Environmental Sciences . 2022 July 14;3(7):748-757. doi: 10.37871/jbres1509.
Biological Activities on Geopolymeric and Ordinary Concretes
Buczkowska KE1,2*, Ruzek V1, Petr Louda1, Bousa M1 and Yalcinkaya B1
2Department of Materials Technology and Production Systems, Faculty of Mechanical Engineering, Lodz University of Technology, Stefanowskiego, Lodz, Poland
Abstract
The primary purpose of the review is to describe the biological growth and its effects on the goepolymeric and ordinary concrete surfaces. As the concrete ages, the surface alkalinity wears off by carbonation and weathering,thus prepares a suitable environment for biological growth. Micro-organisms such as algae, fungi and various types of bacteria start to accumulate on the surface and subsequently penetrate into the micro-cracks of concrete structures, resulting in bursting stresses that can increase the size of cracks and may lead to spalling. Despite the natural resistance of concrete structures against biological growth in the early periods, anti-bacterial additives increase the resistance later.
Introduction
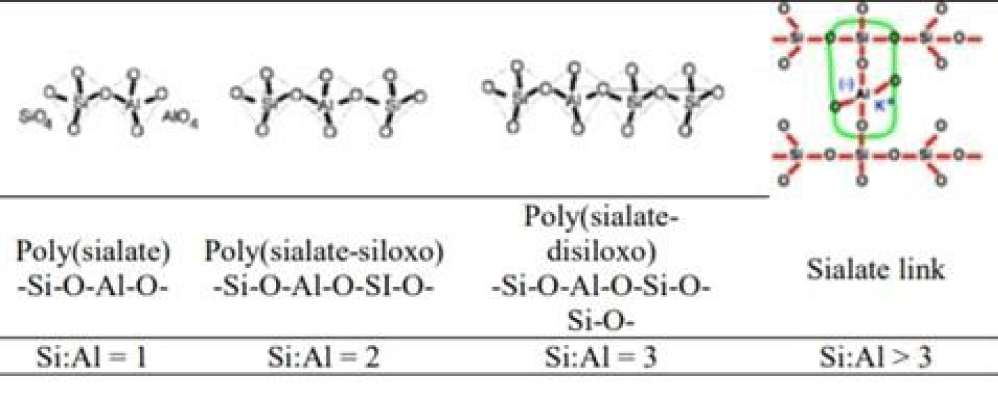
Geopolymers are inorganic materials formed by the polycondensation of aluminosilicates such as rock or clay. The substances are most commonly found in the earth's crust in aalkaline environment under normal temperature and pressure. Covalent Si-O-Al-O bonds are formed, and aluminosilicates are transformed into other forms such as polysulphates. This reaction mimics natural rock consolidation processes and creates a zeolite (microporous) three-dimensional structure [1,2].
The history of the use of geopolymers dates back to ancient times when, for example, the Romans used so-called Roman concrete for construction, which was made from quicklime (calcium oxide), water and volcanic ash (pozzolan) or volcanic glass (tuff). The calcium hydroxide produced by 'ramming' the lime into the mixture creates an alkaline environment and reacts with the pozzolana to form a specific structure. Although Roman concrete did not achieve the mechanical properties of modern products, it was much more resistant to weathering and therefore more durable; it also hardened over time and was more resistant to seawater (which further hardened it) [1,3].
In the 1930s, alkaline activation and curing of sand materials were first attempted in Germany. Further research was carried out in the 1960s at the Glutovsky Institute in Kyiv to dissolve slag using sodium hydroxide and water glass. In the Soviet Union, geopolymers were produced under the name gruntocement (literally 'soil cement', also 'geocements'). Research in the USSR was conducted by V. D. Glukhovsky of Kiev, who published his major publication (Geocements) in 1953. In Eastern Europe, geocements have been used, for example, for railway sleepers, but they have also found applications in the construction of residential houses. In 1990, a prefabricated house was built in Novokuznetsk using concrete made from an alkali-activated mixture of slag and fly ash from power stations. Joseph Davidovits first introduced the name geopolymer in the 1980s. He defined it as "a substance formed by inorganic polymerisation", and his definition was later extended to include alkaline activation of aluminosilicate substances, not just any inorganic polymer. The first research on geopolymers was carried out in Czechoslovakia around 1970. Today they are produced in the Czech Republic, for example, by the company České Lupkové závody, under the trade name Baucis. They are produced from locally mined kaolinite [1,4,5].
