to generate hydroxyl radicals, thereby achieved chemo-dynamic therapy. The PEG-TiO1+x NRs could be effectively passively retained in tumor after intravenous injection. Furthermore, no significant long-term toxicity was observed in the mouse model experiments. It used polymers or high mass molecular weight soft materials as outer shell in order to increase its biocompatibility in cancer therapy.
In addition to the oxygen-deficient structure, it increased the possibility that emphasizes the significance of metal coordination by porphyrins in SDT. As mentioned in previous discussion, three kinds of metal 4-methylphenylporphyrin (TTP) complexes (MnTTP, ZnTTP, and TiOTTP) had been proved to yield large amounts of ROS under ultrasound irradiation and demonstrated superb abilities of ultrasound activation with tissue depths. MnTTP complex illustrated optimal ROS activation, owing to the smallest energy gap between the highest occupied molecular orbital and the lowest unoccupied molecular orbital based on the density functional theory [34]. Metal Organic Framework (MOF)-derived mesoporous carbon nanostructure containing porphyrin-like metalcenters were also an exceptional sonosensitizer with high ROS productivity and superior stability [35]. Different from MnTTP, the superior sonosensitization effect of PMCS might be related to its energy gap. These discoveries would help intensify the comprehension of the sonochemistry mechanism of metal-porphyrin complexes in SDT augmentation.
Metal based magnetic nanocomposites in ultrasound therapy
Currently, the combination of magnetic materials with other functional materials had been well investigated and used in cancer diagnosis and related treatment [36]. Especially to magnetic template as one coherent part, its inherent characteristics played important roles while making integrated structure, such as nickel, iron, and cobalt elements, which could be used in synthesis dual-metal based composites and functional material? In recent studies, a nickel ferrite/carbon nanocomposite (NiFe2O4/C) which combined multiple diagnostic and therapeutic functions, i.e., MRI, magnetic drug delivery, hyperthermia, and SDT was synthesized [37]. Under ultrasound treatment (1.0 W/cm2), NiFe2O4/ C could produce a large amount of ROS due to the catalytic metal compositions (Ni, Fe). In addition, the excellent biocompatibility further expanded the application field of NiFe2O4/ C in cancer treatment [38].
