Medicine Group . 2022 June 29;3(6):722-725. doi: 10.37871/jbres1503.
An Infant with Hydrocephalus and Hepatomegaly
Patricia Pichilingue-Reto1,4*, Lara Pavageau2,5, Venkat Kakkilaya2, Naseem Uddin3 and Amanda S Evans1
2Department of Pediatrics, Division of Neonatal-Perinatal Medicine, UT Southwestern Medical Center, Dallas, TX, USA
3Division of Pathology, UT Southwestern Medical Center, Dallas, TX, USA
4Department of Pediatrics, Division of Pediatric Infectious Diseases, Louisiana State University Health Sciences Center, Shreveport, LA, USA
5Neonatal-Perinatal Medicine, Dallas Regional Medical Center, Dallas, TX, USA
- Hydrocephalus
- Microophthalmia
- Placenta
- Pseudocyst
Abstract
We report a histologic and PCR-confirmed case of symptomatic congenital toxoplasmosis in a premature infant with optic nerve atrophy and severe ventriculomegaly requiring ventriculoperitoneal shunt in the absence of cerebral calcifications. A significant risk factor in acquisition of this congenital infection was mother’s consumption of raw deer meat during pregnancy.
Case Report
A male infant born at 33 weeks of gestational age was admitted to the neonatal intensive care unit due to respiratory distress, prematurity and prenatal diagnosis of multiple abnormalities including ventriculomegaly and hepatomegaly. His mother was a 25-year-old female, G2P2A0, with an uncomplicated prenatal course and normal fetal ultrasound at 20 weeks of gestation. Prenatal screening labs included nonreactive RPR (rapid plasma reagin), nonreactive Hepatitis B surface antigen, nonreactive HIV antibody/antigen screen and Rubella immune.
On mother’s admission to labor and delivery, fetal ultrasound showed evidence of severe ventriculomegaly, ascites, and hepatomegaly. Mother presented with prolonged rupture of membranes (25 hours) and received ampicillin and gentamicin prior to spontaneous vaginal delivery with clear fluid. Birth weight was 2030 g (25th percentile), length was 45 cm (50th percentile) and fronto-occipital circumference was 32.5 cm (75th percentile). The infant was classified as adequate for gestational age with low birth weight; however, not growth restricted.
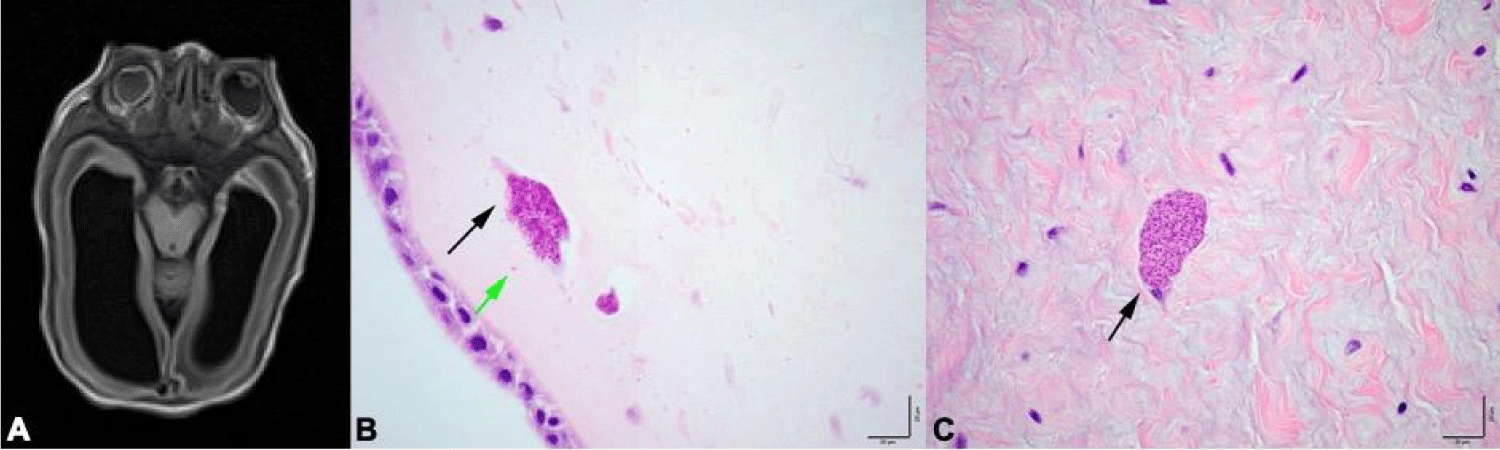
Initially, the infant required positive pressure ventilation. He was started on Continuous Positive Airway Pressure (CPAP) and weaned to room air by 2 hours of life. On physical exam, he was noted to have ballotable sutures and flat and enlarged anterior fontanelle, hepatomegaly, palpable spleen, and uncoordinated suck. Eye exam demonstrated absent red reflex, however he otherwise appeared grossly normal. Initial laboratory results were positive for mild anemia (hemoglobin 10.5 g/dL) and thrombocytopenia (platelets 86,000/µL). Hepatic function panel showed direct hyperbilirubinemia (direct bilirubin 1.0 mg/dL) and elevated gamma-glutamyl transferase (GGT 419 U/L). Head ultrasound on first day of life showed significant enlargement of the lateral ventricles and mild prominence of third ventricle; however, an etiology for the enlarged lateral ventricles was not identified. Brain Magnetic Resonance Imaging (MRI) on day three of life showed severe sequela of intraventricular hemorrhage with associated parenchymal injury, enlarged third and lateral ventricles, regions of bifrontal polymicrogyria, multifocal