Medicine Group . 2022 February 24;3(2):181-188. doi: 10.37871/jbres1419.
Evaluation of South African Equisetum arvense as an Antidiabetic, Antioxidant and Immune Booster using Economical Thin Layer Chromatography Bio-autography
Adrian Svogie1, Mamza Mothibe2 and Kokoette Bassey1*
2Faculty of Pharmacy, Rhodes University, Grahams Town, South Africa
- Anti-diabetic
- Anti-oxidant
- Immune booster
- Equisetum arvense
- TLC-bioautographic
Abstract
Introduction: Equisetum arvense (E. arvense) is an herbal medicinal plant that is mostly found in Limpopo and the North West Provinces of South Africa. Traditionally, the infusions and decoction of this plant are used in the management of type 2 diabetes, compromised immune system and oxidative stress related disorders. We investigated E. arvense in an attempt to authenticate its traditional use by cost-effective thin layer chromatography.
Methods: The anti-diabetic activity was assayed by spraying a developed TLC plate with a buffered β-glucosidase enzyme and bovin solution and incubating at 36.9°C for 20 mins. A freshly prepared solution of 2-naphthyl-β-D-glucopyranoside and fast blue salt (1:4 v/v) was used for detecting how the plant extract inhibited the enzymes activity. Immune boosting potentials were evaluated by analysing for the presence or absence of β-sitosterol, a known immune booster in the 3 extracts (chloroform, ethanol, and water) and enriched fractions E and F. The antioxidant activity was investigated using 2,2-Diphenyl-1-Picryl-Hydrazyl (DPPH), H2O2 solution free radical scavenging as well as the reducing power of iron (+3) to (+2) by the extracts and enriched fractions. Standards and positive controls were used for the respective assays conducted.
Results: Results obtained indicated the potentials of E. arvense extracts and enriched fractions as antidiabetic (inhibits β-glucosidase implicated in type 2 diabetes), immune boosting (presence of β-sitosterol) and antioxidant (IC50 values of 5.48 ± 0.13 - 12.38 ± 0.09 for FRAP, 12.00 ± 0.02 – 14.01 ± 0.23 for DPPH and 18.07 ± 0.06 - 62.01 ± 0.18 for H2O2) assays. The quantified amount of β-sitosterol and the antidiabetic compound in the plant ethanol extract were determined as 323.85 ng/mg and 130.04 mg/ml or 1.3 x 1011 ng/ml respectively.
Conclusions: This study has preliminarily under score authenticated the traditional uses of E. arvense by rural South Africans.
Abbreviations
DPPH: 2,2-diphenyl-1-picrylhydrazyl; FRAP: Ferric Reducing Antioxidant Power Assay; TLC: Thin Layer Chromatography
Introduction
Diabetes is a metabolic and endocrine disorder that results from insulin deficiency (type 1), ineffective use of insulin by the body (type 2) or abnormal high blood glucose value (gestational diabetes). As a global burden, the number of diabetic patient rose from 108 to 422 million between 1980 and 2014 [1] and this number would rise to 629 million adults (48% increase) by the year 2045 if necessary and adequate actions are not taken [2]. An estimated 1.6 million people died from diabetes as of 2016 and the World Health Organization predicted in the same year that diabetes was the seventh leading cause of death globally. Africa and Asia are the two continents endangered with an annual two -to- three-fold increase burden of the disease and South Africa in particular experiences a high mortality rate, especially among the black population from diabetes [3]. Available information suggests that diabetes is emerging as a significant health problem in Africa, including South Africa [2].
The treatment of and management of any category of diabetes involves the use of exorbitant orthodox medications like insulin and pramlintide, cost-saving non-drugs prevention lifestyle including healthy diets, regular exercise, lowering of blood sugar, a normal body weight, foot care and a cigarette free life. Between both treatment types, majority of people from developing countries like South Africa struggle to afford them. Couple with cultural beliefs, religion, the perception that “natural products derived complementary and Alternative medicines are less toxic and safer”, plants and other natural products based medicines and their accessories are better embraced for the management of diabetes.