In addition to natural materials (e.g. metakaolin), geopolymers can be synthesised via emission-free fly ash from thermal power plants. Fly ash mainly consists of silica, alumina, iron oxide and calcium oxide and is commonly used to produce cement or filler materials. In addition, materials such as blast-furnace slag (a material formed in iron production and rich in silicon, aluminium and calcium oxides) or materials produced synthetically by the sol-gel method can be used as the basis for geopolymers; in particular, synthetic kaolinite can be obtained by the reaction of aluminium isopropoxide and Tetraethyl Orthosilicate (TEOS) [6-8].
Geopolymer
Geopolymers can be obtained by polycondensation process from various materials, both natural and anthropogenic. In particular, these materials must have a high alumina and silica content to allow reaction with a reagent such as sodium hydroxide, potassium hydroxide or sodium silicate and potassium silicate. A particular requirement is that these oxides must be in amorphous form, i.e. glassy. Metakaolin, fly ash and blast furnace slag are most commonly used, but the material for geopolymers can also be produced synthetically, for example, by the sol-gel method [1].
These are so-called latent hydraulic substances that solidify and harden when a reagent in water is added; they can also be in the form of a suitable aqueous solution. This phenomenon is used in conventional cement production as well, where Calcium Oxide (CaO), the essential component of cement, is used as an activator [1].
Geopolymerisation process
They are also referred to as curing. This reaction occurs when the aluminosilicate material is in a solution with a pH greater than 12, which is achieved by using a 'reagent', usually consisting of a solution of hydroxide and an alkali metal oxide (potassium or sodium). Although this process is similar to the production of plastics, geopolymers have properties more similar to ceramic materials. The most commonly used mechanism details several reaction steps [1,2].
- Dissolution of aluminosilicates by reaction with hydroxyl ions.
- Reorientation of ions and formation of aluminosilicate oligomers from supersaturated solution.
- Polycondensation of oligomers and formation of three-dimensional structure.
- Bonding of solid particles and formation of a polymeric structure.
At the beginning of the process, hydroxyl ions react with the aluminosilicate surface and break the covalent bonds of Si-O-Si, Si-O-Al and Al-O-Al, assuming that the material is amorphous (glassy). Silicon and aluminium ions are released into the solution, with aluminium ions being released more readily. The higher the pH, the higher the concentration of hydroxyl ions, and the faster the reaction proceeds [1].
The alkali metal cation also influences the dissolution rate and depends explily on the ionic radius, whose smaller size leads to the faster formation of stable ionic pairs with the oligomers present in the solution. Hence, dissolution proceeds faster in NaOH solution than in KOH. It is also true that a larger amount of silicon in the reactant solution increases the concentration of ions released [1]. The polymerisation then proceeds by polycondensation, the formation of covalent bonds between oligomers with the release of water molecules. This final stage of geopolymerisation leads to the formation of a zeolite structure, which is also the last stage of the natural transformation of aluminosilicates containing a sodium or potassium component, commonly referred to as zeolites or boiling stones (Figure 1). The name derives from retaining water in their pores, which escapes when heated, giving the impression of 'boiling'. Geopolymerisation is thus essentially an imitation and significant acceleration of the natural rock hardening process [1].
Comparison of geopolymers and Portland cement
Portland cement is the most commonly used type of cement and the primary ingredient in producing concrete and mortar. It is a ground, a stony mixture consisting mainly of calcium oxide (quicklime - 65%) and silica (silica fume - 21%), as well as e.g. alumina (5.6%) or iron oxide (3.4%), with these substances mainly in the following forms [10]:
- Tricalcium silicate - (CaO)3. SiO2, commonly called allite, is commonly referred to as C3S.
- Dicalcium silicate - (CaO)3. SiO2, trivially called belite, is commonly referred to as C2S.
- Tetracalcium aluminateferrite - (CaO)4. Al2O3. Fe2O3, trivially called celite, is commonly referred to as C4AF.