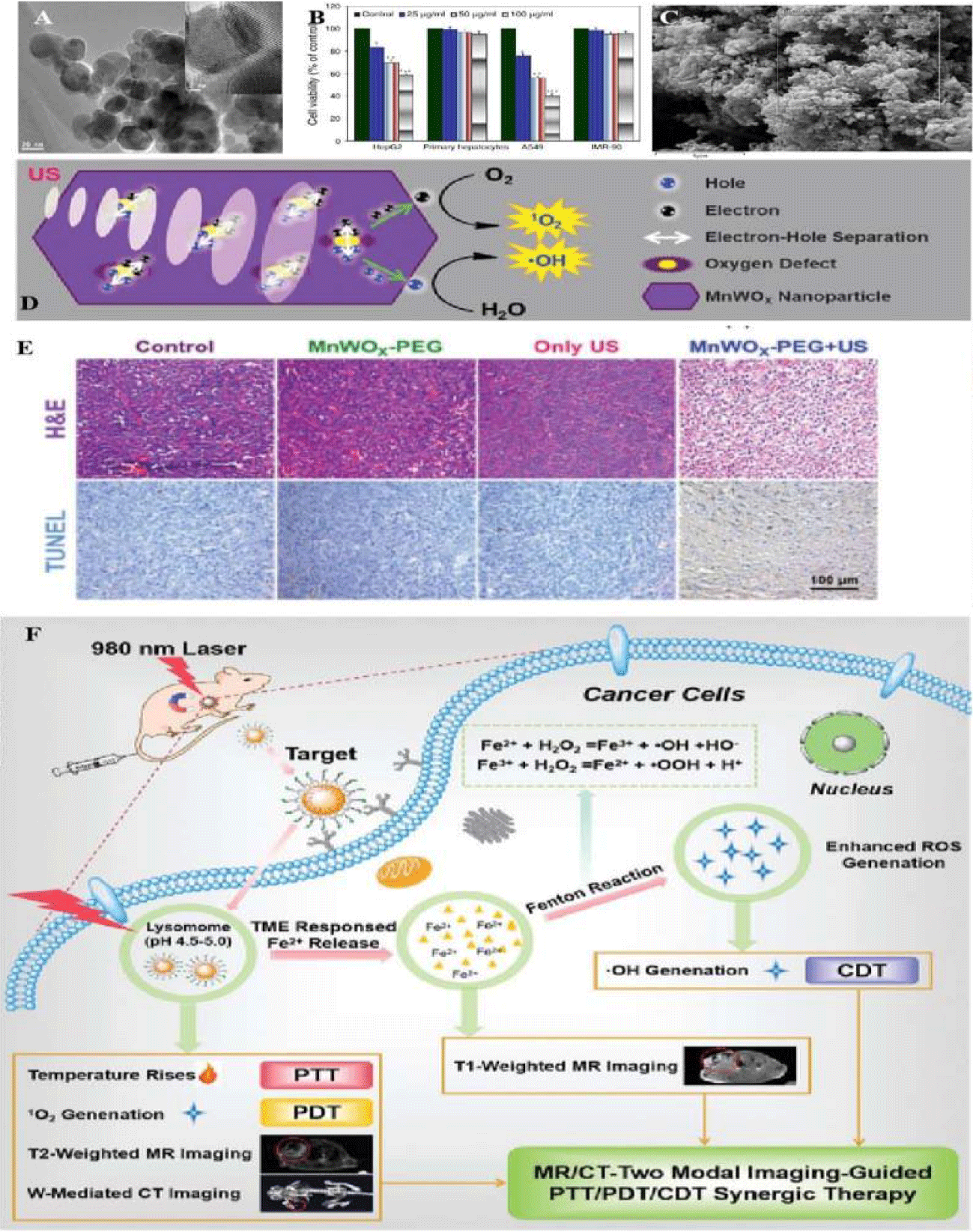
Similarly, there was ultra-small MnWOX-PEG that can serve as a novel sonosensitizer for multi-modal imaging-guided SDT application [39]. Due to its higher hydrophilicity in water mediums, its surface functionality should be well understood in water soluble or buffer solution dispersion (Figure 6). Among the synthetic processes, W(CO)6 precursor, Mn(acac)3 powder and other auxiliary agents reacted at 260°C for 30 min under the protection of nitrogen. The amphiphilic polymer was used to synthesize MnWOX and improved their water suspension stability in further measurement. The unique oxygen defect structure of MnWOX-PEG provided electron capture sites which can prevent the recombination of (e−) and (h+), resulting a high ROS generation efficiency [40,41]. Moreover, MnWOX-PEG prevent Glutathione (GSH) from clearing ROS, and further enhance the effect of SDT treatment. The presence of Mn and W in this Nano platform provided two diagnostic methods (magnetic resonance and computed tomography) that can be used to track the accumulation of materials in tumors applications [42].
Among distinctive sorts of metal oxide NMs, manganese-based oxides including manganese dioxide had been synthesized through appropriate approach [43] and had considerable importance in several technologies. It had medical applications such as energy storage, imaging, theranostics, hyperthermia, drug/ gene delivery, biosensing, ultrasound-promoted tumor chemotherapy and SDT. This kind of manganese-based nanostructures had tunable morphology and structures, inherent magnetic resonance and biodegradable character with excellent biosecurity. It had ability to degrade hydrogen peroxide in the cancer micro-environment to relieve hypoxia. During this, the oxygen generation as well as the ability of GSH depletion, could enhance chemodynamic, PDT, SDT and starvation therapies which might provide favorable application in T1-MR imaging [44].