A comprehensive review by Afolayan and co-worker [3], underscored 32 medicinal plant species used for the in vivo management of diabetes across South Africa. In a separate review conducted by Odeyemi and Bradley [2], 25 medicinal plant families among them, Asteraceae, Asphodelaceae and Alliaceae were used in the management of diabetes in the Eastern Cape Province alone. Most of these plants with anti-diabetic potentials in vivo and in vitro, were characterized with higher percentage of insulin release and inhibition against carbohydrate digesting enzymes as compared with insulin mimetic and peripheral glucose uptake. Similar results was reported for 24 plant species belonging to 20 families, mostly from the Asteraceae (13%), Cucurbitaceae and Sapotaceae (8%), used to treat diabetes mellitus by Bapedi in the Limpopo Province [4] of South Africa. Mimusops zeyheri (29%), Helichrysum caespititium (25%), Plumeria obtusa (21%), ,em>Aloe marlothii var. marlothii, Hypoxis iridifolia and Moringa oleifera
(17% each), were repeatedly mentioned by the traditional healers as most used for the management of diabetes mellitus in the study area [4].Equisetum arvense (E. arvense) of the South African ecotype was not mentioned anywhere in literature as of the time of compiling this report for use in the management of diabetes. Perhaps to corroborate with the claims that there is insufficient scientific evidence to support the effectiveness of E. arvense as a medicine to treat any human condition in addition to the plant being toxic to pregnant women and children because it contains nicotine [5]. In contrast, the medicinal uses of E. arvense L. as reported in countries like Brazil, Romania, Germany, Serbia, China, Greece, Portugal, Iran, and Thailand as diuretic, anti- inflammation, among other are summarized in a review by Boeing and co-worker [6]. Phytochemicals including but not limited to silicic acid, linoleic acid, oleic acid, stearic acid, linolenic acid and traces of equisetin, nicotine, palustrine, and palustrinine alkaloids, flavonoids, saponosides, triterpenoids, phytosterols present in E. arvense are thought to be responsible for the plethora of biological activities of the extracts. In particular, the methanol extract of E. arvense L. from Iran has been reported to exhibit significant antidiabetic effect [7] in support of a personal communication we had with a South African Indigenous Knowledge System (IKS) practitioner that purportedly uses South African E. arvense L. for the management of diabetes. We investigated E. arvense in an attempt to authenticate its traditional use by rural South African as antidiabetic plant drug using cost effective thin layer chromatography bio-autography.
Materials and Methods
Plant material acquisition
Aerial plant material (1.3 Kg) was purchased from Mountain Herb Estate (117, Van Der Hoff Rd, Pretoria, 0068). The plant sample was identified and certified (item number: BS-0645) as Equisetum arvense by the South African National Biodiversity Institute (SANBI) Pretoria. The sample was air dried at room temperature and grinded to a fine powder using a grinder (Kinematica AG, Lauzern, Switzerland). The fine powder was stored in a dark room until use.
Extract preparations
The powdered leaves (20.05 g) was measured into a conical flask and 300 ml of cold distilled water was added into the conical flask. The flask was placed in a shaking water bath (shaker bath SBS30, Stuart Scientific-United Kingdom) set to rotate at 84 RPM for 30 minutes at a temperature of 100°C. The mixture was filtered with a cotton wool after decantation. The extraction process was repeated two more times and to the pooled filtrate was added quantitative amount of methanol. The water extract-methanol solution was air dried to afford a dark brown dried extract. The same protocol was utilized to afford the ethanol and chloroform extract but filtration was done using Whatman filter paper number 4 and evaporation of the pooled filtrate under pressure with a Stuart rotary evaporator (Cole Parmer Ltd., UK) connected to Vacubrand MZ 2C NT pump (Vacuubrand GmBH + Co Kg, Wertheim, Germany). Semi-pure fractions arbitrarily named E and F rich in antioxidants were purified from column chromatography fractionations. Details of the fractionation and purification is not given here to stay within the context and content of this report.
Thin layer chromatography bio-autography antidiabetic assay
Aliquot of 10 mg/ml stock solution of each extract (water, ethanol and CHCl3), enriched fractions E, F and 1 mg/ml of conduritol B epoxide, a known irreversible inhibitor of β-glucosidase enzyme according to Grabowski and co-workers [8] dissolved in methanol, used as a positive control in this experiment were separately prepared. β-glucosidase enzyme dissolved in sodium acetate buffer (1000 u/100 ml), adjusted to pH of 7.5 using sodium hydroxide was also separately prepared. All the prepared test solutions were kept in the fridge at 4°C. Five (5 µl) of the extract (chloroform, ethanol, water), enriched fraction E and F and standard solutions were separately spotted on silica pre-coated aluminium plate at 2 mm x 5 mm away from the length and the breadth of the plate respectively. The TLC plate was developed in a suitable mobile phase, sprayed with β-glucosidase enzyme solution and incubated for 20 minutes at a temperature of 36.9°C. For detection of the active E. arvense extracts against the diabetes mellitus implicated β-glucosidase enzyme, freshly prepared solution of 2-naphthyl-β-D-glucopyranoside (2 mg/ml dissolved in ethanol) and fast blue salt (2.5 mg/ml dissolved in distilled water) were mixed at a ratio of 1:4 respectively. The solution was sprayed onto the developed and dried TLC plate after incubation. Different parameters were tested: temperature, incubation period and pH of the buffer solution. Favorable conditions were selected to afford the strongest color contrast to inhibition zones on the TLC plate as described by Simões‐Pires, et al. [9]. The quantitative anti-diabetic assay was evaluated using the method available in literature [9] with minor changes by making use of JustTLC®, a Sweday (Sweden) software for quantitation.