- Tricalcium aluminate - (CaO)3. Al2O3 is commonly known as C3A.
It is produced by grinding and burning a mixture of calcium carbonate (in the form of limestone, chalk or other rocks, silica (in the form of sand or clay, but old glass can also be used), bauxite (as a source of alumina, but recycled aluminium can also be used), iron ore (as a source of iron oxide, but recycled iron or power station fly ash can also be used), gypsum and other additives. The mixture is hydrated when dissolved in water and solidifies by forming calcium hydroxide (which allows the cement to solidify under water). Geopolymer, therefore, differs from Portland cement mainly in the reaction occurring during solidification. While polycondensation takes place in geopolymer, hydration takes place in cement. Cement only needs to be mixed with water, whereas geopolymers require reactant solutions to achieve a high pH. These solutions both increase the cost of the geopolymer and pose logistical and potential health problems due to their corrosiveness [11,12].
Geopolymers have better mechanical properties than concretes made from Portland cement, especially a much higher compressive strength (about 100 MPa for geopolymers, 30 MPa for cement and up to 60 MPa with special modifications). In contrast, tensile and flexural strengths are lower. However, this can be compensated by using reinforcement used in conventional concretes, such as carbon or corrosion-resistant steel bars. However, lightweight metals, alloys, or even plain glass, cannot be used for geopolymer composites. These substances quickly lose their strength due to the strong alkalinity of geopolymers. To produce a glass fibre-based geopolymer composite, alkali-resistant glass fibres are needed to be used, such as those made from organic polymers [1].
One of the most important advantages is the higher thermal resistance of geopolymers. Concrete, on the other hand, degrades rapidly when heated to temperatures above 300°C due to thermal decomposition, which also causes the release of toxic substances. Geopolymers are stable up to a melting point of about 1265°C [1].
Geopolymers also have low thermal conductivity, which can be further reduced by foaming them with powdered aluminium, which reacts with alkaline substances such as hydroxides in the liquid geopolymer mixture to release hydrogen, making geopolymers ideal materials for passive fire protection [1,13].
Geopolymers are also much more resistant to chemical attack, making them suitable for degrading conditions such as acid rain, sewage treatment plants and chimneys. In addition, they also exhibit significant adsorption capacities, making them potentially useful, for example in wastewater treatment, where they can serve as adsorbents or filter media. For this purpose, they can also be functionalised with anti-microbial agents such as metal nanoparticles. Furthermore, they can encapsulate various particles introduced into the structure during geopolymerisation. Therefore, they can be used to store hazardous waste or other waste such as exhaust flue gases from industrial plants or power stations [1,2,14].
However, the main advantage of geopolymers is the much lower energy consumption during production and lower emissions. The production of geopolymer cements requires about 1230-1310 MJ/t, with most of the energy used in the firing of clay materials, while almost three times as much, about 3500 MJ/t, is used in the production of Portland cement, for example in the firing of lime. Portland cement production also produces a large amount of carbon dioxide (about 1 tonne CO2 per 1 tonne of cement), which cannot be significantly reduced because carbon dioxide is an unavoidable by-product of lime firing. In 2005, cement production was the source of 1.8 billion tonnes of carbon dioxide, equivalent to about 8% of total emissions. Geopolymer production emissions are 50-80% lower, mainly due to lower temperature requirements (which also reduces energy requirements). Cement production requires temperatures up to 1500°C, while 600-700°C is sufficient for geopolymer production and heat treatment of clay components [1].
On the other hand, the main disadvantage of geopolymers is the need for their alkaline activation, which, on the one hand, poses a logistical problem due to the need to purchase or manufacture them, distribute them and for more complex on-site application due to their strong alkalinity and thus corrosiveness. Materials based on ordinary Portland cement only require mixing with water. A second disadvantage is the risk of variability in the composition and structure of the materials used in production, especially fly ash and slag. Variable composition leads to heterogeneous properties of the resulting material, which is problematic, for example in terms of standards or safety. [1].
Biogenic threats to building materials
One of the main threats to commonly used building materials, especially cement, is Microbiological Degradation (MID), which results in the gradual erosion of their integrity under the influence of acids (both organic and inorganic) produced by micro-organisms. This phenomenon occurs mainly when building materials are exposed to significant moisture and biological contamination, such as in wastewater tanks or sewage systems [15].