cystic encephalomalacia (predominantly in the frontal and parietal region), and atrophic appearance of the optic nerves with abnormal morphology of the bilateral globes (Figure 1A). Initial ophthalmologic exam showed signs of congenital glaucoma and cataract in both eyes (right worse than left). Echocardiography was unremarkable and abdominal ultrasound confirmed hepatosplenomegaly. Ophthalmology exam showed signs of congenital glaucoma and cataract in both eyes (right worse than left).
Further history revealed the infant’s parents were born in Burma but had lived in the United States for over five years. Mother visited a relative who owned a cat, but she did not pet it or handle its litter box. When asked about dietary habits, the mother reported consuming raw deer meat during pregnancy on several occasions, prepared in a traditional Burmese dish. Infant has one sibling who is 2 years old with normal perinatal and medical history.
Hospital Course
Urine Cytomegalo Virus (CMV) culture was negative, and maternal serology was consistent with past infection for CMV and Parvovirus. Maternal Toxoplasma IgG was markedly elevated at > 900 IU/ml, and IgM was 15.4 AU/ml (IgM > 10 AU/ml considered positive). These initial serologic tests were done at Associate Regional and University Pathologists, Inc. (ARUP) Laboratories (Salt Lake City, Utah). Additional samples were sent to Palo Alto Medical Foundation Toxoplasma Serology Laboratory (PAMF-TSL; Palo Alto, CA), a reference laboratory for Toxoplasma serologic assays and their interpretation. CerebroSpinal Fluid (CSF) analysis showed white blood cell count of 14 cells/µL (lymphocytes 74%), red blood cells of 1 cell/µL, protein > 600 mg/dL and glucose of 34 mg/dL.
Notably, placental pathology showed multiple pseudocysts in fetal membranes (Figure 1B) and presence of individual organisms consistent with Toxoplasma gondii, as well as rare pseudocysts in the umbilical cord, containing numerous organisms (Figure 1C). Infant’s antibodies for Toxoplasmosis IgM, IgG and IgA were positive, as well as Toxoplasmosis PCR in serum, urine and CSF.
Therapy was started with pyrimethamine, sulfadiazine and leucovorin. Follow up ophthalmoscopic examination showed chorioretinitis in both eyes with microphthalmia, cataract and optic nerve hypoplasia on right eye and optic nerve hypoplasia on left eye, for which infant received prednisolone for two months. Subsequently, the patient was transferred to a pediatric facility for neurosurgical management of hydrocephalus. He had right frontal ventricular reservoir placement on 9th day of life. Due to persistent ventriculomegaly, a VentriculoPeritoneal shunt (VP) was placed at 6 weeks of age, without complications. Patient was discharged home at 9 weeks of age.
Outpatient follow up with Neurology, Neurosurgery, Ophthalmology and Audiology are ongoing. He completed 12 months of combination therapy with pyrimethamine, sulfadiazine and leucovorin. On his last follow up visit, Ophthalmology reported significant microphthalmia with posterior synechiae and congenital cataract on right eye; left micropthalmia and resolved bilateral panuveitis with a very poor visual prognosis. He has global developmental delay with intractable epilepsy and continues to have close follow up with Neurology.
