Medicine Group . 2022 April 30;3(4):436-444. doi: 10.37871/jbres1462.
Pathophysiology, Treatment and Long-Term Consequences of Heart Failure in Infancy
Reiner Buchhorn*
Abstract
Introduction: Infants have the highest risk to die from heart failure. However, innovations like beta-blocker treatment introduced more than 50 years ago are not recorded in the guidelines if the clinical trials are missing or be ignored, like propranolol in infants with severe heart failure to congenital heart disease.
Methods: We re-analyse our data with propranolol and the ACE-inhibitor captopril in infants with severe heart failure due to congenital heart disease as published 20 years ago and the current long-term follow up data.
Results: Propranolol but not Captopril significantly reduces clinical heart failure and neurohormonal activation of the renin angiotensin aldosterone system. Propranolol significantly improve dysautonomia measured by heart rate variability. In contrast to grown up with congenital heart disease – preoperatively treated with digoxin and diuretics - our patients up to the age of 15 years – preoperatively treated with propranolol without frusemide – have normal myocardial function and heart rate variability.
Discussion: The evidence-based data of propranolol to treat severe heart failure in infants with congenital heart disease are the best we have. There is no reason to withheld infants from this effective therapy of early life stress due to heart failure.
Conclusion: Further studies are needed to proof the impact of propranolol in infants with severe heart failure on long-term neurodevelopment, endothelial- and myocardial function.
Introduction
Recently, the master of heart failure treatment – Milton Packer – passed a devasting judgement about therapy of pediatric heart failure (https://bit.ly/37VbAtk).
"There is one group of patients with heart failure who are being deprived of beta-blockers entirely -- even though they are treated by specialists in cardiology. Sadly, some children develop heart failure. For most, it results from a genetic condition, a viral infection or after the treatment of cancer. Many children with a cardiomyopathy would benefit from treatment with a beta-blocker. These drugs are considered accepted therapy in children, but typically, pediatric cardiologists do not prescribe beta-blockers to children with cardiomyopathy.
Why not? In 2007, a small trial reported that beta-blockers did not work in children, but it enrolled only 157 patients and treated them for only 8 months. However, to show dramatic benefits in adults with heart failure, the trials needed to enroll tens of thousands of patients who were treated for many years. That kind of evidence does not exist with beta-blockers in children. Yet, children with heart failure routinely receive other drugs (e.g., digitalis, diuretics, and ACE inhibitors), even though none of them have ever been evaluated in a pediatric clinical trial."
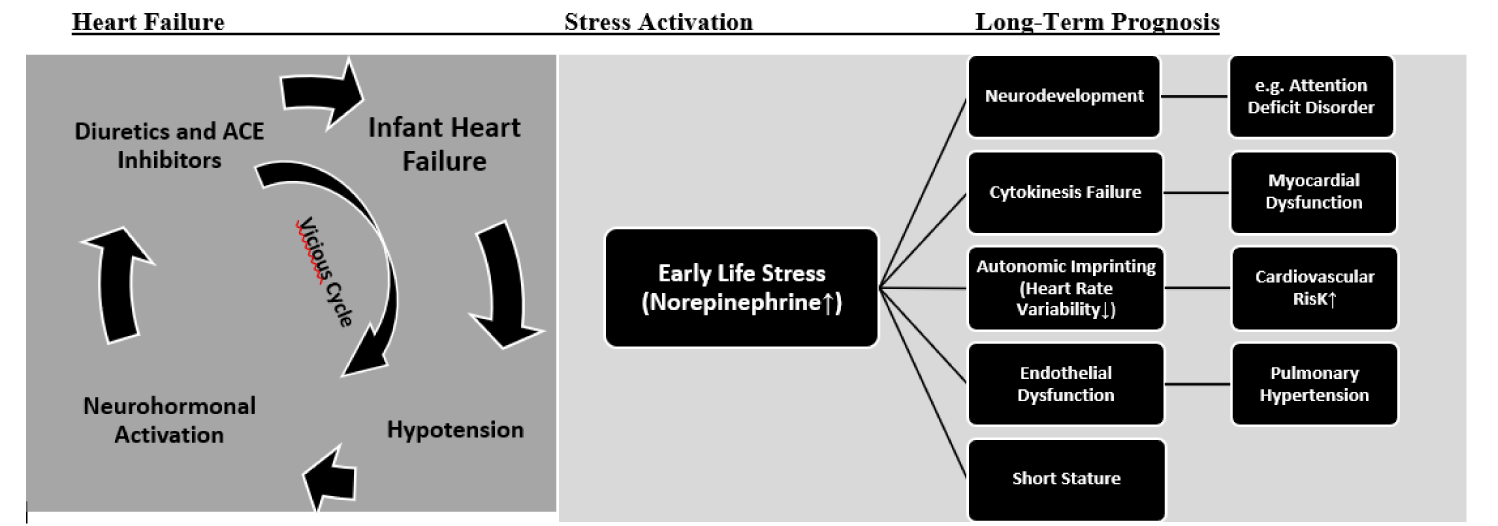
In addition to these facts, the greatest omission of pediatric cardiologists is, that – more than 50 years after Finn Waagstein’s first reports about the benefits of beta-blockers in severe heart failure – they did not recognize the chance of this therapy for one of their most important clinical challenges: The very high mortality in infants with severe heart failure1. The probability of death by 42 years of age in patients with congenital heart disease increases from 11% to 63% in the presence of a heart failure diagnosis [1]. This risk is the highest in early childhood among patients with complex congenital defects (Hazard Ratio >300).
Only one infant was recruited in the above trial [2] and our promising results of propranolol in infants with severe heart failure in a prospective randomized trial [3] were not checked in Europe and US within the last twenty years. Moreover, the publication of an Indian study that confirmed our results was blocked for more than 7 years [4].
The interplay between heart failure and early life stress is illustrated in figure 1. Although our primary goal of propranolol in infants with severe heart failure was a better symptom control and improvement of survival, we realize that there are important long-term benefits based on an effective treatment of early life stress indicated by analysis of heart rate variability.
Clinical symptoms
Robert Ross and our group independently introduce a heart failure scores to quantify the clinical symptoms of heart failure in infants that were used in our prospective trial. In the first step of the CHF-Pro-Infant trial, we analyze the effect of the traditional therapy with digoxin and diuretics on the Ross Score and found a slight clinical improvement of the Ross Score from 8.5 ± 1.7 to 6.8 ± 2.3 (Table 1). After introduction of Propranolol, we confirm a further significant clinical improvement to 3.3±2.3 in this prospective trial3 as before shown in the first published six cases in a compassionate use trial [5]. There was no further clinical improvement with Captopril (Ross Score: 7.4 ± 2.5) in a retrospective analysis [6]. We stopped this compassionate used trial with angiotensin converting enzyme inhibitors in infants with severe heart failure, who failed to improve clinical symptoms, as later confirmed in a prospective randomized trial [7].