Qualitative thin layer chromatography bio-autography assay and quantitative antioxidant determination
The antioxidant activity was investigated using 2,2-diphenyl-1-picryl-hydrazyl (DPPH) solution. After developing solution of each plant extract on TLC plate and allowing to dry, the plate was derivatized with 0.2 % DPPH in methanol solution. Quantitative antioxidant was determined by first preparing 1.0 mg/mL of each extract (water, ethanol and chloroform), semi-pure antioxidant enriched fractions E, F and positive control vitamin C. Serial dilution of 20, 40, 80, 120 and 160 ng/mL were made from the stock solution, Briefly, 1.0 mL of the each test sample was mixed with 1.0 mL of 0.2% DPPH solution. The mixtures were allow to react at room temperature in the dark for 30 minutes. Blank DPPH solution was used as the negative control while L-ascorbic acid (Vitamin C) and 0.4 Mm of the DPPH + plant extract were used as the positive control. The tests were carried in triplicates and the mean values were determined. Hydrogen peroxide free radical scavenging assay was done with the same protocol using 20 mM of 30% w/v H2O2 mixed with PBS (pH = 7.67). Two milliliters (2.0 ml) of the hydrogen peroxide mixed with 1.0 ml each of the serially diluted (20, 40, 80, 120 and 160 ng/mL) test samples solution were separately allowed to react before reading the absorbance. The negative control was H2O2-PBS solution with no test samples while vitamin C was the positive control. The decrease in absorbance was measured at 230 nm using spectrophotometer (spectrophotometers Nanocolor® UV-vis, Macherey-Nagel GmbH & Co. KG, Germany). To 1 mg/ml of the each extract, enriched fractions E and F and vitamin C was added 2.5 ml of 0.2 M PBS (pH = 6.6), 2.5 ml 1% (w/v) K3[Fe(CN)6] solution. Each mixture was vortex and incubated in a water bath at 50°C for 20 mins. To the resulting solution, was added 2.5 ml of 10% (w/v) tricloacetic acid and centrifuge at 300 rpm for 10 mins to afford a bi-layer mixture. 2.5 ml of the upper layer was transferred into a test tube and 2.5 ml of distilled water and 0.5 ml of 0.1% FeCl3 solution was added and vortex again. The absorbance of the final solution was recorded at 700 nm. Values obtained were converted to percentage antioxidant activity (AOXA %) using equation 1.
% scavenging capacity = (A0 – As / A0) x 100 (1)
where A0 indicates absorbance of the negative control 1.0 mL of DPPH solution + 1.0 ml of methanol, 2.0 ml of H2O2 PBS or 0.2 M PBS + 1% (w/v) K3[Fe(CN)6] +. 2.5 ml of 10% (w/v) tricloacetic + 2.5 ml of distilled water and 0.5 ml of 0.1% FeCl3 and As represents the absorbance of the positive control 1.0 ml of DPPH solution + 1.0 ml, 2.0 ml H2O2-PBS solution of + 1.0 ml of extracts, or 0.2 M PBS + 1% (w/v) K3[Fe(CN)2] +. 2.5 ml of 10% (w/v) tricloacetic acid + 2.5 ml of distilled water and 0.5 ml of 0.1% FeCl3 + test samples, standards or vitamin C solution.
Thin layer chromatography bio-autography immune boosting assay
The in vitro qualitative immune boosting potentials was evaluated by analysing for the presence or absence of β-sitosterol, a known immune booster in the three extracts. The TLC conditions previously described, were used and extracts and standard β-sitosterol were spotted on the same TLC plate and developed. JustTlc® (Sweday, Sweden) software was used for the quantitative evaluation of β-sitosterol directly on the TLC plates.
Statistical analysis
Data were expressed as mean ± Standard Deviation (SD). For statistical evaluation, a One-Way Analysis of Variance (ANOVA) was conducted using Excel® version 16.0 and p values < 0.05 were considered as significant.