Under good conditions (low moisture and pollution), concrete has natural anti-bacterial properties due to its alkalinity, which results from the formation of calcium hydroxide during the hydration process. On the other hand, geopolymers are strongly alkaline due to the presence of alkali metal ions in their structure. However, water erosion or abrasion of other materials leads to a roughening of the surface, which, combined with available moisture and nutrients, allows certain micro-organisms, mainly sulphur-oxidising bacteria to colonise the surface and gradually lower the pH by producing acidic sulphur compounds. The gradual lowering of the pH of the surface allows the colonisation of the surface by other micro-organisms, such as certain types of bacteria, fungi, algae and lichens, which form a biofilm on the surface and produce other acidic compounds (e.g. acetic acid, lactic acid, butyric acid, etc.) that accelerate the decrease in pH. The biogenic sulphuric acid then reacts with the substances in the concrete to form gypsum (calcium sulphate), which can act as a protective layer, similar to the corrosion of metals. However, if this layer is removed (e.g. by water if the material is exposed to rain or running water), the material will gradually degrade. In addition, micro-organisms can penetrate the internal structure of concrete through microcracks or capillary structures that form within it, causing degradation of its internal structure [15].
Sulfuric acid is also destructive to geopolymers. It is believed to react with cations in the geopolymer (e.g. sodium, calcium, etc.) and directly break the Si-O-Al polymer bonds. As in Portland cement, the reaction of sulphate anions and calcium cations produces calcium sulphate, which forms a protective layer on the surface. Unlike cement, calcium sulphate does not form a uniform layer but forms on the surface in more layers parallel to the surface. Furthermore, its crystals also form in the pores of the geopolymer, where they induce internal stresses that cause the corroded layer to crack, but this effect is not strong enough to cause peeling. The effect of sulfuric acid on geopolymers is similar to its effect on cement, but geopolymers may be more susceptible to damage due to their more porous structure, especially if they are also foamed [16] (Figure 2).
Bacteria
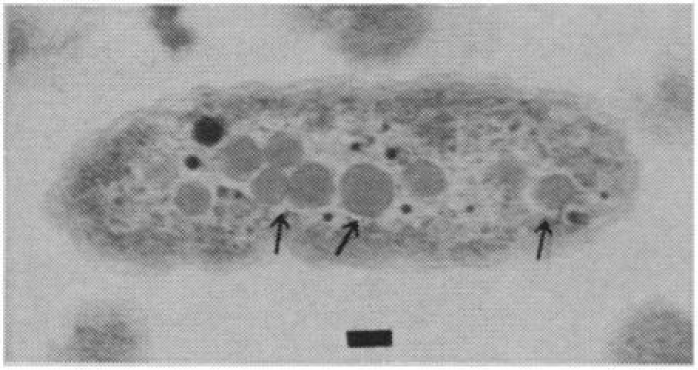
Bacteria are single-celled organisms with sizes of tens or units of micrometres. They are prokaryotic organisms and therefore do not have a cell nucleus (their nucleoid floats freely in the cytoplasm) or organelles, and their ribosomes differ from those of eukaryotic cells. They are virtually ubiquitous in nature and play an irreplaceable role (e.g. in the decomposition of organic matter and recycling of nutrients or the capture and fixation of atmospheric nitrogen into forms usable by other organisms). The main threat to building materials is bacteria that obtain energy by oxidising sulphur (sulphur-oxidising bacteria), as some species are able to oxidise it to biogenic sulphuric acid, which then lowers the pH of the surface (allowing other micro-organisms to colonise it), while also forming calcium sulphate on the surface or in cracks, which can be easily washed away by water (Figure 3). This degradation is most commonly caused by the genus Thiobacillus (intermedius, neapolutanus, novellus, etc.), which is a major participant in the sulphur cycle in nature but can also be caused by bacteria such as Acidithiobacillus thiooxidans or Thiomonas perometablis [19].
