Medicine Group . 2022 February 24;3(2):195-197. doi: 10.37871/jbres1421.
Importance of Organophosphorus Compounds in Medicinal Chemistry Field
Petri A Turhanen*
- Organophosphorus
- Phosphonate
- ATP
- Anti-viral
- Bisphosphonate
Introduction
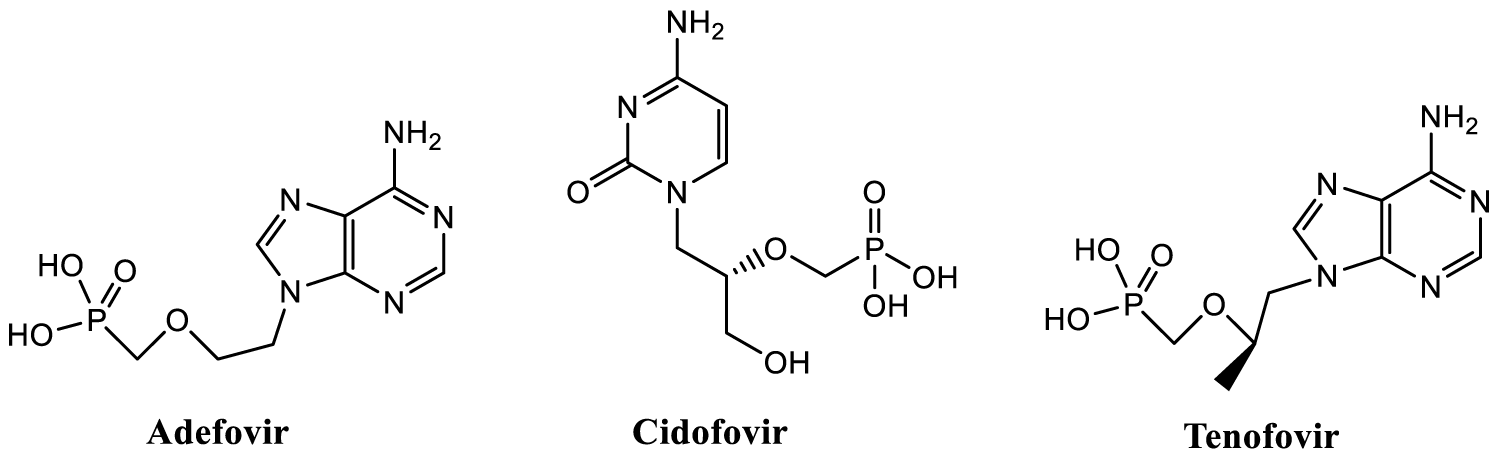
Organophosphorus chemistry has a crucial role in the field of drug research [1]. As generally well known, the Adenosine Triphosphate (ATP) plays a key role in the energy processes in all living cells of mammals. There are also natural phosphorus containing organic compound, Phosphocitrate (PC), found in mammalian mitochondria, likely produced by cytosolic phosphorylation of citrate, and which has important role in calcium metabolism, e.g. inhibiting hydroxyapatite precipitation in cells [2-4]. Phosphonates are analogs of natural phosphates, having characteristic C-P bond(s) and phosphonic acid moiety (R-PO3H2). These compounds present as analogs of carboxylic acids, amino acids and peptides [5]. An interest related to these compounds has increased tremendously because of their potential as drugs (also pro-drugs). Phosphonate compounds were observed to have e.g. antibacterial, anticancer and antiparasitic activity [6]; they can be used in medical imaging and diagnostics, and they have phosphoantigen properties [7]. Several compounds containing phosphonate group are already in use as antiviral drugs, like for the treatment of hepatitis B virus (Adefovir or its pro-drug Adefovir dipivoxil), cytomegalovirus (Cidofovir) and HIV/AIDS (Tenofovir or its pro-drug Tenofovir disoproxil) (Figure 1) [6,8]. There is also at least one organophosphorus compound, Remdesivir, under investigation for the treatment of COVID-19 [9].