Furthermore, Superparamagnetic Iron Oxide (Fe3O4) or it’s oxidized formed maghemite (γ-Fe2O3) with diameters ranging between 1 and 100 nm. As illustrated in previous studies, the consisted iron oxide cores could be targeted to a specific location via externally applied magnets. A magnet was placed externally over the targeted area producing a strong magnetic field that attracted the Fe3O4 to the desired location. It was promising as one kind of carriers capable of delivering drugs to the body ascribed to being biodegradable and simple to synthesize [45]. The different types of functional groups included carboxylgroups (-COOH) and carbodiimides (N=C=N), that could be grafted on the particle surface. Chemotherapeutic drugs could then be conjugated to these functional groups. However, because drugs were only loaded on their surface, magnetic particles had been found to release their drug load soon after injection into the bloodstream (burst effect). The anti-neoplastic agents then failed to reach their therapeutic levels at the desired site [46]. In order to reduce their premature drug release, biocompatible polymers were usually used to coat the metal cores. There had various polymers such as PEG molecules, polylacticco-glycolic acid, polyethylene-co-vinylacetate, and polyvinylpyrrolidone, which had been used as coating materials in aqueous solutions also [47].
Through the surface modification, these coatings protected magnetic core and allowed drug binding by forming covalent bonds, adsorption or entrapment on particle surface. For example, coating Fe3O4 with the cross-linked polymer, poly (ethylene glycol)-co-fumarate, caused a significant reduction in premature released (21%) compared to the non-coated magnetic particles [48]. For the purpose of increasing its targeting abilities, and improving the surfaces ability of the magnetic particles, they could be crafted with targeting molecules such as folic acid, RGD, proteins, transferrin, and hyaluronic acid, and so on [49]. Several in vitro studies had shown no, or low, cytotoxic effects of Fe3O4 on cell cultures. However, others in vivo studies showed controversial toxicity patterns of particle itself; from negative to positive toxicity in several preclinical animal models in form of surface molecule exchanged or charged transfers. Generally, the toxicity of Fe3O4 depends on their size, dose, surface coating, and species.
It investigated the effect of exposing Fe3O4 particles to ultrasound (1 MHz and intensity of 2 W/cm2) on the viability of cancer cells [50]. The study showed that the level of ROS in the cells was enhanced by following ultrasound treatment. It reported that high concentrations of Fe3O4 (above 100 mg/mL) behave as sonosensitizers generating ROS (synergic effect). Coating Fe3O4 with sonosensitizers, e.g., titanium dioxide (TiO2), was a promising technique for enhancing ultrasound-assisted stimulation by inducing the formation of ROS, including hydrogen peroxide and super-oxides. There also had one report by Shen, et al. [51] investigating the cytotoxicity while DOX loaded titanium dioxide-encapsulated Fe3O4 (Fe3O4-TiO2) coupled with ultrasound (1 MHz, 1 W/cm2). This study reported that the magnetic based heterogeneous structure produced ROS following the exposure to ultrasound. Incubation of cancer cells with Fe3O4-TiO2-DOX followed by sonication showed higher toxicities compared to the treatment with free DOX or Fe3O4-TiO2-DOX alone.
Conclusion and Outlook
In this review, we had concluded the summary of metal based materials as functional candidates in cancer therapy. Especially in constructing the functional materials, the metal elements were mixed together and the metal diffusion in alloyed process. Such incorporation of metal materials, conveyed the new conception about biological applications and the further investigation in cancer therapies, offered one possibility for medical clinical works and new drug delivery systematic fabrications. For example, in spite of the charming future of the research field, the developed metal based materials and its system for the SDT applications was very limited at present. Over past 20 years, there were plenty of SDT materials candidate that have been developed, one of which is photo-sensitive candidate which is highly desired, which would be the further direction to advance the functional materials and ultrasound integration. In addition, the loading of sound sensiagent for achieving was highly efficient, selective, and steady ultrasound transfer conversion. The sound sensiagent played multifunctional roles in whole systems: 1) boosted the combination and bond-efficiency of metal materials and ultrasound corresponded molecules, which enhanced the final functions, 2) preventing the dual steps construction of compositional combination (metal materials and ultrasound)and 3) expanded the promotion in activation of the inducing processes. Till now, great progresses were achieved in the design and synthesis of metal-based NMs incorporated with ultrasonic applications. Thus, the tailoring of active functional materials would greatly contribute to achieving high-efficiency ultrasound conversion system.