| Table 1: Clinical signs of heart failure, neurohormonal activation, hemodynamics and myocardial function in infants with heart failure. | ||||
| Baseline | Digoxin/Diuretics | Propranolol | Captopril | |
| CHF-Pro-Infant (prospective randomized) | retrospective | |||
| Heart Failure Score [Ross] | 8.5 ± 1.7 | 6.8 ± 2.3* | 3.3 ± 2.3** | 7.4 ± 2.5 |
| Weight Gain [g/week] | 57 ± 35 | 122 ± 38 | 86 ± 84 | |
| Hospital Stay [days] | 55 ± 40 | 19 ± 9 | 52 ± 24 | |
| Feeding Tube | 6/10 | 1/10 | 6/11 | |
| Neurohormonal Activity | ||||
| Renin [µU/ml] | 139 ± 102 | 704 ± 490** | 338 ± 236** | 2424 ± 1680 |
| Aldosterone [pg/ml] | 1437 ± 1035 | 2268 ± 1538 | 921 ± 586* | 2531 ± 1666 |
| Norepinephrine [ng/l] | 666 ± 322 | 1272 ± 1103 | 997 ± 576 | |
| Endotheline [fmol/ml] | 2.8 ± 3.1 | 2.5 ± 3.3 | ||
| Hemodynamics | ||||
| Qp/Qs | 3.9 ± 2.5 | 3.5 ± 3.9 | ||
| MAP [mmHg] | 58 ± 14 | 61 ± 6 | ||
| PAP [mmHg] | 31 ± 11 (53%) | 27 ± 13 (44%) | ||
| Myocardial Function | ||||
| Heart Rate [bpm] | 149 ± 8 | 148 ± 10 | 118 ± 10*** | |
| EF [%] | 70 ± 6 | 68 ± 6 | ||
| LAP [mmHg] | 9.5 ± 3.0 | 6.4 ± 2.5* | ||
| FAC [%] | 43 ± 11 | 52 ± 9 | 49 ± 10* | |
| VcFc[s-0.5] | 1.1 ± 0.3 | 1.0 ± 0.1 | ||
| ESSm [kdyn/cm2] | 45 ± 17 | 65 ± 18* | ||
| MyF/ESF | 3.3 ± 1.7 | 1.9 ± 0.8* | ||
| Plus–minus values are means ± standard deviation; T-test between baseline data and treatment groups in the prospective randomized trial: *p-value < 0.005; **p-value < 0.001; ***p-value < 0.0001; ns = not significant. Abbreviations: Qp/Qs: Ratio of Pulmonary to Systemic Flow; PAP: Mean Pulmonary Artery Pressure; MAP: Mean Arterial Pressure; EF: Ejection Fraction of Systemic Ventricle; LAP: Mean Left Atrial Pressure; FAC: Fractional Shortening; VcFc: Circumferential Fiber Shortening; ESSm: Ventricular Endsystolic Wall Stress; MyF/ESF: Myocardium Wall to Cavity Area in Echocardiographic Short Axis of the Left Ventricle. |
||||
In 2013 our data were confirmed in the prospective VSD-PHF trial of the All-India Institute of Medical Sciences in 80 infants with moderate to large ventricular septal defects completed on August, 2014 and published in Annals of Pediatric Cardiology in 2021 [4]. These data showed significant lesser hospitalizations in propranolol treated infants compared to digoxin/diuretics treated infants.
Renin angiotensin aldosterone system
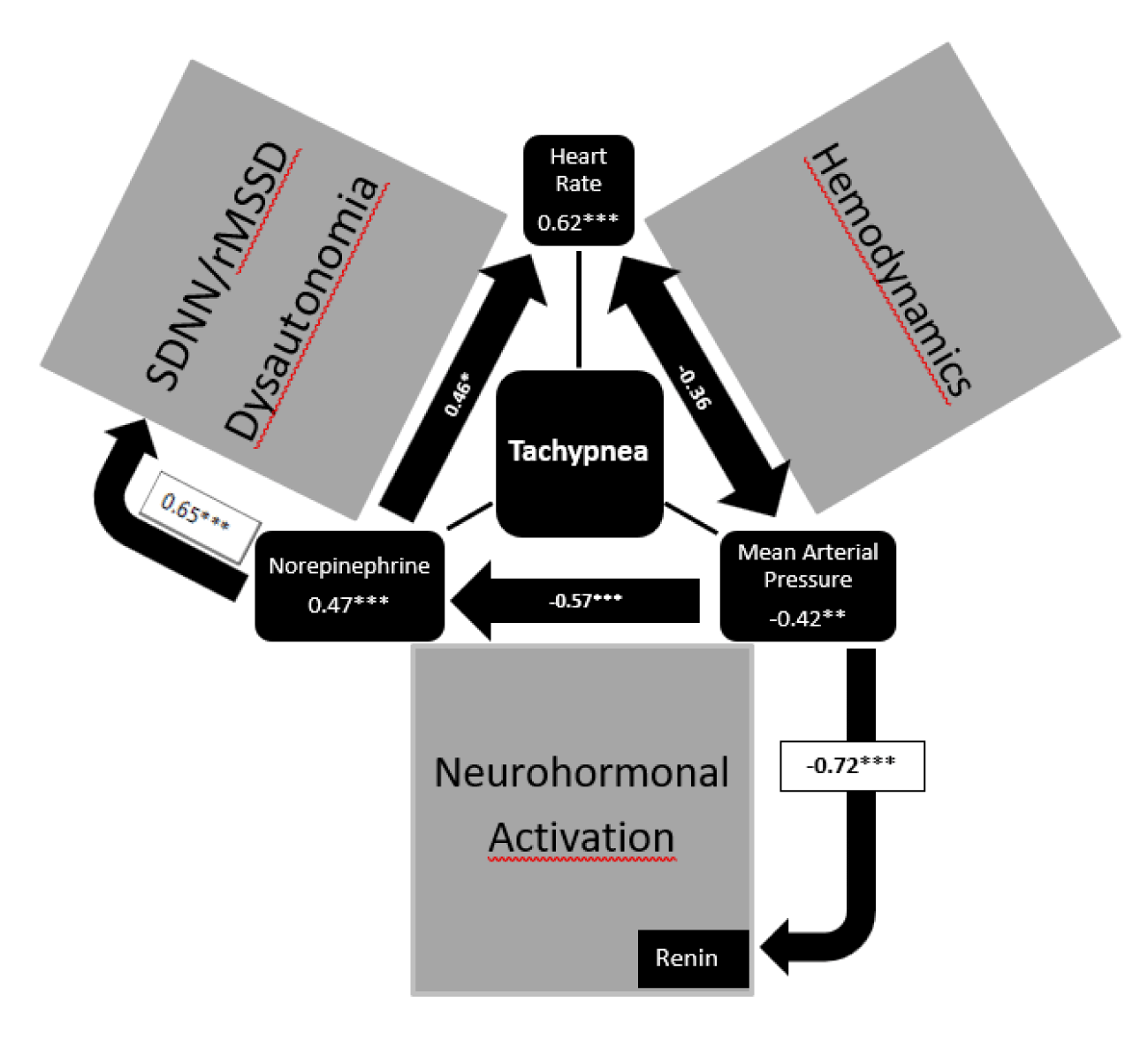
In the CHF-Pro-Infant trial, we analyze the effect of the traditional therapy with digoxin and diuretics on the Ross Score and neurohormonal activation [3]. Beside a slight clinical improvement, we found a significant activation of the renin angiotensin aldosterone system after digoxin and diuretics. After additional propranolol and termination of frusemide renin and aldosterone levels significantly decrease (Table 1). We anticipate arterial hypotension as cause of an aldosterone escape in infants with severe heart failure who received Captopril who show the highest renin and aldosterone values [6]. We anticipate neurohormonal activation induced by low mean arterial pressures as investigated in our hemodynamic studies about neurohormonal activation in infants heart failure [8] (Figure 2).