Results and Discussion
Qualitative determination of antidiabetic compounds in E. arvense
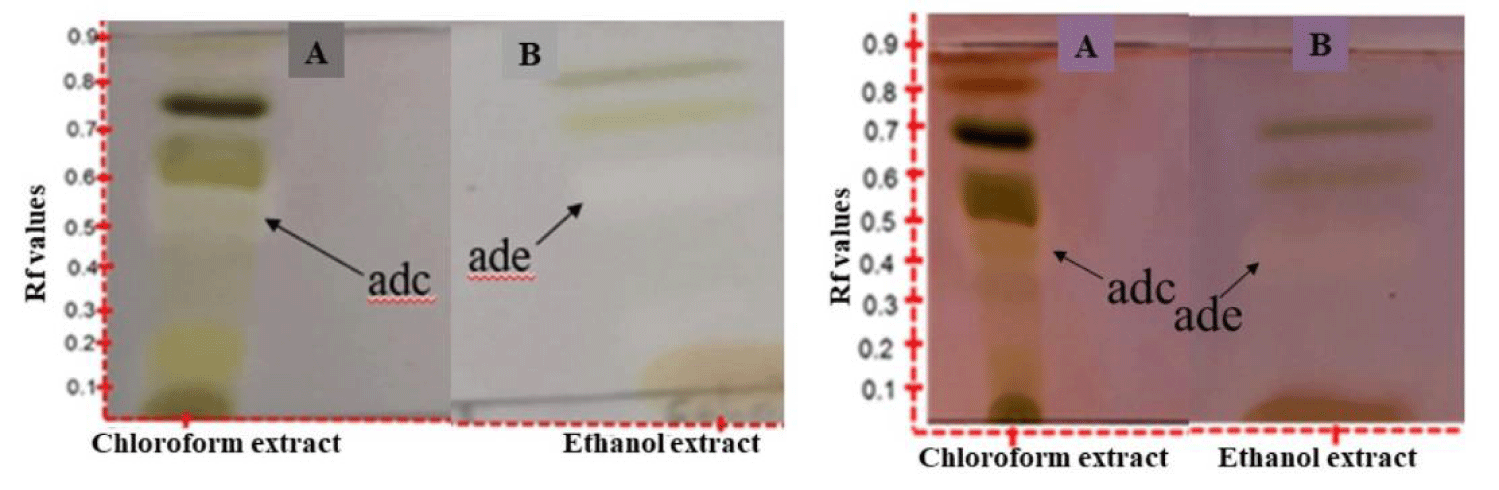
A TLC bioautographic technique was developed and used to identify antidiabetic activity of the plant extracts (water, ethanol and chloroform) of Equisetum arvense. The method was based on the principle of detecting β-glucosidase inhibition by the crude plant extracts. TLC-bioautographic assay play a huge role in the search of active compounds in plant extracts [10]. The TLC bioautography assay was carried out using the method by Simões-Pires and co-workers [9] with slight modifications. The assay deploys either α/β-glucosidase enzymes because of the therapeutic importance of their inhibitors in type 2 diabetes and anti-viral infection [11]. The test depends on the cleavage of 2-naphthyl-α-D-glucopyranoside or 2-naphthyl-β-D-glucopyranoside to form 2-naphthol, which in turn reacts with Fast Blue B salt to give a purple-colored diazonium dye. Plant extract inhibitors of these enzymes will reveal creamy coloration against the purple background within 2-5 minutes [9]. β-glucosidase was the substrate of choice in this study due to its commercial availability. The chloroform and ethanol extracts of E. arvense were resolved by the same solvent system of n-hexane: dichloromethane:ethyl acetate (6:6:2 v/v/v) using the same TLC plate. The chloroform and ethanol extracts (Figure 1) revealed 7.0 and 4.0 phytochemical bands respectively.
The Retardation factors (Rf) and colors of these bands on the left plate (capture after 2 mins) and right plate (captured after 5 mins) of treating the plates with buffered (pH 7.5) β-glucosidase enzyme solution, incubating at 36.9°C for 20 mins and finally spraying with a solution of 2-naphthyl-β-D-glucopyranoside and fast blue salt (1:4 v/v) are displayed in table 1. In accordance with the observation reported by Simões-Pires, et al. [9], the chloroform and ethanol extracts revealed one compound band each that inhibited the β-glucosidase and in turn should exhibit antidiabetic potentials. The compound in the ethanol extract was arbitrarily named D since this extract is of commercial and traditional uses interest. This compound was characterized by the cream coloration against the purple background and localized at Rf value of 0.58 (hexane:CHCl3:EtOAc 6:2:2 v/v/v). The ethanol extract was of outmost importance to us for the isolation and purification (underway study in our laboratory) of the active antidiabetic component D because the traditional concoction prepared from South African E. arvense for oral administration involves the soaking of the whole dry plant material in consumable alcohol like ethanol. The water and chloroform extracts were therefore not investigated for its antidiabetic potential for this reason and partly due to limited supply of enzymes required for the analysis.