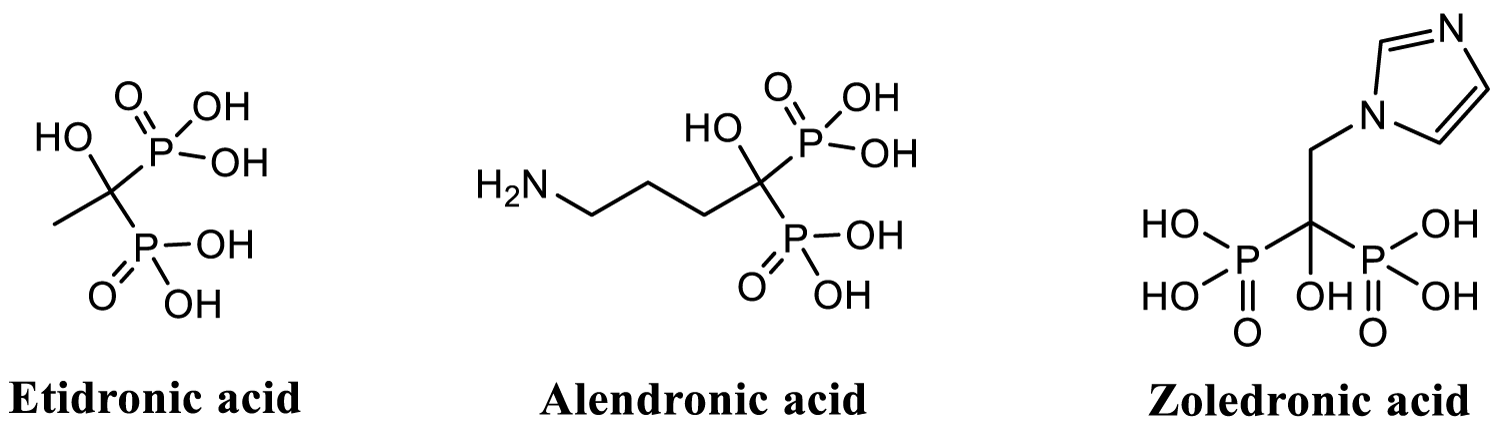
One very interesting group of organophosphorus compounds are, Bisphosphonates (BPs), which are analogs of natural pyrophosphate formed by hydrolysis of ATP and found in cells [10]. BPs are active drugs in many kinds of bone or calcium metabolism related diseases like osteoporosis and Paget´s disease [10,11]. They can also be used as bone targeting agents. BPs can be classified as non-nitrogen BPs (non-NBPs) and nitrogen containing BPs (NBPs); they are also categorized to different generations. In figure 2, structures of three different kind of BPs are presented: etidronic acid (1st generation, non-NBP), alendronic acid (2nd generation, NBP) and zoledronic acid (3rd generation, NBP) [12]. BPs present rich chemistry and biology. Applications of BPs cover much more than just medicinal chemistry field; and that is because of their very high affinity to almost any metal cations (alkali metal cations are exception because of their oxidation state +1); so they are potential compounds e.g. for removing/adsorbing metals from different kinds of water solutions [13-16]. Metal contaminated waters can be purified using BPs. As we know, pure water is crucial for all life on earth, and it is definitely health issue also.