Norepinephrine
It is Robert Ross, who demonstrate highly elevated norepinephrine levels in infants with heart failure due to congenital heart disease in 1987 [9]. As shown in figure 2, the heart rate (0.62***) and norepinephrine levels (0.47***) have a significant impact on the clinical symptom tachypnea analysis as well as the mean arterial pressure (-0.42**) in univariate regression analysis [10]. Our data show that clinical symptoms of infants with heart failure depends on the heart rate with subnormal low heart rates in infants who tolerate significant left-to-right shunts without heart failure (Table 2). However, there is a low significant correlation between heart rate and norepinephrine levels probably of cause down-regulation of the beta adreno-receptors to protect the heart to tachycardia. We could not show a significant decrease of elevated norepinephrine levels after propranolol but significantly lower heart rates and higher heart rate variabilities (Table 2).
| Table 2: Clinical signs of heart failure indicated by the ROSS Score, heart rate variability and plasma norepinephrine in infants with heart failure according to pharmacotherapy. | ||||
| Healthy Control | Congenital Heart Defect without Heart Failure | Congenital Heart Defect with Digoxin/Diuretics | Congenital Heart Defect with Propranolol | |
| Heart Failure Score [Ross] | 2.7 ± 2.5 | 6.8 ± 2.3 | 3.3 ± 2.3 | |
| Norepinephrine [ng/l] | 646 ± 238 | 560 ± 247 | 1272 ± 1103 | 997 ± 576 |
| Heart Rate [bpm] | 149 ± 8 | 128 ± 11** | 148 ± 10 | 118 ± 10*** |
| SDNN [ms] | 57.4 ± 18.9 | 56.6 ± 16.5 | 37.4 ± 11.7*** | 45 ± 18.7 |
| rMSSD [ms] | 22.2 ± 8.7 | 20.2 ± 8.6 | 14.7 ± 6.9*** | 23.1 ± 14.8 |
| VLF Power [ms] | 26.0 ± 7.1 | 14.1 ± 5.0*** | 9.7 ± 5.6*** | 14.7 ± 8.5*** |
| LF Power [ms] | 17.5 ± 5.1 | 12.9 ± 8.3** | 6.8 ± 3.5*** | 13.8 ± 11.1 |
| HF Power [ms] | 10.0 ± 5.1 | 7.5 ± 5.2 | 5.0 ± 3.3*** | 9.6 ± 11.3 |
| Abbreviations: SDNN (ms): Standard Deviation of All NN Intervals; rMSSD (ms): The square root of the mean of the sum of the squares of differences between adjacent NN intervals; VLF (ms2): Very low frequency power spectrum between 0.003 and 0.04 Hz; LF (ms2): Low frequency power spectrum between 0.04 and 0.15 Hz; HF (ms2): High frequency power spectrum between 0.15 and 0.4 Hz; T-test between healthy control and patient groups or between patient groups; *p-value < 0.005; **p-value < 0.001; ***p-value < 0.0001; ns = not significant. |
||||
Analysis of Heart Rate Variability (HRV)
To proof the impact of propranolol on early life stress, we used 24-hours HRV analysis to have a look on dysautonomia independent from norepinephrine plasma levels (Table 2) [11]. Indeed, HRV is highly reduced in infants with congenital heart defects with heart failure treated with digoxin/diuretics but not in untreated infants with significant congenital heart defects without heart failure. Despite a comparable left-to-right shunt these infants without heart failure had spontaneously lower heart rates and norepinephrine levels (Table 2). After 2mg/kg propranolol in the infants with heart failure, we measured the lowest heart rates and nearly normal heart rate variabilities.
In summary, propranolol but not digoxin/diuretics improve clinical symptoms and dysautonomia in infants with severe heart failure. Moreover, a significant iatrogenic neurohormonal activation probably of cause a lowering effect on blood pressure was induced by captopril and diuretics. Paediatric cardiologists heart failure therapy focussed on hemodynamic and ignored neurohormonal activation and dysautonomia in the past (Figure 2).
Long-term cardiovascular prognosis and HRV in children with congenital heart disease
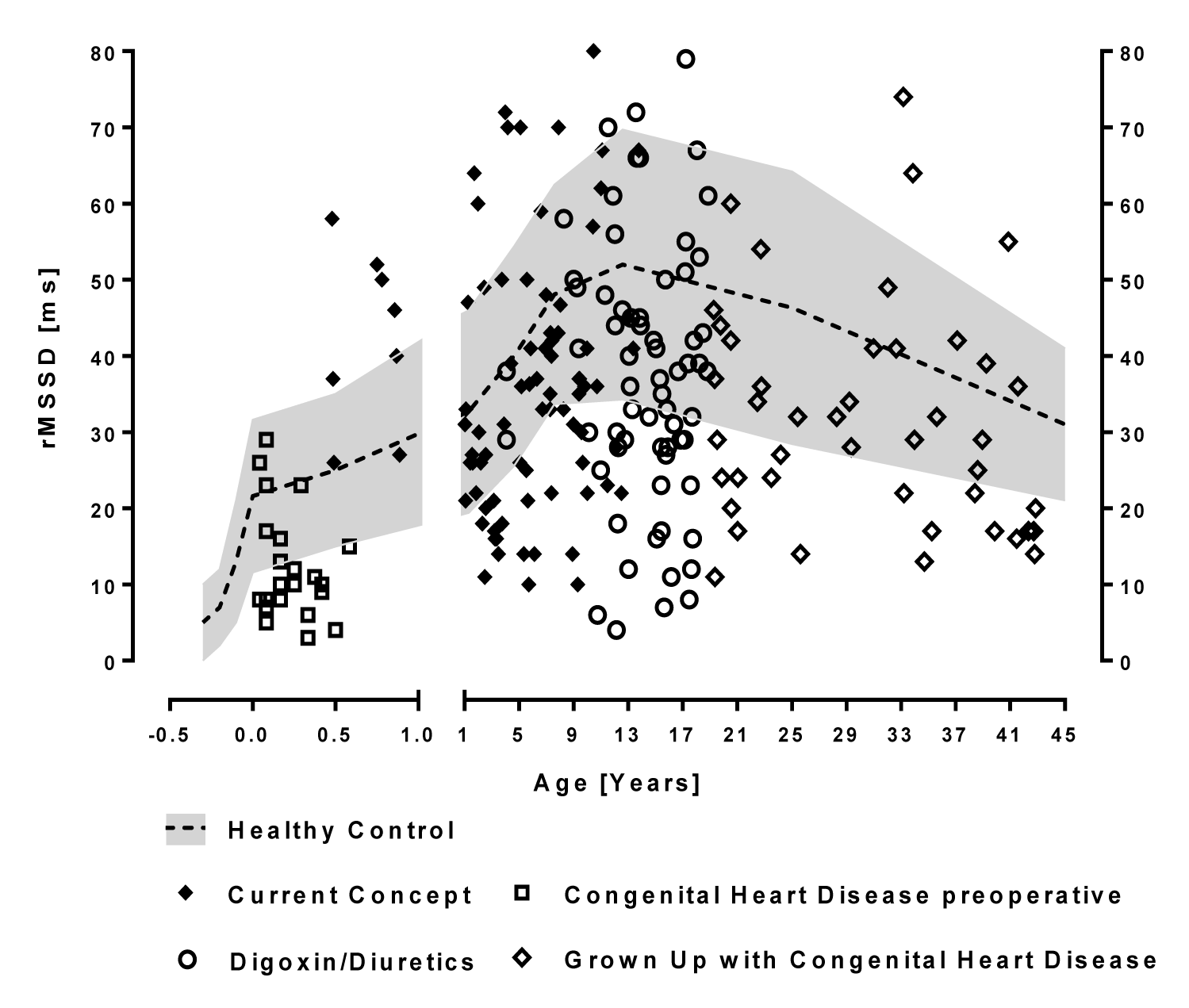
There is a significant long-term cardiovascular mortality in patients with operated congenital heart disease, even the mildest lesions who have no residual hemodynamic abnormalities [12]. We anticipate a lifelong dysautonomia due to early life stress induced by heart failure in infancy that can probably be measured by HRV analysis (Figure 3). We called the impact of early life stress on the autonomic nervous system “autonomic imprinting” as published in PlosOne [13] based on the Developmental Origins of Health and Disease Hypothesis (DOHaD). However, this hypothesis is difficult to proof if we nearly never use diuretics and ACE-inhibitors preoperatively after the CHF-Pro-Infant trial and have no control group in the current generation.
