Medicine Group . 2022 February 26;3(2):205-209. doi: 10.37871/jbres1423.
CNS Demyelination Diseases Following Exposure to Urban Air Pollution
Mojtaba Ehsanifar1* and Zeinab Montazeri2
2Institute of Endocrinology and Metabolism Research and Training Center, Iran University of Medical Sciences, Tehran, Iran
- Air pollution nanoparticles
- ROS
- Neuroinflammation
- CNS demyelination diseases
- Multiple sclerosis
Abstract
Epidemiology findings show that exposure to urban air pollutants as a source of oxidative stress and neuroinflammation is associated with the Central Nervous System (CNS) demyelinating diseases, such as Multiple Sclerosis (MS). An autoimmune response involving increased inflammation and demyelination in the CNS leads to the pathophysiology of MS, which is more common in adult young females. Particulate Matter (PM), including fine particles (PM <2.5μm, PM 2.5) and very fine particles (PM <0.1μm, PM 0.1), transition metals, and ozone are of potent or oxidant capable of producing Reactive Oxygen Species (ROS). Redox-sensitive pathways can be caused by oxidative stress, leading to various biological processes, including inflammation and other harmful outcomes in the brain. Exposure to Diesel Exhaust Particles (DEPs) mediates significant alterations in myelination across various regions in the brain. There is also an increase in ROS production in the CNS of DEPs exposed mice. Thus, targeting neuroinflammation and oxidative stress can be a useful strategy to eliminate the obvious symptoms of the CNS demyelinating diseases. Overall, in the current mini-review, we examined the exposure to air pollutants nanoparticles associated with the CNS demyelinating diseases, such as MS.
Abbreviations
Apo E-/-: Apolipoprotein E Null Mouse; AD: Alzheimer’s Disease; PD: Parkinson's Disease; BBB: Blood-Brain Barrier; CNS: Central Nervous System; EAE: Experimental Autoimmune Encephalomyelitis; ERα: Estrogen Receptor Alpha; ERβ: Estrogen Receptor Beta; MOG: Myelin Oligodendrocyte Glycoprotein; MHC: Major Histocompatibility Complex; MS: Multiple Sclerosis; DEPs: Diesel Exhaust Particles; Ov+: Female Mice with Ovaries; OV-: Ovariectomized Female Mice; PRO: Progesterone Receptors; ROS: Reactive Oxygen Species; TNF-α: Tumor Necrosis Factor Alpha; TLR: Toll-Like Receptor; WHO: World Health Organization; PAHs: Polycyclic Aromatic Hydrocarbons
Introduction
The WHO reported that exposure to air pollution is responsible for around 7 million deaths per year [1]. Exposure to air pollution has previously has been characterized to contribute to detrimental CNS occurrences such as neuroinflammation, neurodegeneration, and stroke [2-4]. While a variety of environmental factors are involved in neuronal inflammation leading to CNS disease, air pollutants may be the most common source of environmental oxidative stress and inflammation. In the chronic nature and pathology of CNS disease, inflammation is recognized as a risk factor [5-7]. Ambient air contains a complex combination of toxins, including gases, benzene, and Particulate Matter (PM) that can be irritating. Chemical composition of particles varies widely depending on geographic, meteorological, and specific source variables [8,9]. In general, ambient particles include elemental and organic carbon, inorganic components (trace metals, nitrates, sulfates, chloride, and ammonium), biological components (pollens, bacteria, and spores), volatile and semi-disintegrating organic compounds [10]. Furthermore, when the ambient particles are mixed with atmospheric gases (carbon monoxide, sulfur, ozone, and nitric oxides), they can form airborne particles. Environmental particles are commonly characterized by aerodynamic properties and their size and defined as PM2.5 and PM10 with diameters of less than 2.5 and 10μm: PM with an aerodynamic diameter of 2.5 to 10μm (PM10), PM smaller than 2.5μm (PM2.5) and very small PM less than 0.1μm or ultrafine PM (UFPs; <100 nm). This particles are acceptable fractions from different sources such as agricultural dust, wood combustion, road, vehicles emission, tire wear propagation, construction, mining operations, and demolition work [11,12]. PM2.5 due to heavy metals absorbed in pores and particle surfaces produces more hydroxyl radicals, while larger particles (PM10) are mainly in the upper airways and it is purified by the mucosal system [13,14]. Diesel Exhaust Particles (DEPs), from vehicle emissions especially diesel engines emission, are a great source of UFPs that may penetrate the home if poorly ventilated, where