Final Diagnosis
Congenital toxoplasmosis.
Discussion
We report a rare occurrence of a confirmed case of severely symptomatic congenital toxoplasmosis in Dallas, Texas, resulting in complications of obstructive hydrocephalus requiring VP shunt placement, optic nerve atrophy and blindness, developmental delay and seizure disorder. Toxoplasma gondii, a protozoal parasite that causes toxoplasmosis, has a worldwide distribution and infects warm-blooded animals. Globally, toxoplasmosis seroprevalance rates vary widely, depending on the geographic location, and can range from less than 10 to 90% [1].
Toxoplasmosis is not a reportable disease in the United States; hence, data regarding actual prevalence and incidence rates is limited. Evidence that is available includes serum samples from the National Health and Nutrition Examination Survey (NHANES) that are periodically tested for Toxoplasma gondii antibodies. The overall seroprevalence among women of childbearing age (15-44 years old) from these surveys has slightly declined over time, at 9.1% on the 2009-2010 analysis, decreasing to 7.5% in NHANES 2011-2014 [2]. A similar trend has been noted in the overall participants studied in the NHANES surveys (15.3% vs. 11.9%, respectively) [3]. The most recent analysis indicates that more than 97% of women in the United States are susceptible to initial T. gondii infection during pregnancy [3]. In the United States, according to data from the New England Newborn Screening Program, the incidence of congenital toxoplasmosis was approximately 0.23 cases per 10, 000 live births by 2015 [4]. The last case of congenital toxoplasmosis in Texas was reported in Houston in 2003: a one-month old Asian girl with fever, irritability and asymmetric red reflexes found to have congenital toxoplasmosis with panuveitis and total retinal detachment. Infant's mother had a history of fever and lymphadenopathy and she reported eating undercooked meat early in pregnancy [5].
A case-control study of adults infected with T. gondii selected from the Palo Alto Medical Foundation Toxoplasma Serology Laboratory from August 2002 through May 2007, showed that T. gondii infection was associated with the following factors: eating raw ground beef, rare lamb and locally produced, cured, dried, or smoked meat; working with meat; drinking unpasteurized goat’s milk; and having 3 or more kittens [6]. In our case, undercooked deer meat was recognized as a probable risk factor of transmission. Epidemiological surveys of several areas of the United States have reported 31-76% seropositivity to T. gondii in white-tailed deer [7]. Raw deer meat consumption has been reported in cases of acquired toxoplasmosis infections. A more recent study in Quebec, found that out of 10 Canadian deer hunters who attended a hunting retreat in Illinois, United States, 6 returned with symptoms of fever, severe headache, myalgia, and articular pain, presenting similar illness onset dates. Further serologic testing indicated a recent toxoplasmosis infection for all 6 symptomatic hunters, and the risk factor identified was consumption of undercooked deer meat [8]. Additionally, in a family cluster epidemiologic survey of congenital toxoplasmosis infections, 2 of 6 mothers with recent primary infection had report of preparing and/or consuming raw deer meat [9].
Congenital toxoplasmosis causes a wide spectrum of clinical manifestations, from asymptomatic to severely affected [10]. It can be subclinical in ~75% of infected newborns [11]. The classic triad of signs of congenital toxoplasmosis are chorioretinitis, hydrocephalus, and intracranial calcifications [4]. Recent data from PAMF-TSL showed that the majority of infants with congenital toxoplasmosis (84%) demonstrated one or more severe clinical manifestations of congenital toxoplasmosis, including eye disease (92.2%), brain calcifications (79.6%), and hydrocephalus (67.7%). Additional signs seen included microcephaly, seizures, hearing loss, lymphadenopathy, hepatomegaly, splenomegaly, pneumonitis and meningoencephalitis [10]. Further analysis from this cohort showed a lower prevalence of eye findings (62.5%) and hydrocephalus (38.5%) in the group of infants born to treated mothers (25 women) compared to that reported in infants born to untreated mothers [12]. Hydrocephalus in congenital toxoplasmosis has traditionally been attributed only to obstruction of the aqueduct, being the most predominant cause in a cohort, accounting for 43% of cases and 54% when mixed aqueductal and foraminal obstruction are included; but hydrocephalus has also been reported to occur without anatomic obstruction of CSF circulation [13,14].