As shown in table 3, we compare the vagus activity indicated by the HRV parameter rMSSD of our complete group of patients with operated congenital heart disease preoperatively treated with the current concept (Propranolol in infants with heart failure) born after 2004 with a historical group treated with digoxin/diuretics up to 2004. The low preoperative rMSSD values significantly increase to normal values and remain normal on average in children up to 16 years treated with the current concept (rMSSD 36.6 ± 17.5 versus 35.7 ± 12.5ms in age related healthy controls) as well as the global HRV indicated as SDNN (126.3 ± 58.3ms versus 120.6±36.4ms in age related healthy controls). The older children up to 18 years and Grown Up with congenital heart disease who are treated preoperatively with digoxin/diuretics show significantly lower vagus activities indicated as rMSSD (39.4 ± 19.1 versus 46.1 ± 11.8*ms in age related healthy controls) and global HRV indicated as SDNN (154.6 ± 63.0ms versus 178.1 ± 44.5**ms in age related healthy controls).
| Table 3: Comparison of Children with Congenital Heart Defects according preoperative heart failure therapy in infancy. | ||||
| Parameter | Current Concept | Digoxin/ Diuretics | p-value | GUCH |
| N | 86 | 71 | 45 | |
| Age [Years] | 5.8 ± 3.7 | 14.5 ± 3.2*** | <0.001 | 30.2 ± 8.3 |
| Height [Percentile] | 33.0 ± 28.5 | 33.5 ± 32.1 | ns | 48.2 ± 29.6 |
| BMI [Percentile] | 38.0 ± 32.3 | 53.2 ± 33.4** | 0.0045 | 55.0 ± 31.4 |
| Systolic Blood Pressure [Percentile] | 71.1 ± 23.1 | 67.9 ± 30.3 | ns | 60.3 ± 32.8 |
| Diastolic Blood Pressure [Percentile] | 55.0 ± 30.9 | 41.0 ± 30.8** | 0.0055 | 60.3 ± 31.4 |
| Aristoteles Score | 6.8 ± 3.1 | 6.5 ± 2.9 | ns | 8.7 ± 2.13 |
| Age at Operation [years] | 2.9 ± 3.3 | 3.5 ± 3.9 | ns | 6.9 ± 7.2 |
| VO2max | 35.8 ± 6.9 | 33.1 ± 1.3 | ns | 22.2 ± 4.7 |
| NT-BNP [pg/ml] | 208 ± 215 | 147 ± 165 | ns | 594 ± 1180 |
| FS | 37.6 ± 6.9 | 36.6 ± 6.2 | ns | 33.4 ± 6.3 |
| LVIMP | 0.26 ± 0.14 | 0.33 ± 0.18 | ns | 0.43 ± 0.14 |
| RVIMP | 0.15 ± 0.12 | 0.21 ± 0.16 | ns | 0.35 ± 0.12 |
| Heart Rate [bpm] | 94.7 ± 17.7 | 78.5 ± 11.8 | 74.5 ± 7.8 | |
| SDNN [ms] | 126.3 ± 58.3 | 154.6 ± 63.0** | versus Healthy Control | 128.8 ± 36.3 |
| Healthy Control | 120.6 ± 36.4 | 178.1 ± 44.5 | ||
| RMSSD [ms] | 36.6 ± 17.5 | 39.4 ± 19.1* | versus Healthy Control | 31.8 ± 14.7 |
| Healthy Control | 35.7 ± 12.5 | 46.1 ± 11.8 | ||
| PVC [1/24h] | 33.8 ± 18.6 | 182 ± 69 | ns | 432 ± 205 |
| Abbreviations: BMI: Body Mass Index; NT-BNP: Brain Natriuretic Peptide; SDNN: Standard Deviation of All NN Intervals; RMSSD: The square root of the mean of the sum of the squares of differences between adjacent NN intervals; TP: Total Power VLF: Very Low Frequency Power; LF: Low Frequency Power; HF: High Frequency Power; HF/LF: Ratio HF to LF T-test between healthy control and patient groups or between patient groups: *p-value < 0.005; **p-value < 0.001; ***p-value < 0.0001; ns = not significant |
||||
The impact of early life stress on myocardial function
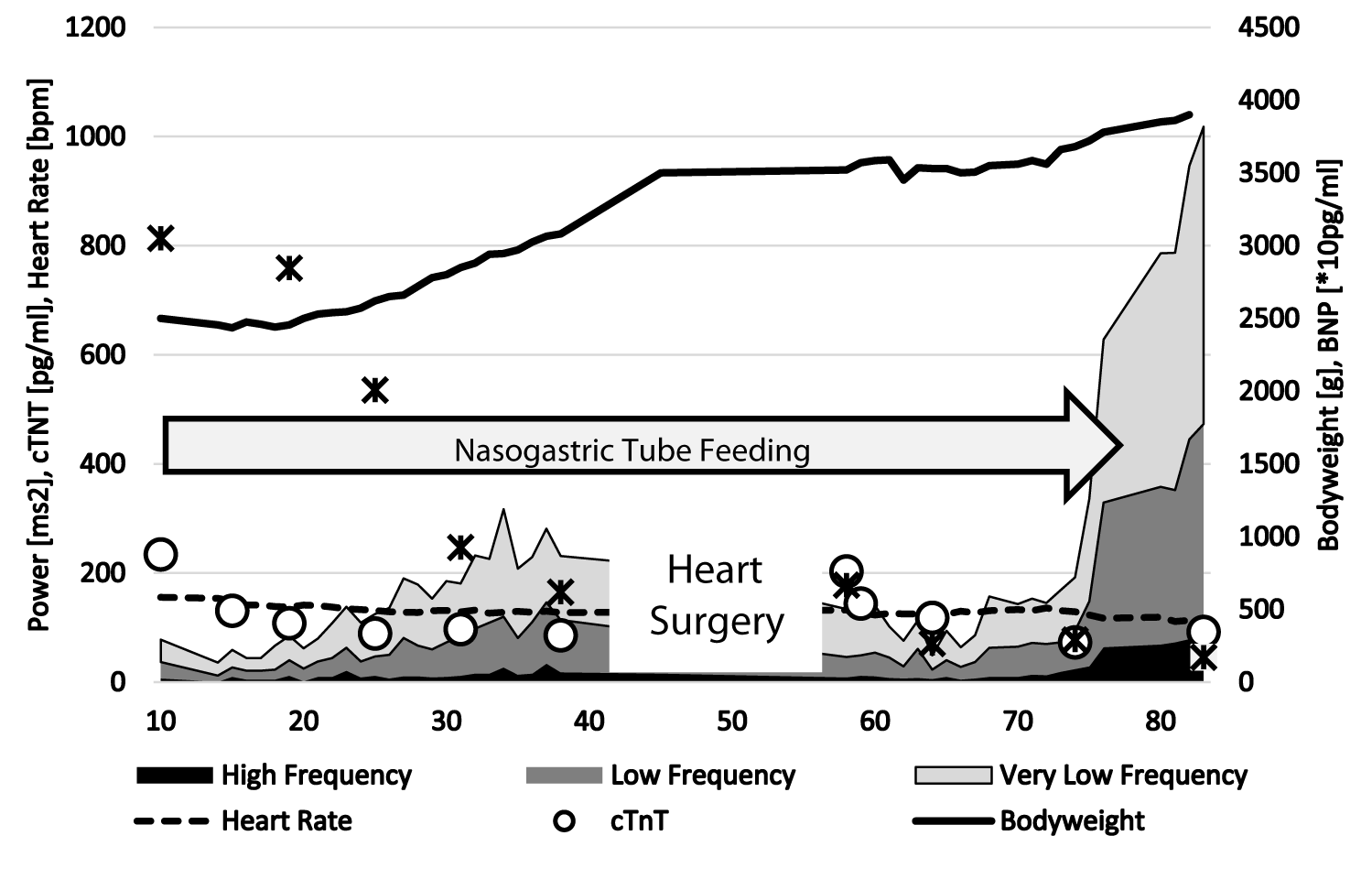