additional sources such as burning candles, cooking, tobacco smoke, and chemical reactions are also available [15]. Although, interest has also focused on nanoparticles with diameters less than 100 nm; UFPs are of great importance due to their health effects such as the high alveolar deposition fraction, chemical composition, large area, and ability to enter bloodstream and cause inflammation [10,16]. The main route of air pollutant exposure is inhalation. The fine particles (PM2.5) are deposited in lungs, while the coarse particles (PM10) are filtered usually out by nose and the upper airways. Air pollutants are multifaceted ambient poison that can attack the CNS through a variety of routes [10,17,18]. Exposure to air pollutants is associated with CNS diseases including stroke, Alzheimer's Disease (AD), Parkinson's Disease (PD), and autoimmune disorders of the CNS, such as MS [19-21]. To date, very little information exists on the effects of traffic-generated pollutant-exposures in the CNS of females. Exposure to DEPs causes alterations in the integrity of the Blood-Brain Barrier (BBB) and increases inflammation in the CNS of female mice [2,22,23]. We shod be investigate additional whether additional pathophysiological changes also occur in the CNS from DEPs exposure? And whether there are sex-specific differences occurring in the CNS of females vs. males [24,25]? The aim of this mini-review is to investigate whether exposure to urban air pollutants increases CNS outcomes associated with the progression or exacerbation of demyelinating diseases.
Multiple Sclerosis associated with exposure to urban air pollution
The findings suggest that exposure to inhaled urban air pollutants may cause demyelination in certain brain regions. In addition, the presence or absence of ovaries and possibly sex hormone signaling appear to play a role in the rate of DEPs-mediated demyelination observed, as evidenced by decreased levels of myelination observed in the brains of DEPs exposed ov-, compared to DEPs exposed ov+ female mice [26]. CD8+ cells are cytotoxic cells that stimulate the apoptotic signals of the myelin sheath, while CD4+ cells have been reported to be involved in direct destruction of the myelin sheath [27]. The results of studies of neuroinflammation in the CNS, caused by prolonged DEPs exposure, report that areas of the brain, including the frontal cortex, hippocampus, cerebellum, striatum, and olfactory bulb, are more exposed to proinflammatory signals. From exposure to air pollution [28], there are currently conflicting reports as to whether exposure to air pollution contributes to pathology in the CNS associated with the cause of demyelinating diseases, including MS [29]. In addition, the role of steroid hormone receptor contributions in these mediated outcomes of exposure to air pollution is less understood [22]. Changes in BBB have been reported to mediate the MS pathogenesis [24,25]. MS is a CNS disease that is associated with demyelination and the neuronal damage [30]. Exposure to DEPs mediates increases inflammatory signaling in the CNS of female ApoE-/- mice [22], and exposure to urban air pollutants have been shown to directly lead to the activation of microglia in animal and human studies [31]. Estrogen receptors are nuclear transcription proteins that regulate the immune system and are involved in altering innate and compatible immune responses through dendritic cell activation and Toll-Like Receptor (TLR) signals [32]. Therefore, it is plausible that different brain regions are more “susceptible” to detrimental outcomes in the CNS, related to the progression of MS, resulting from inhalation urban air pollutants exposure. MOG is present exclusively in the CNS and has been reported to play a role in demyelination. The presence of MOG antibodies (MOG-Abs) is a biomarker used to diagnose both MS and Optic Neuromyelitis Optica Spectrum Disorder (NMOSD) [33].
The effect of gender on multiple sclerosis
It is also believed that sex hormones are involved in the onset or progression of MS. The highest prevalence of MS has been reported in young women (20 to 40 years old) with a diagnosis rate of approximately 2-3 times higher than men [34]. Manifestations of MS appear to be relatively rare early in life, but occur more frequently in adolescence and then decrease around the age of 50 [35]. It has also been reported that in the third trimester of pregnancy in pregnant MS patients, when estrogen and progesterone levels rise, recurrences are decline by up to 80% [36].