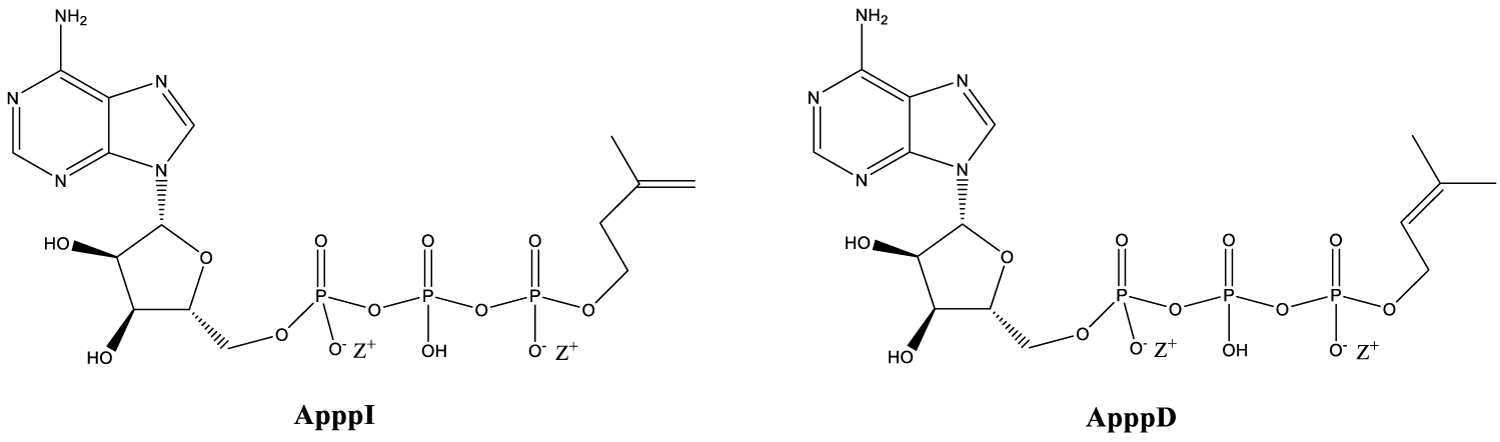
When NBPs are used as drugs, they will induce to formation of ATP analogs ApppI and ApppD (Figure 3) in cells. Non-NBPs will be metabolized also in cells but little bit different way to form adenosine monophosphate (AMP) attached to non-NBP used (ATP analogs also) [17]. These ATP analogs have observed to inhibit cell signaling pathways [18]. Nowadays, ATP analogs can be chemically synthesized, so more options for further research are available [18-20].
Conclusion
As a conclusion, it is more than clear that organophosphorus chemistry and compounds are very important in medicinal chemistry field and they offer an extremely fascinating “world”, which is constantly expanding. Organophosphorus compounds can be found in our bodies either as natural compounds (like ATP and PC) or induced by other compounds; and we really still do not know much of their biological functions and possibilities e.g. as drug LEADs.
Acknowledgment
I want to share my greatest gratitude and dedicate this paper to my grandmother Aino Kuivalainen, who just passed away. I also want to thank chief laboratory technician, Ms Maritta Salminkoski (retired) for her tremendous work in the field of organophosphorus chemistry.
References
- Tajti Á, Keglevich G. The importance of organophosphorus compounds as biologically active agents". Organophosphorus Chemistry: Novel Developments, edited by György Keglevich, Berlin, Boston: De Gruyter. 2018:53-65. doi: 10.1515/9783110535839-003
- Turhanen PA, Demadis KD, Peräniemi S, Vepsäläinen JJ. A novel strategy for the preparation of naturally occurring phosphocitrate and its partially esterified derivatives. J Org Chem. 2007;72(4):1468-1471. doi: 10.1021/jo061709c
- Moro L, Stagni N, Luxich E, Sallis JD, de Bernard B. Evidence in vitro for an enzymatic synthesis of phosphocitrate. Biochem Biophys Res Commun. 1990 Jul 16;170(1):251-258. doi: 10.1016/0006-291x(90)91267-v. PMID: 2372290.
- Howard JE. Studies on urinary stone formation: A saga of clinical investigation. Johns Hopkins Med J. 1976 Dec;139(6):239-52. PMID: 12399.