Due to pulmonary over-circulation, the infant with a truncus arteriosus communis suffer from severe heart failure preoperatively as shown in figure 4. In this case, we demonstrate successful treatment with propranolol and digoxin with a weight gain up to 3.5 kg that was wished by the surgeons for cardiac surgery. Of course severe postoperative heart failure, the baby needs again propranolol to treat heart failure. The cardio selective beta blocker metoprolol was not effective in this infant. The figure 4 demonstrates the impact of pharmacotherapy on the highly reduced heart rate variability. At 75 days of life -two weeks after corrective surgery- we observe a spontaneous increase of heart rate variability to normal values and the baby completely recover and could be weaned from pharmacotherapy and nasogastric tube feeding. Beneath the well-known neurohormonal activation, heart failure was accompanied by elevated NT-BNP and troponin T values that indicate myocardial damage [14]. Our CHF-Pro-Infant trial showed normal ejection fractions in both treatment groups but a lower left atrial pressure and lesser myocardial hypertrophy in propranolol treated infants (Table 1) [15]. Myocardial gene expression in intraoperative biopsies showed a significant downregulation of beta-2-receptor and angiotensin-2 receptor genes, and up-regulation of endothelin A receptor and connective tissue growth factor genes, that were partially prevented by additional treatment with propranolol [15]. Our data indicate that propranolol treatment protects the heart against pathologic cardiac remodeling due to heart failure in infants with congenital heart disease.
Our long-term data illustrated in table 3 show a progressive decline of Fractional Shortening (FAC) and the left- and Right Ventricular Index of Myocardial Performance (LVIMP, RVIMP) that indicates ventricular dysfunction with increasing age that probably have an impact on long-term prognosis. There is no significant difference between the NT-BNP values of patients treated with current concept compared to patients treated with digoxin/diuretics (208 ± 215pg/ml versus 147 ± 165pg/ml). However, NT-BNP values in grown up with congenital heart defects are even higher with a large standard deviation (594 ± 1180pg/ml). Recently, Liu H, et al. [16] demonstrate that inactivation of the β-adrenoreceptor genes decreased cardiomyocyte division in neonatal mice, which decreased the number of cardiomyocytes (endowment). The β-blocker propranolol improves cardiomyocyte endowment and conferred benefit after myocardial infarction in adult mice. As shown in single cases with Tetralogy of Fallot, these results suggest that β-blockers could be evaluated for increasing cardiomyocyte division in patients with congenital heart disease using intraoperative biopsies after randomization [17].
Endothelial dysfunction and pulmonary vascular disease
Pulmonary hypertension and pulmonary vascular disease are the detrimental long-term consequences of untreated congenital heart defects with left-to-right shunts. We anticipate a dysregulated interaction of beta-adrenergic pathways and the cytokine system and measured significantly elevated tumor necrosis factor receptor levels in infants with left-to-right shunts that correlates with nitrate/nitrite levels that indicates inflammation [18]. Indeed, the mean pulmonary to mean arterial pressure ratio is lower with additional propranolol treated infants (44%) compared to only digoxin/diuretics (53%) treated infants in the CHF-Pro-Infant trial (Table 1). We published a case report in 1999 showing a significant decrease of pulmonary hypertension in an infant after propranolol treatment [19]. However, current treatment of pulmonary hypertension in pediatric cardiology focus on expansive vasodilators like bosentan and sildenafil and neglect the promising beneficial effects of beta blockers in pulmonary hypertension [20].