In addition, estrogen has been reported to have neuroprotective effects; treatment with beta estrogen Receptor Agonist (ERβ) effectively improves the clinical course of MS and provides neuroprotection in EAE [37]. Increased expression of ERα and ERβ receptors is associated with decreased demyelination and axonal loss in the animal model of EAE MS [38]. Together, the results show that changes in sex steroid hormone production and signaling play a key role in the etiology and pathology of the disease. Further observations confirm the hypothesis that pregnancy appears to provide "protective effects" of recurrence in MS patients by reducing estrogen-mediated proinflammatory cytokine expression [39]. In addition, exposure to DE causes neuroinflammation and increased ROS production in male mice [24,25]. Mechanical / inhibitory studies are needed to further elucidate the role of female sex hormones and /or receptor-mediated signaling in the mediating effects of inhaled environmental pollutants in autoimmune and CNS disorders. Inhaled urban air pollutants have been reported to inflammation, increase ROS production, and BBB permeability in the CNS of wild type male C57BL/6 and Apo E-/- mice, in addition to promoting microglial activation, they have all been reported to contribute to the progression of MS [31,40].
Multiple Sclerosis associated with Inflammatory and ROS
In MS, increased ROS production is related to demyelination and axonal damage. This premise is further corroborated by reports showing elevated ROS in the CNS of both MS patients and EAE mice [41].
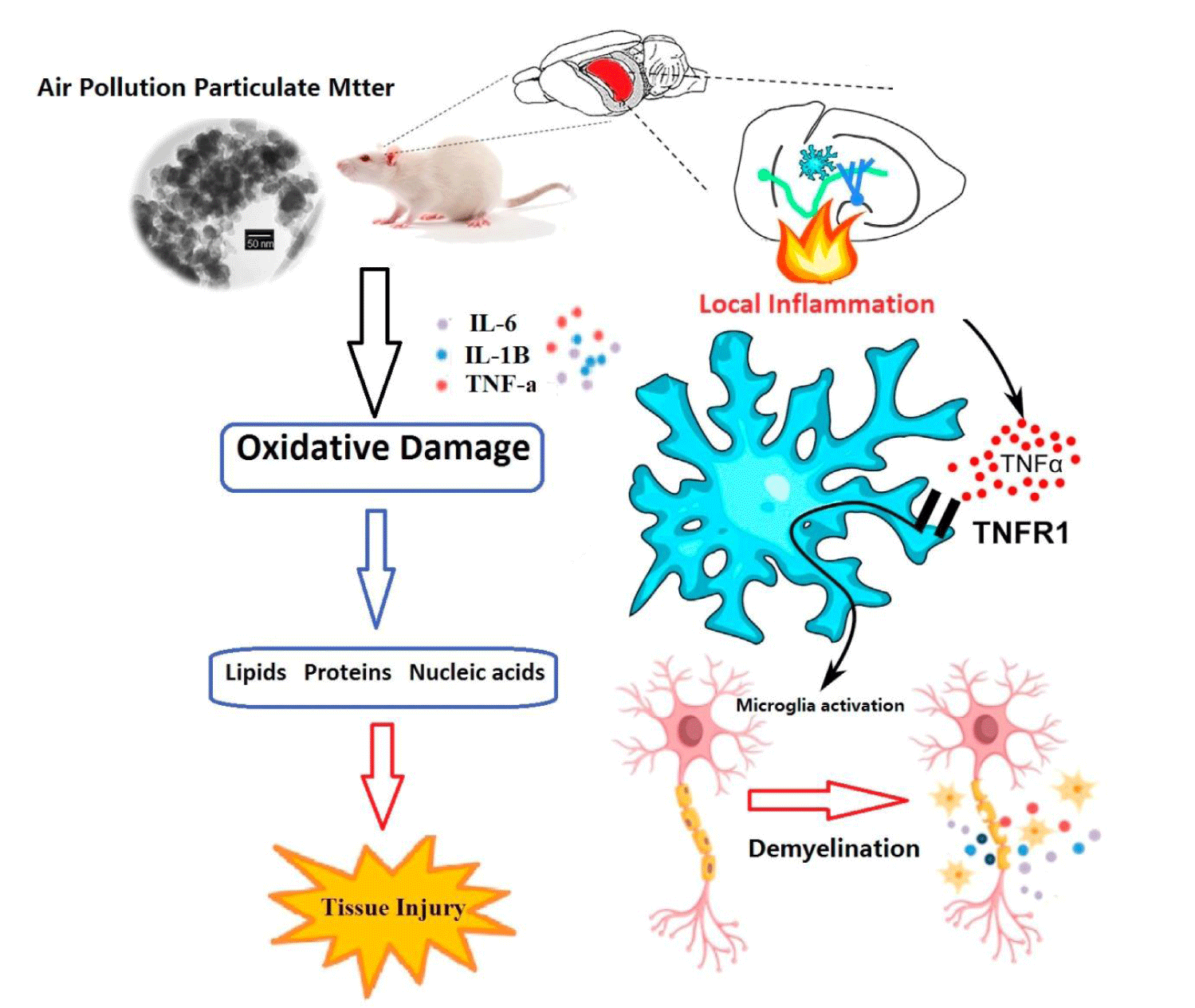
Increased ROS production is associated with the incidence and severity of MS, possibly due to activation of myelin sheath phagocytosis [42]. Microglial activation in the CNS has been shown to increase ROS production and expression of inflammatory agents [43]. The myelin sheath is a layer of fat produced by oligodendrocytes in the CNS that wraps around nerves [44]. Myelin Oligodendrocyte Glycoprotein (MOG) is located in the outer layer of the CNS myelin sheath and is expressed by oligodendrocytes [45] although the MOG function is not yet fully understood, it is believed to act as a cell adhesion molecule in regulating the stability of oligodendrocyte microtubules to facilitate a complementary cascade [46]. It is known that MOG can destruction of the myelin sheath in the CNS. As MOG antibody disease is now diagnosed as a distinct demyelinating disease from MS, it has been reported that the progression of MS and the relapse rate in patients with antibodies to MOG increase [47]. Inflammatory signaling and ROS are associated with demyelination in CNS [48]. ROS Overproduction can accelerate the onset of the lipid peroxidation cascade, leading to demyelination and oligodendrocyte death [49]. ROS plays a vital role in signaling molecules that target T cell differentiation and activation, while ROS overproduction causes damage to these cellular organelles and biomolecules, resulting