| Table 1: Phytochemical present in E. arvense chloroform and ethanol extracts with their Rf values and coloration. | ||||
| Band # | Equisetum arvense extracts | |||
| CHCl3 | EtOH | |||
| Coloration | Rf value | Coloration | Rf value | |
| 1 | Dark brown | 0.6 | pink | 0.5 |
| 2 | gold | 0.18 | Nd | Nd |
| 3 | grey | 0.45 | Nd | Nd |
| 4 | cream | 0.58 | cream | 0.58 |
| 5 | gold | 0.68 | gold | 0.68 |
| 6 | Dark green | 0.77 | Dark green | 0.77 |
| 7 | Dull green | 0.89 | Nd | Nd |
| Nd: Not detected; CHCl3: Chloroform; EtOH: Ethanol | ||||
Qualitative determination of antioxidant compounds in E. arvense
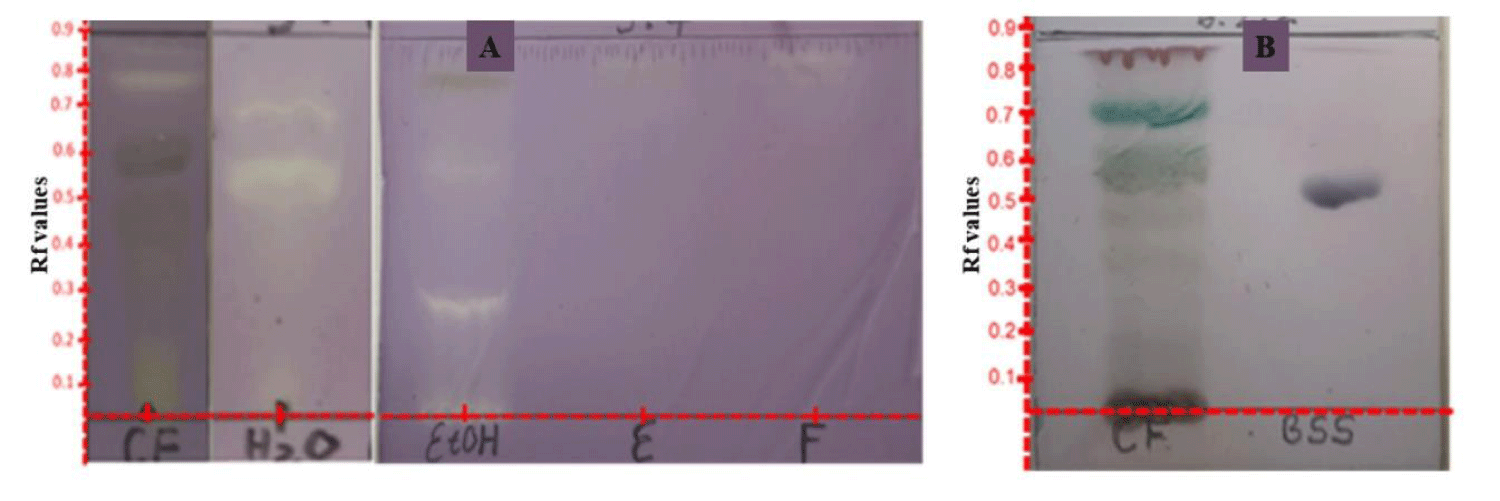
Antioxidants such as N-acetylcysteine, vitamin C and α-lipoic acid are effective in reducing diabetic complications, thus underlining the health benefits of consuming natural antioxidants by ingestion or through dietary supplementation [12]. The indication of antidiabetic activity of the ethanol and chloroform extracts of E. arvense prompted us to analyze these extracts for their antioxidant potentials. The water extracts was also investigated due to ease of availability of the reagents for this analysis. Our results (Figure 2) revealed that the three extracts analyzed exhibited DPPH free radical scavenging property, a function of creamy bands against purple background. Whereas the chloroform extract (Figure 2A: CF) bare creamy bands for antioxidant compounds at Rf = 0.68 and 0.75 in n-Hex:DCM:EtAOc (6:2;2 v/v/v), the ethanol (Figure 2A: EtOH) in MeOH:H2O (5:4 v/v) indicated antioxidant spot at Rf = 0.50 and the water extract (Figure 2A: H2O) exhibited two antioxidant compounds spots at Rf = 0.59 and between 0.67 in MeOH: H2O (5:4 v/v). The enriched fractions E and F on other hand indicated antioxidant compounds at Rf = 0.86 and 0.87 respectively with less color intensity.
Qualitative determination of β-sitosterol in E. arvense
Patients suffering from diabetes, especially the type 2 often have compromised immune system [13]. Such systems is mostly characterized by the destruction of beta cells in the pancreas that is responsible for the in vivo synthesis of insulin. Food rich in dietary immune boosting herbs are deem pivotal in the prevention of diabetes. E. arvense whole plant is marketed across South Africa as such plant drug and our result here authenticates its use in that regard. As evident in Figure 2B, the qualitative analysis of the chloroform extract of E. arvense shows the presence of β-sitosterol (BSS). According to Wei and co-workers [13], this known immune boosting sterol can also be extracted using solvents like methanol, ethanol, acetone, ethyl acetate, and n-hexane at temperatures ranging from 278.15 to 333.15. Therefore the ethanol extract of E. arvense should contain this valuable sterol as well. The detection of the antidiabetic, antioxidant and immune boosting sterol in the extracts of E. arvense are in support of the traditional use of the plant in the wholesome management of diabetes. Factoring in the perspective that these biological activities are of clinical importance, we determined their quantitative amount in the extracts using the IC50 values and calibration standard methods.