With respect to the systemic blood pressure, the occasional systolic blood pressure decreases with the increasing age of the patient groups (Table 3; current concept: 71.1 ± 23.1%, digoxin/diuretics: 67.9 ± 30.3%, GUCH: 60.3 ± 32.8%). This difference probably depends on the fear of the younger children during the examination that probably explain significantly higher but normal diastolic blood pressures percentile in the current concept group (55.0 ± 30.9% versus 41.0 ± 30.8%**)
Early life stress and neurodevelopment
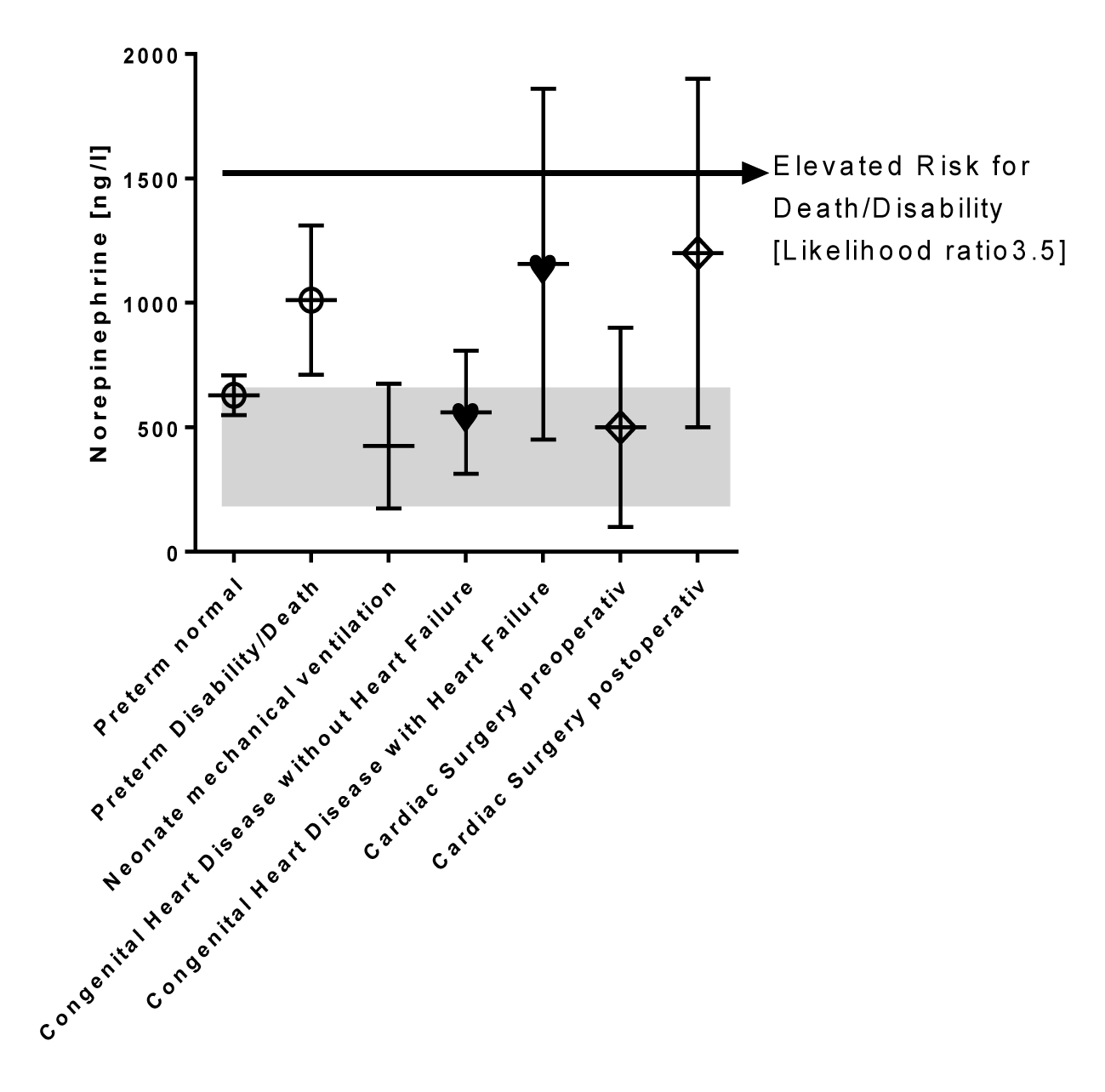
Evans DJ, et al. [21] showed that elevated norepinephrine levels are associated with adverse outcome in preterm infants in 2001. Figure 5 shows the norepinephrine levels of preterm infants stratified for the outcome data assessed at 4-5 years of life. Those infants who died or suffer from disabilities had significantly higher norepinephrine plasma level at the first day of life (1011 ± 300 ng/l). The author proposed a norepinephrine cut off plasma level of 1530 ng/l to estimate a worse outcome in preterm infants [21]. Norepinephrine levels of preterm infants with a favorable outcome are in the high normal level (629 ± 80 ng/l) comparable to 126 neonates who need mechanical ventilation (425 ± 250 ng/l) [22]. Our published norepinephrine levels from infants with congenital heart disease without heart failure (560 ± 247 ng/l) are not elevated [11]. In contrast, infants with heart failure have significantly elevated norepinephrine levels (1156 ± 705 ng/l) in a daily life setting that is comparable to the norepinephrine levels immediately after cardiac surgery (1200 ± 700 ng/l) as published by Gruber, et al. [23]. A retrospective analysis from 86 of our infants with congenital heart disease treated 20 years ago at the university hospital of Göttingen shows that 15 from 86 infants (17.4 %) had a norepinephrine level in a daily life setting above the proposed cut off value of 1530 ng/l to estimate a worse outcome in preterm infants.
Survival of congenital heart disease improved due to early heart surgery but there is a significant burden of neurodevelopmental deficits: Acute neurologic complications in children undergoing congenital heart surgery occur in 1.75% patients in a recent retrospective study and are related to hypoxia, brain bleeding or embolism [24]. The cumulative incidence rates of attention deficit (hyperactivity) disorder and autism spectrum disorder were even higher in children with congenital heart disease than in a control group (4.55 vs. 1.26/1000 person years for attention deficit hyperactivity disorder and 0.99 vs. 0.2/1000 person-years for autism spectrum disorder) [25].
Recent cerebral MRI scans using voxel-based cortical thickness and morphometry analysis showing brain volume reductions that correlated significantly with cognitive, motor and executive functions [26]. König J, et al. [27] showed association between autonomic nervous system function and brain morphology across the lifespan. Greater cortical thickness may be vital for the maintenance of healthy cardiac regulation via the autonomic nervous system or greater cardiac vagal activity as indirectly reflected in HRV may slow brain atrophy.