in abnormal function [50]. T cells, CD4+ and CD8+, are part of the adaptive immune system. CD4+ cells bind to major histocompatibility complex (MHC) class II, which are antigen-presenting cells that act as macrophage cells present in human MS lesion, while CD8+ bind to MHC class I and promotes a cytotoxic immune response [51]. Demyelinating diseases, such as MS, are associated with T cell infiltration and inflammation in the CNS, leading to lesions characterized by demyelination and axonal loss [52]. During MS, BBB integrity is often disrupted, leading to increased active T cells transmission such as CD8 + and CD4 +, from the circulation into the parenchyma [48]. Increased activated T cells penetration is related to an inflammatory response and activation of microglia, which can damage the myelin sheath and neuronal apoptosis [53,54]. ERα down regulation is associated with increased expression of TNF-α and macrophage infiltration, which may be a mechanism that helps increase the incidence of autoimmune diseases in women compared to men [55]. ERα signaling has been reported to offer protective effects on the CNS by inhibiting the uptake of inflammatory cells in EAE mice [56]. The observed decrease in ERα receptor mRNA expression in the brain of ov+ mice exposed to DEPs may be result from negative feedback due to the presence and signaling of estrogen-mimetics in urban air pollutants. These include compounds such as PAHs, phthalates, metalloestrogens, and alkylphenols [57], many of which act as ligands in estrogen receptors, thereby altering signaling activities [58]. Progesterone can contribute to the pathophysiology of MS by inhibiting T helper cells [59]. However, the components of air pollution mediating these harmful consequences in the CNS have not yet been fully characterized as in (Figure 1). In addition, these exposures are not detected in the MS model to confirm that the observed adverse outcomes promote MS pathology.
Conclusion
Based on the results of research done, inhalation exposure to DEPs resulted in the promotion of demyelination in the cerebrum of female mice. Also, the degree of demyelination appears to be mediated by the presence of female sex hormones, as demyelination has been observed to be exacerbated in the cerebrum of DEPs-exposed ovariectomized female mice. DEPs exposure induces demyelination, T cell infiltration, and ROS production, associated with increased demyelination. Each of which is a factor involved in or contributes to the pathogenesis of MS in the cerebrum of mice.
References
- Organization WHO. WHO global ambient air quality database (update 2018). World Health Organization: Geneva, Switzerland. 2018. https://tinyurl.com/2p86kb63
- Ehsanifar M, Jafari AJ, Montazeri Z, Kalantari RR, Gholami M, Ashtarinezhad A. Learning and memory disorders related to hippocampal inflammation following exposure to air pollution. J Environ Health Sci Eng. 2021 Jan 22;19(1):261-272. doi: 10.1007/s40201-020-00600-x. PMID: 34150234; PMCID: PMC8172730.