Quantitative antioxidant activity evaluation
DPPH free radical scavenging: It is of nutritional and clinical importance to know what extract or compound thereof that exhibit the best free radical scavenging activity in E. arvense. In addition, the concentration of a plant extract or phytochemical(s) required to inhibit or scavenged 50% of the free radical in vitro is a valuable tool when formulating herbal medicaments. The free radical scavenging potentials of the E. arvense extracts and enriched fraction are display in Table 2. The decreasing absorbance of the DPPH for the test samples and control, were converted to percentage DPPH free radical scavenging (% DPPH antioxidant) and using regression analysis, IC50 Values (Table 2) were calculated for test samples and control. Results revealed that the E. arvense water, chloroform extracts and fraction E displayed percentage radical scavenging properties that are comparable to that of the ascorbic acid, while the ethanol extract and fraction F exhibited a slightly better antioxidant activity with IC50 = 13.21 and 13.03 ng/ml than the positive control with IC50 = 13.00 ng/ml (Table 2).
| Table 2: Percentage antioxidant (DPPH and H2O2 free radical scavenging, FRAP) and IC50 (ng/mL) values of test samples and control. | ||||||
| Anlyte | Percentage free radical scavenging | Calculated IC50 values | ||||
| %DPPH (ng/mL) | %H2O2 (ng/mL) |
% FRAP | DPPH IC50 (ng/mL) |
H2O2 IC50 (ng/mL) | FRAP IC50 (ng/mL) |
|
| Fraction E | 80.64 | 36.09 | 74.63 | 14.01 ± 0.01 | 62.01 ± 0.18 | 5.52 ± 0.23 |
| Fraction F | 85.11 | 62.09 | 63.72 | 13.21 ± 0.11 | 18.07 ± 0.09 | 5.48 ± 1.21 |
| Water extract | 80.41 | 62.77 | 64.65 | 12.00 ± 0.02 | 20.2 ± 0.02 | 8.32 ± 0.07 |
| Ethanol extract | 84.68 | 61.94 | 72.51 | 13.03 ± 0.11 | 24.90 ± 0.62 | 6.56 ± 0.91 |
| Chloroform extract | 80.68 | 58.57 | 73.32 | 14.15 ± 0.00 | 26.06 ± 0.07 | 8.89 ± 0.04 |
| Vitamin C | 80.84 | 49.48 | 58.07 | 13.00 ± 0.03 | 19.05 ± 0.13 | 12.38 ± 0.08 |
Hydrogen peroxide free radical scavenging property: The hydrogen peroxide free radical scavenging assay results (Table 2) was similar to that obtained from the DPPH assay. The best antioxidant activity were recorded for fraction F, water extract and ethanol extract with IC50 = 18.07 ng/mL, 20.20s ng/mL and 24.90 ng/mL respectively. These results are also in agreement with the qualitative antioxidant results obtained from the TLC-bioautography, which highlighted two antioxidant compounds for the water and ethanol extract and only one for the chloroform extract.
Ferric acid reducing power: The percentage ferric reducing power of the test extracts, antioxidant-enriched fractions E, F and vitamin C agreed with the DPPH and hydrogen peroxide results for the same test samples. The reducing power IC50 (Table 2) values concur to the observations that compound F (IC50 = 5.48 ng/ml) exhibited the best antioxidant activity that is comparable with the positive control, ascorbic acid. The water and ethanol extracts exhibited the second and third best reducing ability one-to-one.
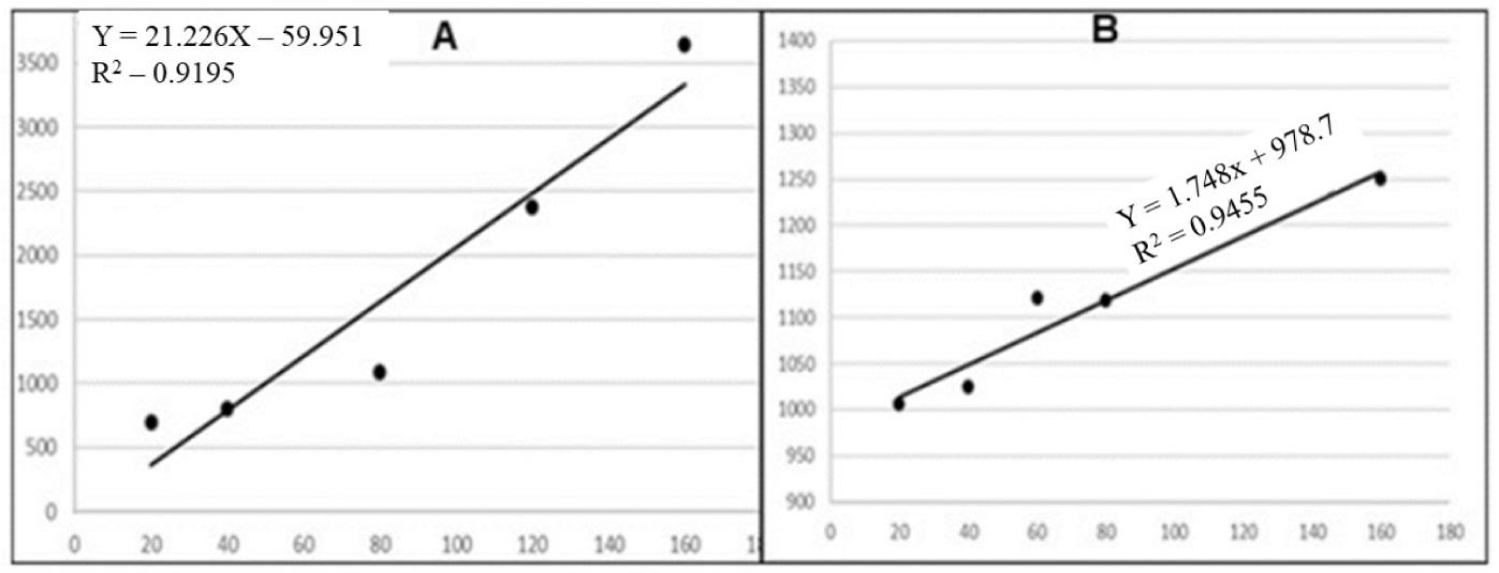
Quantitative antidiabetic evaluation of compound D using JustTLC®: A calibration cure (Figure 3A) was created using standard concentrations of 20, 40, 80, 120 and 160 ng/ml of compound D just like the concentration range used to quantify the antioxidant compounds from the spectrophometry method. A linear regression with R2 = 0.9195 confirm this was a good concertation range for use in the quantification of D from the ethanol extract of E. arvense.