Short stature in congenital heart disease
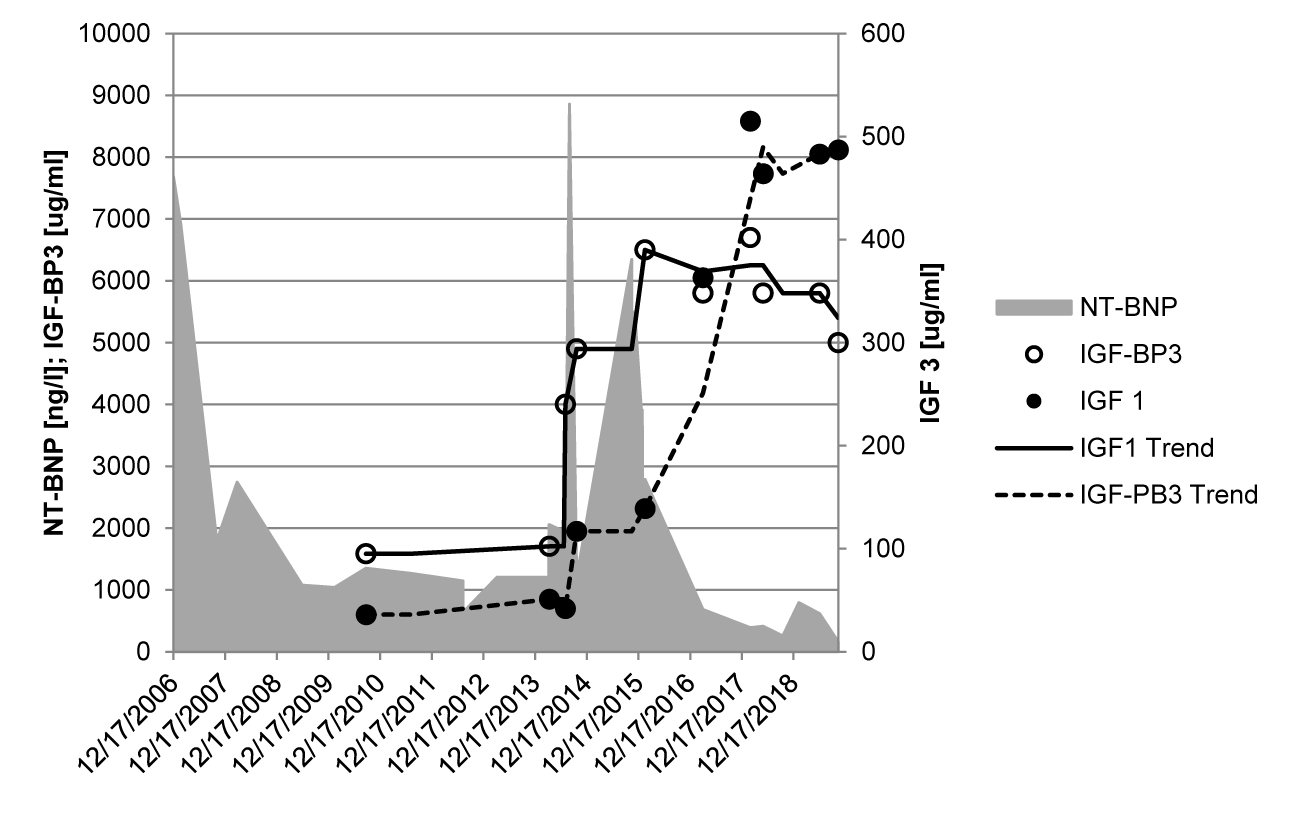
Short stature is a frequent symptom in children with congenital heart disease and we anticipate a pathophysiological model of autonomic imprinting by early life stress model due to heart failure as shown in children with intrauterine growth retardation or social deprivation [13]. However, there are important difference if most children with severe heart failure due to congenital heart defects have successful cardiac surgery and catch-up growth until adolescence. Our long-term data show a comparable height in children treated with the current concept and the digoxin/diuretic concept (33.0 ± 28.5% versus 33.5 ± 32.1%). There seems to be a significant constitutional growth delay in patients with operated congenital heart defects [28], if grown-ups with congenital heart defects have nearly normal heights (48.2 ± 29.6%). The impact of heart failure on height and IGF-1 values is well documented in one boy, who have heart transplantation at the age of 14 years with a dramatic catch up growth after transplantation [29] (Figure 6).
Discussion
In 1996, we introduce propranolol treatment in infants with severe heart failure to improve prognosis in children with congenital heart disease. The CHF-Pro-Infant and VSD-PHF trials are the first prospective randomized trials to this topic that clearly showed that propranolol improves clinical symptoms in infants with heart failure due to congenital heart disease. Moreover, we clearly showed an iatrogenic neurohormonal activation probably of cause a lowering effect on blood pressure that was induced by captopril and diuretics.
We further proof the effect of propranolol on cardiac remodeling and dysautonomia in infants with severe heart failure. Using analysis of heart rate variability, we are able to show a significant preoperative improvement of dysautonomia and normal postoperative values on average in the current generation up to 15 years after preoperative treatment with propranolol without diuretics.
Our preoperative data indicate that propranolol treatment protects the heart against pathologic cardiac remodeling. The intraoperative biopsies showed a significant downregulation of beta-2-receptor and angiotensin-2 receptor genes, and up-regulation of endothelin. A receptor and connective tissue growth factor genes, that were partially prevented by propranolol. The postoperative data in the current generation up to 15 years (Table 3) indicates a normal myocardial function with left ventricular fractional shortening of 37.6 ± 6.9% and normal indices of myocardial performance (LVIMP: 0.26 ± 0.14, RVIMP: 0.15 ± 0.12, reference < 0.4) and normal heart rate variability in the current generation.
However, we are not able to demonstrate a better preoperative survival, a lower incidence of short stature or a better neurodevelopment. The cause is the low number of propranolol treated children and the missing of a control group after the CHF-Pro-Infant trial. These limitations based upon the fact that we are not able to convince our colleagues to use propranolol to treat infants with severe heart failure and to get financial support for proper methods e.g., to measure neurodevelopment.
Conclusion
25 years after our first reports about this promising therapy, new methods are used to proof propranolol for increasing cardiomyocyte division in children with congenital heart disease. The first group of infants in this trial with tetralogy of Fallot has a low risk to die from heart failure but if the therapy is successful the high-risk patients with complex defects and single ventricle will follow.
50 years after Waagstein’s first reports about the treatment of heart failure with beta blockers, this most successful treatment is far away from routine in pediatric cardiology. There was enough time to perform the urgently needed prospective, randomized trials but of cause the missing will and financial support the children have no chance to get a better treatment of heart failure. However, the evidence-based data of propranolol to treat severe heart failure in infants with congenital heart disease are the best we have. There is no reason to withheld infants from this effective therapy of early life stress due to heart failure.
References
- Gilljam T, Mandalenakis Z, Dellborg M. Development of heart failure in young patients with congenital heart disease: a nation-wide cohort study. Open heart. 2019;6(1):e000858. doi: 10.1136/openhrt-2018-000858
- Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA, Rosenthal DN, Burr J, LaSalle B, Holubkov R, Lukas MA, Tani LY. Pediatric Carvedilol Study Group. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007 Sep 12;298(10):1171-1179. doi: 10.1001/jama.298.10.1171. PMID: 17848651.
- Buchhorn R, Hulpke-Wette M, Hilgers R, Bartmus D, Wessel A, Bürsch J. Propranolol treatment of congestive heart failure in infants with congenital heart disease: The CHF-PRO-INFANT trial. Congestive heart failure in infants treated with propanol. Int J Cardiol. 2001 Jul;79(2-3):167-173. doi: 10.1016/s0167-5273(01)00413-2. PMID: 11461738.
- Ramakrishnan S, Ghati N, Ahuja RS. Efficacy and safety of propranolol in infants with heart failure due to moderate-to-large ventricular septal defect (VSD-PHF study) - A prospective randomized trial. Annals of pediatric cardiology. 2021;14(3):331-340. doi: 10.4103/apc.APC_94_21
- Buchhorn R, Bartmus D, Siekmeyer W, Hulpke-Wette M, Schulz R, Bürsch J. Beta-blocker therapy of severe congestive heart failure in infants with left to right shunts. Am J Cardiol. 1998 Jun 1;81(11):1366-1368. doi: 10.1016/s0002-9149(98)00175-1. PMID: 9631979.