- Ehsanifar M, Banihashemian, Farokhmanesh. Exposure to ambient ultra-fine particles and stroke. J Biomed Res Environ Sci. 2021;2(10):954-995 https://tinyurl.com/mwhm6ynx
- Ehsanifar M, Montazeri, Rafati. Alzheimer’s disease-like neuropathology following exposure to ambient noise. J Biomed Res Environ Sci. 2021;2(11):1159-1162. https://tinyurl.com/ms43fjz3
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007 Jan;8(1):57-69. doi: 10.1038/nrn2038. PMID: 17180163.
- Ehsanifar M, Tameh AA, Farzadkia M, Kalantari RR, Zavareh MS, Nikzaad H, Jafari AJ. Exposure to nanoscale diesel exhaust particles: Oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol Environ Saf. 2019 Jan 30;168:338-347. doi: 10.1016/j.ecoenv.2018.10.090. Epub 2018 Nov 2. PMID: 30391838.
- Ehsanifar M, Montazeri Z, Taheri MA, Rafati M, Behjati M, Karimian M. Hippocampal inflammation and oxidative stress following exposure to diesel exhaust nanoparticles in male and female mice. Neurochem Int. 2021 May;145:104989. doi: 10.1016/j.neuint.2021.104989. Epub 2021 Feb 12. PMID: 33582162.
- Haghani A. Toxicity of urban air pollution particulate matter in developing and adult mouse brain: Comparison of total and filter-eluted nanoparticles. Environment International. 2020;136:105510. https://tinyurl.com/5458r85r
- Ehsanifar M. Airborne aerosols particles and COVID-19 transition. Environ Res. 2021 Sep;200:111752. doi: 10.1016/j.envres.2021.111752. Epub 2021 Jul 22. PMID: 34302822; PMCID: PMC8295061.
- Forman HJ, Finch CE. A critical review of assays for hazardous components of air pollution. Free Radic Biol Med. 2018 Mar;117:202-217. doi: 10.1016/j.freeradbiomed.2018.01.030. Epub 2018 Jan 31. PMID: 29407794; PMCID: PMC5845809.
- Calderón-Garcidueñas L, Reynoso-Robles R, González-Maciel A. Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: The culprit of Alzheimer and Parkinson's diseases. Environ Res. 2019 Sep;176:108574. doi: 10.1016/j.envres.2019.108574. Epub 2019 Jul 5. PMID: 31299618.
- Cory-Slechta DA, Sobolewski M, Marvin E, Conrad K, Merrill A, Anderson T, Jackson BP, Oberdorster G. The impact of inhaled ambient ultrafine particulate matter on developing brain: Potential importance of elemental contaminants. Toxicol Pathol. 2019 Dec;47(8):976-992. doi: 10.1177/0192623319878400. Epub 2019 Oct 14. PMID: 31610749; PMCID: PMC6911038.
- Cory-Slechta DA, Allen JL, Conrad K, Marvin E, Sobolewski M. Developmental exposure to low level ambient ultrafine particle air pollution and cognitive dysfunction. Neurotoxicology. 2018 Dec;69:217-231. doi: 10.1016/j.neuro.2017.12.003. Epub 2017 Dec 13. PMID: 29247674; PMCID: PMC5999548.
- Jonidi Jafari A, Ehsanifar. The share of different vehicles in air pollutant emission in tehran, using 2013 traffic information. Caspian Journal of Health Research. 2016;2(2):28-36. https://tinyurl.com/mrxkseu3
- Reis H, Reis C, Sharip A, Reis W, Zhao Y, Sinclair R, Beeson L. Diesel exhaust exposure, its multi-system effects, and the effect of new technology diesel exhaust. Environ Int. 2018 May;114:252-265. doi: 10.1016/j.envint.2018.02.042. Epub 2018 Mar 7. PMID: 29524921.
- Russ TC, Reis S, van Tongeren M. Air pollution and brain health: defining the research agenda. Curr Opin Psychiatry. 2019 Mar;32(2):97-104. doi: 10.1097/YCO.0000000000000480. PMID: 30543549.