Using the same experimental conditions like the stationary phase, mobile phase and visualization reagent, a rough quantitation was demonstrated to quantify compound D from the plant extract. The area under the curve (area) from which the amount of D present in the extract was 4238 (Table 3). From the line equation y = 21.22x – 59.91, the amount of D in the ethanol extract of E. arvense was calculated using equation 2.
| Table 3: Post analysis data from the quantitation of (D) from E. arevense extract using JustTLC®. | |||||
| No | Rf | Area | Volume | Notes | X = y + 59.95 / 21.22 |
| 1 | 0.58 | 3645 | 10953002 | 160 ng/ml Standard Level 5 | Standard |
| 2 | 0.61 | 1805 | 5519854 | 120 ng/ml Standard Level 4 | Standard |
| 3 | 0.58 | 1089 | 2619416 | 80 ng/ml Standard Level 3 | Standard |
| 4 | 0.50 | 805 | 1015610 | 40 ng/ml Standard Level 2 | Standard |
| 5 | 0.49 | 695 | 378244 | 20 ng/ml Standard Level 1 | Standard |
| 6 | 0.43 | 4238 | 8900523 | 1 mg/ml extract | X0 = 202.54 |
| 7 | 0.55 | 2323 | 10246359 | 1 mg/ml extract | X1 = 112.29 |
| 8 | 0.55 | 2381 | 9554498 | 1 mg/ml extract | X2 = 115.03 |
| 9 | 0.50 | 3395 | 964563 | 1 mg/ml extract | X3 = 162.82 |
X = Y + 59.95/21.22 (2)
The amount of D form the rough analysis X0 is equal to 202.54 mg/ml. In a similar manner, X1, X2, and X3 from the first, second and third quantitation were obtained as 112.29, 115.03 and 162.82 mg/ml. The average quantified amount of D from the E. arvense ethanol extract is therefore 130.04 mg/ml or 1.3 x 1011 ng/ml. This amount of the antidiabetic compound D is of clinical importance. Diabetic patients that consumes E. arvense herbal concoctions especially those formulated with consumable alcohol extracts, like ethanol should benefit from compound D. This compound may help inhibit the β-glucosidase enzymatic pathway that is implicated in diabetes [8]. The enzymatic pathway of β-glucosidases enzymes in diabetes involves the cleavage of glycosidically β-linked sugars from polysaccharides thus impacting negatively on carbohydrate digestion in the gastrointestinal tract [14]. We propose that compound D has potential to interfere or inhibit such cleavage by β-glucosidases. Thus implying that it can help in the management of diabetes like it is practice by traditional healers in rural South Africa.
Quantitative determination of β-sitosterol from E. arevnse using JustTLC®: The rapid quantitative evaluation of the immune boosting β-sitosterol from the extracts of E. arvense was conducted using affordable JustTLC® (Sweday, Sweden) software. A linear regression with R2 of 0.9455 (Figure 3B) was obtained for β-sitosterol standard at the same concentration rate of 20-160 ng/ml. The β-sitosterol was quantified by determining the concentration of the Area Under the Curve (AUC) value of 1545 for the spot of the chloroform extract corresponding to β-sitosterol standard. The exact amount of the β-sitosterol (323.85 ng/ml; Table 4) was also calculated from the line equation, obtained from the calibration of the β-sitosterol standard.
| Table 4:Post analysis data from the quantitation of b-sitosterol from E. arvense extract using JustTLC®. | |||||
| No | Rf | Area | Volume | Notes | X = y – 978.7/ 1.7486 |
| 1 | 0.392 | 1251 | 19.15 | 160 ng/ml Standard Level 5 | Standard |
| 2 | 0.386 | 1119 | 18.88 | 120 ng/ml Standard Level 4 | Standard |
| 3 | 0.386 | 1121 | 20.82 | 80 ng/ml Standard Level 3 | Standard |
| 4 | 0.388 | 1025 | 7.38 | 40 ng/ml Standard Level 2 | Standard |
| 5 | 0.388 | 1007 | 4.96 | 20 ng/ml Standard Level 1 | Standard |
| 6 | 0.393 | 1545 | 51.91 | 1 mg/ml CHCl3 extract | 323.85 |
Again, one cannot overemphasized on the clinical status of determining the quantitative amount of β-sitosterol present in the extract of E. arvense. From the results obtain herein one may logically advise investors in the trade of E. avernse herbal medicines including supplements of several dosage forms of the need to standardize their products using the information that is shared in this study.