Furthermore, compared with existed metal materials, how to utilize the metal alloys was the most common difficulty, which would expand the range of metal elements’ fabrications, and also enhance function toward cancer therapy once modified by functional group completely. Among cancer therapy, the medium condition was the special requirement for metal materials; its acquirement should be in the form of an inert candidate without any changes in its properties exhibitions. Moreover, the ultrasound treatment generally induced non-selective agent in cancer treatment, especially to metal platform, the chemical stability was easily influenced and appeared damages in whole constructions. Finally, to implement the ultrasound corresponded with SDT cure from laboratory study to “practical” applications, the construction of fixed ultrasound sensiagent must be taken into account. Particularly, the photosensitizer/ sonosensitizer fixed the whole system as one flexible substrate should be a preferred option. This would not only prevent sedimentation and promotes candidates recycling, but also alleviated the cost of reduced mass transfer between the target cancer issues and reactant. In the long run, only through the collective optimization of the metal based photosensitizer or sonosensitizer, reaction media and reactor, the practical application of cancer therapy valorization can be reliable.
Author Contributions
X. H., and S.C., contributed equally in this review paper. X. M. conceived the idea, X. H, S. C, collected data, prepared and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgement
This work was financed by Science Foundation Project of Chongqing Education Commission (Grants NO: KJQN201900436) and Science and Technology Planning Project of Yuzhong District, Chongqing, China (Grants NO: 20200118).
References
- Fan Y, Lin L, Yin F, Zhu Y, Shen M, Wang H, Du L, Mignani S, Majoral, Shi X. Phosphorus dendrimer-based copper(II) complexes enable ultrasound-enhanced tumor theranostics. Nano Today. 2020;33:100899. https://tinyurl.com/czby7cvh
- Zhang Y, Luo K, Gu Z. Functional dendritic polymer-based nanoscale vehicles for imaging-guided cancer therapy. Springer-Verlag. 2016. https://tinyurl.com/3t8jsx39
- Koziolová E, Goel S, Chytil P, Janoušková O, Barnhart T, Cai W, Etrych T. A tumor-targeted polymer theranostics platform for positron emission tomography and fluorescence imaging. Nanoscale. 2017;30:10906-10918. https://tinyurl.com/c6scmp6p
- Feng L, Gai S, He F, Yang P, Zhao Y. Multifunctional bismuth ferrite nanocatalysts with optical and magnetic functions for ultrasound-enhanced tumor theranostics. ACS Nano. 2020 Jun 23;14(6):7245-7258. doi: 10.1021/acsnano.0c02458. Epub 2020 May 28. PMID: 32432848.
- Guarch P, López R, SerretSalse J, González LJ, Borras, Perez J. Basis for the toxicological evaluation of engineered nanomaterials. Rev Chil Infecto. 2014;31(1):9-22. https://tinyurl.com/uzsv8fe
- Bejarano I, Espino J, Paredes S, Ortiz Á, Lozano G, Pariente J, Rodríguez A. Apoptosis, ROS and calcium signaling in human spermatozoa: Relationship to infertility. In Tech. 2012. https://tinyurl.com/2fa255d5
- Long L, Chen, Xiong Y, Zou M, Deng Y, Chen L, Wang Z. Efficacy of high-intensity focused ultrasound ablation for adenomyosis therapy and sexual life quality. Int J Clin Exp Med. 2015;87:11701-11707. https://tinyurl.com/5byu8mtk
- Schmidt M, Fuchs M. Penetrability in model colloid-polymer mixtures. J Chem Phys. 2002;117:6308-6312. https://tinyurl.com/2dvk5jrh
- Sakuma G, Fukunaka Y, Matsushima H. Nucleation and growth of electrolytic gas bubbles under microgravity. Int J of Hydrog Energy. 2014;39:7638-7645. https://tinyurl.com/by2pskf9
- Greenwood GW, Foreman A, Rimmer D. The role of vacancies and dislocations in the nucleation and growth of gas bubbles in irradiated fissile material. J Nucl Mater. 1959;1:305-324. https://tinyurl.com/spsb7xxh
- Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, Schmid G, Brandau W, Dechent W. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007 Nov;3(11):1941-1949. doi: 10.1002/smll.200700378. PMID: 17963284.