- Ehsanifar M, Montazeri, Rafat. Neurotoxicity related exposure to ambient nanoparticles. 2022. https://tinyurl.com/bdeb3jk7
- Ehsanifar M. Anxiety and depression following diesel exhaust Nano-particles exposure in male and female mice. J Neurophysiol Neurol Disord. 2020;8:1-8. https://tinyurl.com/524f4z66
- Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009 Sep;32(9):506-516. doi: 10.1016/j.tins.2009.05.009. Epub 2009 Aug 26. PMID: 19716187; PMCID: PMC2743793.
- Ehsanifar M. Exposure To urban air pollution nanoparticles and CNS disease. On J Neur Br Disord. 2021;5(5):520-526. https://tinyurl.com/ymd6z3jb
- Ehsanifar M, Banihashemian, Ehsanifar. Exposure to Air Pollution Nanoparticles: Oxidative Stress and Neuroinfl ammation. J Biomed Res Environ Sci. 2021;2(10):964-976. https://tinyurl.com/4eev4cxk
- Adivi A, Lucero J, Simpson N, McDonald JD, Lund AK. Exposure to traffic-generated air pollution promotes alterations in the integrity of the brain microvasculature and inflammation in female ApoE-/- mice. Toxicol Lett. 2021 Mar 15;339:39-50. doi: 10.1016/j.toxlet.2020.12.016. Epub 2020 Dec 26. PMID: 33373663; PMCID: PMC7864563.
- Ehsanifar M, Jafari AJ, Nikzad H, Zavareh MS, Atlasi MA, Mohammadi H, Tameh AA. Prenatal exposure to diesel exhaust particles causes anxiety, spatial memory disorders with alters expression of hippocampal pro-inflammatory cytokines and NMDA receptor subunits in adult male mice offspring. Ecotoxicol Environ Saf. 2019 Jul 30;176:34-41. doi: 10.1016/j.ecoenv.2019.03.090. Epub 2019 Mar 25. PMID: 30921694.
- Oppenheim HA, Lucero J, Guyot AC, Herbert LM, McDonald JD, Mabondzo A, Lund AK. Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part Fibre Toxicol. 2013 Dec 17;10:62. doi: 10.1186/1743-8977-10-62. PMID: 24344990; PMCID: PMC3878624.
- Lucero J, Suwannasual U, Herbert LM, McDonald JD, Lund AK. The role of the lectin-like oxLDL receptor (LOX-1) in traffic-generated air pollution exposure-mediated alteration of the brain microvasculature in Apolipoprotein (Apo) E knockout mice. Inhal Toxicol. 2017 May;29(6):266-281. doi: 10.1080/08958378.2017.1357774. Epub 2017 Aug 17. PMID: 28816559; PMCID: PMC6732220.
- Chitnis T. The role of CD4 T cells in the pathogenesis of multiple sclerosis. Int Rev Neurobiol. 2007;79:43-72. doi: 10.1016/S0074-7742(07)79003-7. PMID: 17531837; PMCID: PMC7112308.
- Patel J, Balabanov R. Molecular mechanisms of oligodendrocyte injury in multiple sclerosis and experimental autoimmune encephalomyelitis. Int J Mol Sci. 2012;13(8):10647-10659. doi: 10.3390/ijms130810647. Epub 2012 Aug 23. PMID: 22949885; PMCID: PMC3431883.
- Gerlofs-Nijland ME, van Berlo D, Cassee FR, Schins RP, Wang K, Campbell A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol. 2010 May 17;7:12. doi: 10.1186/1743-8977-7-12. PMID: 20478040; PMCID: PMC2883965.
- Bai L, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Brook JR, Tu K, Copes R, Goldberg MS, Martin RV, Murray BJ, Kopp A, Chen H. Long-term exposure to air pollution and the incidence of multiple sclerosis: A population-based cohort study. Environ Res. 2018 Oct;166:437-443. doi: 10.1016/j.envres.2018.06.003. Epub 2018 Jun 22. PMID: 29940476.
- Peterson LK, Fujinami RS. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2007 Mar;184(1-2):37-44. doi: 10.1016/j.jneuroim.2006.11.015. Epub 2006 Dec 28. PMID: 17196667; PMCID: PMC1933528.
- Mumaw CL, Levesque S, McGraw C, Robertson S, Lucas S, Stafflinger JE, Campen MJ, Hall P, Norenberg JP, Anderson T, Lund AK, McDonald JD, Ottens AK, Block ML. Microglial priming through the lung-brain axis: The role of air pollution-induced circulating factors. FASEB J. 2016 May;30(5):1880-1891. doi: 10.1096/fj.201500047. Epub 2016 Feb 10. PMID: 26864854; PMCID: PMC4836369.
- Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015 Apr;294(2):63-69. doi: 10.1016/j.cellimm.2015.01.018. Epub 2015 Feb 7. PMID: 25682174; PMCID: PMC4380804.
- Hacohen Y, Banwell B. Treatment approaches for MOG-Ab-associated demyelination in children. Curr Treat Options Neurol. 2019 Jan 22;21(1):2. doi: 10.1007/s11940-019-0541-x. PMID: 30671648; PMCID: PMC6342853.
- Noonan CW, Kathman SJ, White MC. Prevalence estimates for MS in the United States and evidence of an increasing trend for women. Neurology. 2002 Jan 8;58(1):136-138. doi: 10.1212/wnl.58.1.136. PMID: 11781421.
- Ascherio A, Munger KL, Lünemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012 Nov 5;8(11):602-612. doi: 10.1038/nrneurol.2012.198. Epub 2012 Oct 9. PMID: 23045241; PMCID: PMC4467212.
- Harbo HF, Gold R, Tintoré M. Sex and gender issues in multiple sclerosis. Ther Adv Neurol Disord. 2013 Jul;6(4):237-248. doi: 10.1177/1756285613488434. PMID: 23858327; PMCID: PMC3707353.
- Khalaj AJ, Hasselmann J, Augello C, Moore S, Tiwari-Woodruff SK. Nudging oligodendrocyte intrinsic signaling to remyelinate and repair: Estrogen receptor ligand effects. J Steroid Biochem Mol Biol. 2016 Jun;160:43-52. doi: 10.1016/j.jsbmb.2016.01.006. Epub 2016 Jan 14. PMID: 26776441; PMCID: PMC5233753.
- Maglione A, Rolla S, Mercanti SF, Cutrupi S, Clerico M. The adaptive immune system in multiple sclerosis: an estrogen-mediated point of view. Cells. 2019 Oct 19;8(10):1280. doi: 10.3390/cells8101280. PMID: 31635066; PMCID: PMC6829884.
- Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol. 2003 Dec 1;171(11):6267-6274. doi: 10.4049/jimmunol.171.11.6267. PMID: 14634144.
- Suwannasual U, Lucero J, McDonald JD, Lund AK. Exposure to traffic-generated air pollutants mediates alterations in brain microvascular integrity in wildtype mice on a high-fat diet. Environ Res. 2018 Jan;160:449-461. doi: 10.1016/j.envres.2017.10.029. Epub 2017 Nov 5. PMID: 29073573; PMCID: PMC5705467.
- Witherick J, Wilkins A, Scolding N, Kemp K. Mechanisms of oxidative damage in multiple sclerosis and a cell therapy approach to treatment. Autoimmune Dis. 2010 Dec 15;2011:164608. doi: 10.4061/2011/164608. PMID: 21197107; PMCID: PMC3010615.
- van der Goes A, Brouwer J, Hoekstra K, Roos D, van den Berg TK, Dijkstra CD. Reactive oxygen species are required for the phagocytosis of myelin by macrophages. J Neuroimmunol. 1998 Dec 1;92(1-2):67-75. doi: 10.1016/s0165-5728(98)00175-1. PMID: 9916881.
- Lassmann H, van Horssen J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 2011 Dec 1;585(23):3715-23. doi: 10.1016/j.febslet.2011.08.004. Epub 2011 Aug 16. PMID: 21854776.
- Jackman N, Ishii A, Bansal R. Oligodendrocyte development and myelin biogenesis: parsing out the roles of glycosphingolipids. Physiology (Bethesda). 2009 Oct;24:290-297. doi: 10.1152/physiol.00016.2009. PMID: 19815855; PMCID: PMC2854184.
- Narayan R, Simpson A, Fritsche K, Salama S, Pardo S, Mealy M, Paul F, Levy M. MOG antibody disease: A review of MOG antibody seropositive neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. 2018 Oct;25:66-72. doi: 10.1016/j.msard.2018.07.025. Epub 2018 Jul 24. PMID: 30048919.
- Wynford-Thomas R, Jacob A, Tomassini V. Neurological update: MOG antibody disease. J Neurol. 2019 May;266(5):1280-1286. doi: 10.1007/s00415-018-9122-2. Epub 2018 Dec 19. PMID: 30569382; PMCID: PMC6469662.