Conclusion
The results from this study indicates that E. arvense of the South African ecotype, contains active antidiabetic, antioxidants and immune boosting phytochemicals. These phytochemicals may include but not limited polyphenols (antioxidant), phytosterols (immune boosting) and phenol glycosides (antidiabetics). This authenticates to some extent, the use of E. arvense by South African traditional healers in the management of diabetes. In vivo studies should be conducted using extracts of E. arvense to further confirm its potential use in the management of diabetes. In addition, considering that inhibitors α/β-glucosidase are of clinical importance in type 2 diabetes and anti-viral infection, plant drugs like E. arvense should be tested against the deadly COVID-19 strands amongst other viral strands.
Acknowledgment
The authors thank the National Research Foundation (NRF) and the Sefako Makgatho Health Sciences University Research and Development Grant (RDG D113) for financial support.
References
- WHO. Diabetes. World Health Organization. 2018. https://tinyurl.com/yc6hmpd3
- Odeyemi S, Bradley G. Medicinal plants used for the traditional management of diabetes in the Eastern Cape, South Africa: Pharmacology and Toxicology. Molecules. 2018 Oct 25;23(11):2759. doi: 10.3390/molecules23112759. PMID: 30366359; PMCID: PMC6278280.
- J Afolayan A, O Sunmonu T. In vivo Studies on antidiabetic plants used in South African herbal medicine. J Clin Biochem Nutr. 2010 Sep;47(2):98-106. doi: 10.3164/jcbn.09-126R. Epub 2010 Jun 23. PMID: 20838564; PMCID: PMC2935160.
- Semenya S, Potgieter M, Erasmus L. Ethnobotanical survey of medicinal plants used by Bapedi healers to treat diabetes mellitus in the Limpopo Province, South Africa. J Ethnopharmacol. 2012 May 7;141(1):440-5. doi: 10.1016/j.jep.2012.03.008. Epub 2012 Mar 16. PMID: 22430018.
- European Medicines Agency (EMA/HMPC/278089/2015: Committee on Herbal Medicinal Products (HMPC), 2016. Assessment report on Equisetum arvense L, herba. https://bit.ly/3LWIbOI
- Boeing T, Moreno KGT, Junior AG, da Silva LM, de Souza P. Phytochemistry and pharmacology of the genus Equisetum (Equisetaceae): A narrative review of the species with therapeutic potential for kidney diseases evidence-based complementary and alternative medicine. 2021;2021:1-17. doi: 10.1155/2021/6658434.
- Safiyeh S, Fathallah FB, Vahid N, Hossine N, Habib SS. Antidiabetic effect of Equisetum arvense L. (Equisetaceae) in Streptozotocin-induced Diabetes in Male Rats. Pak J Biol Sci. 2007;10:1661-6. doi: 10.3923/pjbs.2007.1661.1666.
- Grabowski GA, Osiecki-Newman K, Dinur T, Fabbro D, Legler G, Gatt S, Desnick RJ. Human acid beta-glucosidase. Use of conduritol B epoxide derivatives to investigate the catalytically active normal and Gaucher disease enzymes. J Biol Chem. 1986 Jun 25;261(18):8263-9. PMID: 3087971.
- Simões-Pires CA, Hmicha B, Marston A, Hostettmann K. A TLC bioautographic method for the detection of alpha- and beta-glucosidase inhibitors in plant extracts. Phytochem Anal. 2009 Nov-Dec;20(6):511-5. doi: 10.1002/pca.1154. PMID: 19774543
- Marston A, Kissling J, Hostettmann K. A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem Anal. 2002 Jan-Feb;13(1):51-4. doi: 10.1002/pca.623. PMID: 11899607.
- Mehta A, Zitzmann N, Rudd PM, Block TM, Dwek RA. Alpha-glucosidase inhibitors as potential broad based anti-viral agents. FEBS Lett. 1998 Jun 23;430(1-2):17-22. doi: 10.1016/s0014-5793(98)00525-0. PMID: 9678587.
- Bajaj S, Khan A. Antioxidants and diabetes. Indian J Endocrinol Metab. 2012 Dec;16(Suppl 2):S267-71. doi: 10.4103/2230-8210.104057. PMID: 23565396; PMCID: PMC3603044.
- Wei D, Lishuo W, Cheng L, Baohe W. β-sitosterol solubility in selected organic solvents. J Chem Eng. 2010;55:2917-2919.
- Bhatia Y, Mishra S, Bisaria VS. Microbial beta-glucosidases: cloning, properties, and applications. Crit Rev Biotechnol. 2002;22(4):375-407. doi: 10.1080/07388550290789568. PMID: 12487426.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.