- Broda J, Schmid G, Simon. U. Size and ligand-specific bioresponse of gold clusters and nanoparticles: challenges and perspectives. Springer Int Pub. 2013. https://tinyurl.com/yhthwaxr
- Emelianov S, Wilson K, Homan K. Nanocarriers for imaging and therapy applications. Curr Pharm Design. 2012. https://tinyurl.com/awy2vzjb
- Brazzale C, Canaparo R, Racca L, Foglietta F, Durando G, Fantozzi R, Caliceti P, Salmaso S, Serpe L. Enhanced selective sonosensitizing efficacy of ultrasound-based anticancer treatment by targeted gold nanoparticles. Nanomedicine (Lond). 2016 Dec;11(23):3053-3070. doi: 10.2217/nnm-2016-0293. Epub 2016 Sep 15. PMID: 27627904.
- Grant CD, Schwartzberg AM, Norman TJ Jr, Zhang JZ. Ultrafast electronic relaxation and coherent vibrational oscillation of strongly coupled gold nanoparticle aggregates. J Am Chem Soc. 2003 Jan 15;125(2):549-553. doi: 10.1021/ja028532y. PMID: 12517170.
- Guillet Y, Rashidi, Prota D, Palpanta B. Gold nanoparticle assemblies: interplay between thermal effects and optical response. Gold Bull. 2008;41:341-348. https://tinyurl.com/2ytx2dnw
- Sazgarnia A, Shanei A, Taheri AR, Meibodi NT, Eshghi H, Attaran N, Shanei MM. Therapeutic effects of acoustic cavitation in the presence of gold nanoparticles on a colon tumor model. J Ultrasound Med. 2013 Mar;32(3):475-483. doi: 10.7863/jum.2013.32.3.475. PMID: 23443188.
- Sazgarnia A, Shanei A. Evaluation of acoustic cavitation in terephthalic acid solutions containing gold nanoparticles by the spectrofluorometry method. Int J Photoenergy. 2012:1-5. https://tinyurl.com/drcnn2rx
- Deng CX, Xu Q, Apfel RE, Holland CK. Inertial cavitation produced by pulsed ultrasound in controlled host media. J Acoust Soc Am. 1996 Aug;100(2 Pt 1):1199-1208. doi: 10.1121/1.416304. PMID: 8759969.
- Kosheleva OK, Lai TC, Chen NG, Hsiao M, Chen CH. Selective killing of cancer cells by nanoparticle-assisted ultrasound. J Nanobiotechnology. 2016;14(1):46. https://tinyurl.com/2xex22jk
- Harada A, Ono M, Yuba E, Kono K. Titanium dioxide nanoparticle-entrapped polyion complex micelles generate singlet oxygen in the cells by ultrasound irradiation for sonodynamic therapy. Biomater Sci. 2013;1(1):65–73. https://tinyurl.com/4254a23s
- Nitta , Kaya A, Yamane T, Hyodo K, Okada M, Furuzono T. Fundamental Study on Activation of Aminated Titanium Dioxide Composite by Low-Intensity Focused Ultrasound Irradiation in Anti-Infective Catheter System. Jpn J Appl Phys. 2010;49. https://tinyurl.com/2dksrsr8
- Paleologou A, Marakas H, Xekoukoulotakis N, Moya A, Vergara Y, Kalogerakis N. Disinfection of water and wastewater by TiO2 photocatalysis, sonolysis and UV-C irradiation. Catal Today. 2007;129:136-142. https://tinyurl.com/pabjrupm
- Ogino C, Shibata N, Sasai R, Takaki K, Miyachi Y, Kuroda S. Construction of protein-modified TiO2 nanoparticles for use with ultrasound irradiation in a novel cell injuring method. Bioorg Med Chem Lett. 2010;20(17):5320–5325. https://tinyurl.com/e59yw5wh
- Ninomiya K, Noda K, Ogino C, Kuroda S, Shimizu N. Enhanced OH radical generation by dual-frequency ultrasound with TiO2 nanoparticles: its application to targeted sonodynamic therapy. Ultrason Sonochem. 2014 Jan;21(1):289-94. doi: 10.1016/j.ultsonch.2013.05.005. Epub 2013 May 24. PMID: 23746399.