- Spadaro M, Gerdes LA, Krumbholz M, Ertl-Wagner B, Thaler FS, Schuh E, Metz I, Blaschek A, Dick A, Brück W, Hohlfeld R, Meinl E, Kümpfel T. Autoantibodies to MOG in a distinct subgroup of adult multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016 Jun 30;3(5):e257. doi: 10.1212/NXI.0000000000000257. PMID: 27458601; PMCID: PMC4949775.
- Ortiz GG, Pacheco-Moisés FP, Bitzer-Quintero OK, Ramírez-Anguiano AC, Flores-Alvarado LJ, Ramírez-Ramírez V, Macias-Islas MA, Torres-Sánchez ED. Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clin Dev Immunol. 2013;2013:708659. doi: 10.1155/2013/708659. Epub 2013 Sep 24. PMID: 24174971; PMCID: PMC3794553.
- Smith KJ, Kapoor R, Felts PA. Demyelination: The role of reactive oxygen and nitrogen species. Brain Pathol. 1999 Jan;9(1):69-92. doi: 10.1111/j.1750-3639.1999.tb00212.x. PMID: 9989453; PMCID: PMC7161906.
- Rashida Gnanaprakasam JN, Wu R, Wang R. Metabolic Reprogramming in Modulating T Cell Reactive Oxygen Species Generation and Antioxidant Capacity. Front Immunol. 2018 May 16;9:1075. doi: 10.3389/fimmu.2018.01075. PMID: 29868027; PMCID: PMC5964129.
- Denic A, Wootla B, Rodriguez M. CD8 (+) T cells in multiple sclerosis. Expert Opin Ther Targets. 2013 Sep;17(9):1053-1066. doi: 10.1517/14728222.2013.815726. Epub 2013 Jul 6. PMID: 23829711; PMCID: PMC3928018.
- Brück W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005 Nov;252 Suppl 5:v3-9. doi: 10.1007/s00415-005-5002-7. PMID: 16254699.
- Ortiz GG, Pacheco-Moisés FP, Macías-Islas MÁ, Flores-Alvarado LJ, Mireles-Ramírez MA, González-Renovato ED, Hernández-Navarro VE, Sánchez-López AL, Alatorre-Jiménez MA. Role of the blood-brain barrier in multiple sclerosis. Arch Med Res. 2014 Nov;45(8):687-697. doi: 10.1016/j.arcmed.2014.11.013. Epub 2014 Nov 26. PMID: 25431839.
- Volpe E, Sambucci M, Battistini L, Borsellino G. Fas-Fas ligand: checkpoint of T cell functions in multiple sclerosis. Front Immunol. 2016 Sep 27;7:382. doi: 10.3389/fimmu.2016.00382. PMID: 27729910; PMCID: PMC5037862.
- Panchanathan R, Shen H, Zhang X, Ho SM, Choubey D. Mutually positive regulatory feedback loop between interferons and estrogen receptor-alpha in mice: implications for sex bias in autoimmunity. PLoS One. 2010 May 28;5(5):e10868. doi: 10.1371/journal.pone.0010868. PMID: 20526365; PMCID: PMC2878324.
- Subramanian S, Matejuk A, Zamora A, Vandenbark AA, Offner H. Oral feeding with ethinyl estradiol suppresses and treats experimental autoimmune encephalomyelitis in SJL mice and inhibits the recruitment of inflammatory cells into the central nervous system. J Immunol. 2003 Feb 1;170(3):1548-1555. doi: 10.4049/jimmunol.170.3.1548. PMID: 12538720.
- Fucic A. Environmental exposure to xenoestrogens and oestrogen related cancers: reproductive system, breast, lung, kidney, pancreas, and brain. Environmental Health. 2012;11(1):1-9. https://tinyurl.com/zsrxs4yj
- Carpenter DO, Arcaro K, Spink DC. Understanding the human health effects of chemical mixtures. Environ Health Perspect. 2002 Feb;110 Suppl 1(Suppl 1):25-42. doi: 10.1289/ehp.02110s125. PMID: 11834461; PMCID: PMC1241145.
- Hughes GC. Progesterone and autoimmune disease. Autoimmun Rev. 2012 May;11(6-7):A502-514. doi: 10.1016/j.autrev.2011.12.003. Epub 2011 Dec 13. PMID: 22193289; PMCID: PMC3431799.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.