- Buchhorn R, Ross RD, Hulpke-Wette M, Bartmus D, Wessel A, Schulz R, Bürsch J. Effectiveness of low dose captopril versus propranolol therapy in infants with severe congestive failure due to left-to-right shunts. Int J Cardiol. 2000 Nov-Dec;76(2-3):227-233. doi: 10.1016/s0167-5273(00)00384-3. PMID: 11104878.
- Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, Barker PC, Ravishankar C, McCrindle BW, Williams RV, Altmann K, Ghanayem NS, Margossian R, Chung WK, Border WL, Pearson GD, Stylianou MP, Mital S. Pediatric Heart Network Investigators. Enalapril in infants with single ventricle: Results of a multicenter randomized trial. Circulation. 2010 Jul 27;122(4):333-340. doi: 10.1161/CIRCULATIONAHA.109.927988. Epub 2010 Jul 12. PMID: 20625111; PMCID: PMC3692364.
- Buchhorn R, Ross RD, Bartmus D, Wessel A, Hulpke-Wette M, Bürsch J. Activity of the renin-angiotensin-aldosterone and sympathetic nervous system and their relation to hemodynamic and clinical abnormalities in infants with left-to-right shunts. Int J Cardiol. 2001 May;78(3):225-230; discussion 230-231. doi: 10.1016/s0167-5273(01)00398-9. PMID: 11376824.
- Ross RD, Daniels SR, Schwartz DC, Hannon DW, Shukla R, Kaplan S. Plasma norepinephrine levels in infants and children with congestive heart failure. Am J Cardiol. 1987 Apr 1;59(8):911-914. doi: 10.1016/0002-9149(87)91118-0. PMID: 3825955.
- Buchhorn R, Hammersen A, Bartmus D, Bürsch J. The pathogenesis of heart failure in infants with congenital heart disease. Cardiol Young. 2001 Sep;11(5):498-504. doi: 10.1017/s1047951101000725. PMID: 11727904.
- Buchhorn R, Hulpke-Wette M, Nothroff J, Paul T. Heart rate variability in infants with heart failure due to congenital heart disease: reversal of depressed heart rate variability by propranolol. Med Sci Monit. 2002 Oct;8(10):CR661-6. PMID: 12388917.
- Spector LG, Menk JS, Knight JH, et al. Trends in Long-Term Mortality After Congenital Heart Surgery. Journal of the American College of Cardiology. 2018;71(21):2434-46. doi: 10.1016/j.jacc.2018.03.491
- Buchhorn R, Meint S, Willaschek C. The Impact of Early Life Stress on Growth and Cardiovascular Risk: A Possible Example for Autonomic Imprinting? PloS one. 2016;11(11):e0166447. doi: 10.1371/journal.pone.0166447
- Buchhorn R. Myocardial Protection with Beta Blocker Treatment in Infants with Heart Failure Due to Congenital Heart Defects and Duchenne Muscular Dystrophy. Open Journal of Thoracic Surgery. 2020;10(4). https://tinyurl.com/4j7ma5wh
- Buchhorn R, Hulpke-Wette M, Ruschewski W, Ross RD, Fielitz J, Pregla R, Hetzer R, Regitz-Zagrosek V. Effects of therapeutic beta blockade on myocardial function and cardiac remodelling in congenital cardiac disease. Cardiol Young. 2003 Feb;13(1):36-43. doi: 10.1017/s1047951103000076. PMID: 12691286.
- Liu H, Zhang CH, Ammanamanchi N. Control of cytokinesis by β-adrenergic receptors indicates an approach for regulating cardiomyocyte endowment. Science translational medicine. 2019;11(513). doi: 10.1126/scitranslmed.aaw6419
- El Khoudary SR, Fabio A, Yester JW. Design and rationale of a clinical trial to increase cardiomyocyte division in infants with tetralogy of Fallot. International journal of cardiology. 2021;339:36-42. doi: 10.1016/j.ijcard.2021.07.020
- Buchhorn R, Wessel A, Hulpke-Wette M. Endogenous nitric oxide and soluble tumor necrosis factor receptor levels are enhanced in infants with congenital heart disease. Crit Care Med. 2001;29(11):2208-2210. doi: 10.1097/00003246-200111000-00026
- Buchhorn R, Hulpke-Wette M, Wessel A. Beta-blocker therapy in an infant with pulmonary hypertension. Eur J Pediatr. 1999;158(12):1007-1008. https://tinyurl.com/ys5cb9zr
- Farha S, Saygin D, Park MM. Pulmonary arterial hypertension treatment with carvedilol for heart failure: a randomized controlled trial. JCI insight. 2017;2(16). doi: 10.1172/jci.insight.95240
- Evans DJ, MacGregor RJ, Dean HG. Neonatal catecholamine levels and neurodevelopmental outcome: a cohort study. Archives of disease in childhood Fetal and neonatal edition. 2001;84(1):F49-52. doi: 10.1136/fn.84.1.f49
- Longin E, Schaible T, Lenz T, König S. Short term heart rate variability in healthy neonates: normative data and physiological observations. Early Hum Dev. 2005 Aug;81(8):663-671. doi: 10.1016/j.earlhumdev.2005.03.015. PMID: 16046085.
- Gruber EM, Laussen PC, Casta A, Zimmerman AA, Zurakowski D, Reid R, Odegard KC, Chakravorti S, Davis PJ, McGowan FX Jr, Hickey PR, Hansen DD. Stress response in infants undergoing cardiac surgery: A randomized study of fentanyl bolus, fentanyl infusion, and fentanyl-midazolam infusion. Anesth Analg. 2001 Apr;92(4):882-890. doi: 10.1097/00000539-200104000-00016. PMID: 11273919.
- Jafri SK, Ehsan L, Abbas Q. Frequency and Outcome of Acute Neurologic Complications after Congenital Heart Disease Surgery. Journal of pediatric neurosciences. 2017;12(4):328-331. doi: 10.4103/jpn.JPN_87_17
- Tsao PC, Lee YS, Jeng MJ. Additive effect of congenital heart disease and early developmental disorders on attention-deficit/hyperactivity disorder and autism spectrum disorder: A nationwide population-based longitudinal study. European child & adolescent psychiatry. 2017;26(11):1351-1359. doi: 10.1007/s00787-017-0989-8
- Meuwly E, Feldmann M, Knirsch W. Postoperative brain volumes are associated with one-year neurodevelopmental outcome in children with severe congenital heart disease. Scientific reports. 2019;9(1):10885. doi: 10.1038/s41598-019-47328-9
- Koenig J, Abler B, Agartz. Cortical thickness and resting-state cardiac function across the lifespan: A cross-sectional pooled mega-analysis. Psychophysiology. 2021;58(7):e13688. doi: 10.1111/psyp.13688
- Menon SC, Al-Dulaimi R, McCrindle BW. Delayed puberty and abnormal anthropometry and its associations with quality of life in young Fontan survivors: A multicenter cross-sectional study. Congenital heart disease. 2018;13(3):463-469. doi: 10.1111/chd.12597
- Buchhorn R. Short stature in severe pediatric heart failure: The deleterious role of growth hormone replacement. Biomed Res. 2019;4:1-3.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.