- Ninomiya K, Fukuda A, Ogino C, Shimizu N. Targeted sonocatalytic cancer cell injury using avidin-conjugated titanium dioxide nanoparticles. Ultrason Sonochem. 2014 Sep;21(5):1624-8. doi: 10.1016/j.ultsonch.2014.03.010. Epub 2014 Mar 19. PMID: 24717690.
- Li C, Li F, Zhang Y, Zhang W, Zhang XE, Wang Q. Real-time monitoring surface chemistry-dependent in vivo behaviors of protein nanocages via encapsulating an NIR-II Ag2S quantum dot. ACS Nano. 2015 Dec 22;9(12):12255-12263. doi: 10.1021/acsnano.5b05503. Epub 2015 Oct 28. PMID: 26496067.
- Li C, Yang XQ, An J, Cheng K, Hou XL, Zhang XS, Hu YG, Liu B, Zhao YD. Red blood cell membrane-enveloped O2 self-supplementing biomimetic nanoparticles for tumor imaging-guided enhanced sonodynamic therapy. Theranostics. 2020 Jan 1;10(2):867-879. doi: 10.7150/thno.37930. PMID: 31903156; PMCID: PMC6929970.
- Deepagan VG, You DG, Um W, Ko H, Kwon S, Choi KY, Yi GR, Lee JY, Lee DS, Kim K, Kwon IC, Park JH. Long-circulating Au-TiO2 nanocomposite as a sonosensitizer for ROS-mediated eradication of cancer. Nano Lett. 2016 Oct 12;16(10):6257-6264. doi: 10.1021/acs.nanolett.6b02547. Epub 2016 Oct 3. PMID: 27643533.
- Seh ZW, Liu S, Zhang SY, Shah KW, Han MY. Synthesis and multiple reuse of eccentric Au@TiO2 nanostructures as catalysts. Chem Commun (Camb). 2011;47(23):6689–6691. https://tinyurl.com/j4b9844u
- Gao F, He G, Yin H, Chen J, Liu Y, Lan C. Titania-coated 2D gold nanoplates as nanoagents for synergistic photothermal/sonodynamic therapy in the second near-infrared window. Nano. 2019;11(5):2374–2384. https://tinyurl.com/4nkuk3hc
- Cao Y, Wu T, Wenhao D, Haifeng D, Zhang X. TiO2 nanosheets with the au nanocrystal-decorated edge for mitochondria-targeting enhanced sonodynamic therapy. Chemistry of Materials. 2019;31:9105-9114. https://tinyurl.com/k6rcdfbk
- Yamaguchi S, Kobayashi H, Narita T, Kanehira K, Sonezaki S, Kudo N, Kubota Y, Terasaka S, Houkin K. Sonodynamic therapy using water-dispersed TiO2-polyethylene glycol compound on glioma cells: comparison of cytotoxic mechanism with photodynamic therapy. Ultrason Sonochem. 2011 Sep;18(5):1197-1204. doi: 10.1016/j.ultsonch.2010.12.017. Epub 2010 Dec 31. PMID: 21257331.
- Ma A, Chen H, Cui Y, Luo Z, Liang R, Wu Z. Metalloporphyrin complex-based nanosonosensitizers for deep-tissue tumor theranostics by noninvasive sonodynamic therapy. Small. 2019;15(5). https://tinyurl.com/2z49k56n
- Pan X, Bai L, Wang H, Wu Q, Wang H, Liu S. Metal-organic-framework-derived carbon nanostructure augmented sonodynamic cancer therapy. Adv Mater. 2018;30(23):e1800180. https://tinyurl.com/2pbkmh2p
- Ahamed M, Alhadlaq HA, Khan MM, Akhtar MJ. Selective killing of cancer cells by iron oxide nanoparticles mediated through reactive oxygen species via p53 pathway. J Nanopart Res. 2013;15(1):1-11. https://tinyurl.com/wt5hj75b
- Bakhshi H, Azari MM, Shokuhfar A. A facile approach for obtaining NiFe2O4@C core-shell nanoparticles and their magnetic properties assessment. Diam Relat Mater. 2020;110:108159. https://tinyurl.com/4dnb6twm
- Galvao WS, Freire RM, Ribeiro TS, Vasconcelos IF. Cubic superparamagnetic nanoparticles of NiFe2O4 via fast microwave heating. J Nano Res. 2014;16(12):1-10. https://tinyurl.com/yp38hhf7
- Cheng Y, Lu H , Yang F , Zhang Y , Dong H . Biodegradable FeWOx nanoparticles for CT/MR imaging-guided synergistic photothermal, photodynamic, and chemodynamic therapy. Nanoscale. 2021 Feb 7;13(5):3049-3060. doi: 10.1039/d0nr07215j. Epub 2021 Jan 29. PMID: 33514969.