Despite the severe neurologic manifestations in this infant, there were no calcifications present on neuroimaging. In congenital toxoplasmosis, the parasite causes cell damage resulting in focal leptomeningitis, inflammation with granuloma formation and brain necrosis [15]. Necrosis can progress to formation of cystic areas with focal calcification. Disseminated calcifications do not necessarily suggest a poor prognosis and have been discovered in “normal” children at 1 year of age, diagnosed with congenital toxoplasmosis by a systematic survey [16]. Calcifications can diminish in size or even resolve with treatment [17].
Diagnostic criteria for congenital toxoplasmosis usually depends on serology and molecular diagnostic studies [4]. In our case, diagnosis was made promptly due to placenta findings, highlighting the need to examine placenta pathology in high risk deliveries. In France, the parasitic analysis of 102 placentas from cases of toxoplasmosis acquired during gestation were reviewed. Congenital toxoplasmosis was diagnosed in 28 of these cases. The positive and negative predictive values of placental examination were 91% and 90%, respectively [18].
Clinical evaluation should include maternal exposure history, physical examination, neurologic, ophthalmologic and brainstem auditory evoked responses evaluation. Head imaging and abdominal ultrasound should be included in the evaluation. Treatment length is 12 months and includes pyrimethamine, sulfadiazine and folinic acid. In the presence of chorioretinitis or if protein in CSF is greater than 1 g/dL, corticosteroids are recommended [19].
Conclusion
Maternal consumption of raw deer meat during pregnancy and infant’s severe obstructive hydrocephalus in the absence of cerebral calcifications, presenting along with optic nerve atrophy and globe abnormalities, were among the unique findings of this case of congenital toxoplasmosis. Culinary educational strategies for toxoplasmosis prevention among pregnant women with epidemiological risk factors should be considered in the United States. It is also important to consider histologic evaluation of the placenta among deliveries with high suspicion for congenital infections.
Disclosures
None of the authors have any conflicts of interest.
References
- Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009 Oct;39(12):1385-94. doi: 10.1016/j.ijpara.2009.04.003. Epub 2009 May 9. PMID: 19433092.
- Jones JL, Kruszon-Moran D, Elder S, Rivera HN, Press C, Montoya JG, McQuillan GM. Toxoplasma gondii Infection in the United States, 2011-2014. Am J Trop Med Hyg. 2018 Feb;98(2):551-557. doi: 10.4269/ajtmh.17-0677. Epub 2017 Dec 14. Erratum in: Am J Trop Med Hyg. 2018 Jul;99(1):241-242. PMID: 29260660; PMCID: PMC5929212.
- Owusu-Dommey A, Pogreba-Brown K, Villa-Zapata L. Seroprevalence of Toxoplasma gondii in the U.S.: Evidence from a representative cross-sectional survey. Parasitol Int. 2020 Dec;79:102175. doi: 10.1016/j.parint.2020.102175. Epub 2020 Aug 5. PMID: 32763362.
- Maldonado YA, Read JS; COMMITTEE ON INFECTIOUS DISEASES. Diagnosis, Treatment, and Prevention of Congenital Toxoplasmosis in the United States. Pediatrics. 2017 Feb;139(2):e20163860. doi: 10.1542/peds.2016-3860. PMID: 28138010.
- Brady-McCreery KM, Hussein MA, Paysse EA. Congenital toxoplasmosis with unusual retinal findings. Arch Ophthalmol. 2003 Aug;121(8):1200-1. doi: 10.1001/archopht.121.8.1200. PMID: 12912703.
- Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis. 2009 Sep 15;49(6):878-84. doi: 10.1086/605433. PMID: 19663709.
- Gerhold RW, Saraf P, Chapman A, Zou X, Hickling G, Stiver WH, Houston A, Souza M, Su C. Toxoplasma gondii seroprevalence and genotype diversity in select wildlife species from the southeastern United States. Parasit Vectors. 2017 Oct 23;10(1):508. doi: 10.1186/s13071-017-2456-2. PMID: 29061166; PMCID: PMC5654087.