- Turhanen PA, Demadis KD, Kafarski P. Editorial: Phosphonate chemistry in drug design and development. Front Chem. 2021, 9:695128. doi: 10.3389/fchem.2021.695128
- Groaz E, De Jonghe S. Overview of biologically active nucleoside phosphonates. Front Chem. 2021;8:616863. doi: 10.3389/fchem.2020.616863
- Sevrain CM, Berchel M, Couthon H, Jaffrès PA. Phosphonic acid: Preparation and applications. J Org Chem. 2017;13:2186–2213. doi: 10.3762/bjoc.13.219
- Wilkens H, Sester M. Treatment of cytomegalovirus infection with Cidofovir and CMV immune globulin in a lung transplant recipient. Case Rep Transplant. 2016;4560745. doi: 10.1155/2016/4560745
- Al Mosawi AJ. Remdesivir research progress: An overview of the emerging evidence. J Biomed Res Environ Sci. 2020;1(6):216-218. doi: 10.37871/jbres1146
- Graham R, Russell G. Bisphosphonates: The first 40 years. Bone. 2011;49(1):2-19. doi: 10.1016/j.bone.2011.04.022
- Park J, Pandya VR, Ezekiel SJ, Berghuis AM. Phosphonate and bisphosphonate inhibitors of farnesyl pyrophosphate synthases: a structure-guided perspective. Front Chem. 2021:612728. doi: 10.3389/fchem.2020.612728
- Popov K, Oshchepkov M, Tkachenko S, Sergienko V, Oshchepkov A. Bisphosphonates: Synthesis, structures, properties, medical and industrial applications. J Mol Liq. 2022. doi: 10.1016/j.molliq.2022.118619
- Turhanen PA, Vepsäläinen JJ, Peräniemi S. Advanced material and approach for metal ions removal from aqueous solutions. Sci Rep. 2015;5:8992. doi: 10.1038/srep08992
- Haluska O, Rahmani A, Salami A, Turhanen P, Vepsäläinen J, Lappalainen R, Lehto VP, Riikonen J. Plant-based nanostructured silicon carbide modified with bisphosphonates for metal adsorption. Microporous Mesoporous Mater. 2021;324:111294. doi: 10.1016/j.micromeso.2021.111294
- Thapa R, Nissinen T, Turhanen P, Määttä J, Vepsäläinen J, Lehto VP, Riikonen J. Bisphosphonate modified mesoporous silicon for scandium adsorption. Microporous Mesoporous Mater. 2020;296:109980. doi: 10.1016/j.micromeso.2019.109980
- Thapa R, Rahmani A, Turhanen P, Taskinen A, Nissinen T, Neitola R, Vepsäläinen J, Lehto VP, Riikonen J. Recovery of uranium with bisphosphonate modified mesoporous silicon. Sep Purif. 2021;272:118913. doi: 10.1016/j.seppur.2021.118913
- Mönkkönen H, Auriola S, Lehenkari P, Kellinsalmi M, Hassinen IE, Vepsäläinen J, Mönkkönen J. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol. 2006 Feb;147(4):437-445. doi: 10.1038/sj.bjp.0706628. PMID: 16402039; PMCID: PMC1616989.
- Malwal SR, Dowd B, Feng X, Turhanen P, Shin C, Yao J, Kim BK, Baig N, Zhou T, Bansal S, Khade RL, Zhang Y, Oldfield E. Bisphosphonate-generated ATP-analogs inhibit cell signaling pathways. J Am Chem Soc. 2018;140:7568–7578. doi: 10.1021/jacs.8b02363
- Turhanen PA. Synthesis of a biologically important adenosine triphosphate analogue, ApppD. ACS Omega 2017;2(6):2835-2838. doi: 10.1021/acsomega.7b00531
- Puljula E, Turhanen PA. Semi-preparative high-performance countercurrent chromatography method for the purification of chemically synthesized ATP analogue, ApppI. J. Chromatogr. B: Anal. Technol Biomed Life Sci. 2017;1063:180-182. doi: 10.1016/j.jchromb.2017.08.038
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.