- Han X, Huang J, Jing X, Yang D, Lin H, Wang Z. Oxygen-deficient black titania for synergistic/enhanced sonodynamic and photoinduced cancer therapy at near infrared-ii biowindow. ACS Nano. 2018;12(5):4545–4555. https://tinyurl.com/6ahhvyw
- Wang, Huang J, Zhou W, Zhao J, Peng Q, Zhang L. Hypoxia modulation by dual-drug nanoparticles for enhanced synergistic sonodynamic and starvation therapy. J Nano. 2021;19(1):87. https://tinyurl.com/banjmbtu
- Gong F, Cheng L,Yang N, Betzer O, Feng L, Zhou. Ultrasmall oxygen-deficient bimetallic oxide MnWOX nanoparticles for depletion of endogenous GSH and enhanced sonodynamic cancer therapy. Adv Mater. 2019;31(23):e1900730. https://tinyurl.com/jffwb3nj
- Heli H, Rahi A. Synthesis and Applications of Nanoflowers. Recent Pat Nanotechnol. 2016;10(2):86-115. doi: 10.2174/1872210510999160517102102. PMID: 27502388.
- Xu F, Zhu J, Lin L, Zhang C, Sun W, Fan Y, Yin F, van Hest JCM, Wang H, Du L, Shi X. Multifunctional PVCL nanogels with redox-responsiveness enable enhanced MR imaging and ultrasound-promoted tumor chemotherapy. Theranostics. 2020 Mar 15;10(10):4349-4358. doi: 10.7150/thno.43402. PMID: 32292499; PMCID: PMC7150492.
- Revia RA, Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater Today (Kidlington). 2016 Apr;19(3):157-168. doi: 10.1016/j.mattod.2015.08.022. PMID: 27524934; PMCID: PMC4981486.
- Bleyer WA. The clinical pharmacology of methotrexate: New applications of an old drug. Cancer. 1978 Jan;41(1):36-51. doi: 10.1002/1097-0142(197801)41:1<36::aid-cncr2820410108>3.0.co;2-i. PMID: 342086.
- Zhao X, Harris JM. Novel degradable poly(ethylene glycol) hydrogels for controlled release of protein. J Pharm Sci. 1998 Nov;87(11):1450-1458. doi: 10.1021/js980065o. PMID: 9811505.
- Tartaj P, Serna CJ. Synthesis of monodisperse superparamagnetic Fe/silica nanospherical composites. J Am Chem Soc. 2003 Dec 24;125(51):15754-15755. doi: 10.1021/ja0380594. PMID: 14677960.
- Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv drug deliver Rev. 2011;63(1-2):24-46. https://tinyurl.com/kfdazkf9
- Fard AE, Zarepour A, Zarrabi A, Shanei A, Salehi H. Synergistic effect of the combination of triethylene-glycol modified Fe3O4 nanoparticles and ultrasound wave on MCF-7 cells. J Magn Mater. 2015;394:44-49. https://tinyurl.com/2yt6xxs7
- Shen Y, Pi Z, Yan F, Yeh CK, Zeng X, Diao X, Hu Y, Chen S, Chen X, Zheng H. Enhanced delivery of paclitaxel liposomes using focused ultrasound with microbubbles for treating nude mice bearing intracranial glioblastoma xenografts. Int J Nanomedicine. 2017 Aug 9;12:5613-5629. doi: 10.2147/IJN.S136401. PMID: 28848341; PMCID: PMC5557914.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.