- Gaulin C, Ramsay D, Thivierge K, Tataryn J, Courville A, Martin C, Cunningham P, Désilets J, Morin D, Dion R. Acute Toxoplasmosis among Canadian Deer Hunters Associated with Consumption of Undercooked Deer Meat Hunted in the United States. Emerg Infect Dis. 2020 Feb;26(2):199-205. doi: 10.3201/eid2602.191218. PMID: 31961291; PMCID: PMC6986818.
- Contopoulos-Ioannidis D, Wheeler KM, Ramirez R, Press C, Mui E, Zhou Y, Van Tubbergen C, Prasad S, Maldonado Y, Withers S, Boyer KM, Noble AG, Rabiah P, Swisher CN, Heydemann P, Wroblewski K, Karrison T, Grigg ME, Montoya JG, McLeod R. Clustering of Toxoplasma gondii Infections Within Families of Congenitally Infected Infants. Clin Infect Dis. 2015 Dec 15;61(12):1815-24. doi: 10.1093/cid/civ721. Epub 2015 Sep 24. PMID: 26405150; PMCID: PMC4657536.
- Olariu TR, Remington JS, McLeod R, Alam A, Montoya JG. Severe congenital toxoplasmosis in the United States: clinical and serologic findings in untreated infants. Pediatr Infect Dis J. 2011 Dec;30(12):1056-61. doi: 10.1097/INF.0b013e3182343096. PMID: 21956696.
- McAuley JB. Congenital Toxoplasmosis. J Pediatric Infect Dis Soc. 2014 Sep;3 Suppl 1(Suppl 1):S30-5. doi: 10.1093/jpids/piu077. PMID: 25232475; PMCID: PMC4164182.
- Olariu TR, Press C, Talucod J, Olson K, Montoya JG. Congenital toxoplasmosis in the United States: clinical and serologic findings in infants born to mothers treated during pregnancy. Parasite. 2019;26:13. doi: 10.1051/parasite/2019013. Epub 2019 Mar 6. PMID: 30838974; PMCID: PMC6402364.
- Hutson SL, Wheeler KM, McLone D, Frim D, Penn R, Swisher CN, Heydemann PT, Boyer KM, Noble AG, Rabiah P, Withers S, Montoya JG, Wroblewski K, Karrison T, Grigg ME, McLeod R. Patterns of Hydrocephalus Caused by Congenital Toxoplasma gondii Infection Associate With Parasite Genetics. Clin Infect Dis. 2015 Dec 15;61(12):1831-4. doi: 10.1093/cid/civ720. Epub 2015 Sep 24. PMID: 26405147; PMCID: PMC4657535.
- Khan K, Khan W. Congenital toxoplasmosis: An overview of the neurological and ocular manifestations. Parasitol Int. 2018 Dec;67(6):715-721. doi: 10.1016/j.parint.2018.07.004. Epub 2018 Jul 21. PMID: 30041005.
- Popli MB, Popli V. Neuroimage: Congenital toxoplasmosis. Neurol India. 1999 Mar;47(1):74. PMID: 10339716.
- Remington JS, McLeod R, Wilson CB, Desmonts G. Toxoplasmosis. In: Remington JS, Klein JO, Wilson, Victor Nizet, Maldonado YA, editors. Infectious diseases of the fetus and newborn. 7th ed. Saunders WB; 2011. p.918-1041. doi: 10.1016/B978-1-4160-6400-8.00031-6.
- Patel DV, Holfels EM, Vogel NP, Boyer KM, Mets MB, Swisher CN, Roizen NJ, Stein LK, Stein MA, Hopkins J, Withers SE, Mack DG, Luciano RA, Meier P, Remington JS, McLeod RL. Resolution of intracranial calcifications in infants with treated congenital toxoplasmosis. Radiology. 1996 May;199(2):433-40. doi: 10.1148/radiology.199.2.8668790. PMID: 8668790.
- Robert-Gangneux F, Dupretz P, Yvenou C, Quinio D, Poulain P, Guiguen C, Gangneux JP. Clinical relevance of placenta examination for the diagnosis of congenital toxoplasmosis. Pediatr Infect Dis J. 2010 Jan;29(1):33-8. doi: 10.1097/INF.0b013e3181b20ed1. PMID: 19858771.
- Toxoplasma gondii Infections. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red Book: 2018 Report of the Committee on Infectious Diseases. American Academy of Pediatrics; 2018. p.809-819.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.