Moreover, dangerous to building materials are nitrifying bacteria (e.g. the genus Nitrosomonas), which in nature ensure the oxidation of ammonia (released during certain decomposition processes) to nitric or nitrous acid (HNO2 and HNO3), which then react with ammonia to form ammonium nitrite (NH4NO2) and ammonium nitrate (NH4NO3). If these bacteria are found on concrete exposed to water (e.g. in sewerage systems, where ammonia is also abundant, or on the facades of buildings exposed to rain), with the sulphur-oxidising bacteria, the resulting acids react with calcium to form highly soluble salts, namely calcium nitrite (Ca(NO2)2) and calcium nitrate (Ca(NO3)2), which are then washed away by water, leading to the gradual degradation of the cement. This phenomenon was observed, for example, in Czechoslovakia on the roofs of agricultural buildings used to house animals and constructed with asbestos cement. The likely cause is the high concentration of ammonia in these buildings. Nitrifying bacteria also cause the degradation of sandstone monuments and facades of historical buildings such as cathedrals [20,21].
Gram staining
This frequently used method (Gram staining) is named after the Danish microbiologist Hans Christian Gram, who discovered it in 1884. It involves using specific dyes to stain bacteria, which are stained dark purple (Gram-positive) or red (Gram-negative). Gram-positive and Gram-negative bacteria differ in the composition and structure of the bacterial wall [22].
Different staining solutions are applied sequentially to the bacterial sample in Gram staining, according to the abbreviation VLAS (or VLAK).
- Crystal violet (also hexamethylparosaniline chloride or gentian violet)
- Lugol's solution (solution of elemental iodine and potassium iodide)
- Alcohol
- Rinse with water
- Safranin or Carbolfuchsin
The cell wall of Gram-positive bacteria (also G+ bacteria) consists of peptidoglycan and polysaccharides, through which teichoic acid (polysaccharides) passes. Crystal violet passes through the cell wall and forms a blue coloured complex with the lye solution. Alcohol does not penetrate the cell wall and cannot dissolve the complex. Safranin gives the bacteria a dark purple colour. Gram-positive bacteria include the genera Streptococcus, Clostridium, Listeria and Bacillus.
The cell wall of Gram-negative bacteria consists of a peptidoglycan layer and a lipopolysaccharide layer, through which alcohol can penetrate, thus leaching the complex in the third step and decolourising the bacteria. Safranin then stains them red. Gram-negative bacteria (also G-bacteria) are Enterobacter, Pseudomonas, Salmonella, Escherichia coli and Helicobacter pylori.
Some bacteria can change status from G+ to G- after long culture or surviving exposure to certain antibiotics. Bacteria with a high content of fatty acids and waxes in the cell wall (e.g. Mycobacterium tuberculosis) may not stain at all on Gram staining.
Gram staining is used in microbiology or medicine to label bacteria to facilitate their observation under a microscope or as a test for their presence, especially in the diagnosis of an ongoing bacterial infection, for example, to enable the rapid determination of the appropriate antibiotic [23].
Algae
Algae are aquatic, eukaryotic organisms capable of photosynthesis. Although they are often compared to plants, the similarities are few. They are a polyphyletic group of organisms that do not share a common ancestor. Their species can vary considerably, but they have in common primarily their ability to photosynthesise (and therefore their chlorophyll content), their eukaryotic cell and their marked susceptibility to desiccation. Most can only live in water or very moist environments (making them an ecological rather than a taxonomic group). However, some can exist in symbiosis with the fungus with which they form a lichen under dry conditions. Algae are sometimes classified as cyanobacteria or photosynthetic bacteria, but they are prokaryotic organisms. There are unicellular, multicellular, and species that form a stem and are therefore very similar to plants. Although they have no roots, usually only microscopic algae can survive on land [24].
Concrete on land is particularly susceptible to colonisation by algae if it is wet for long periods. Although algae do not produce harmful corrosive substances in concrete, they are able to penetrate the entire concrete structure and, among other things, increase its porosity, which can accelerate surface wear and promote degradation caused by other micro-organisms. In particular, diatoms (Diatomeae) possess this property [15].
If concrete is underwater, especially seawater, algae can directly cause its degradation. This is caused by species such as Chaetomorpha antennina or Ulva fasciata, which can decompose and digest concrete to extract calcium or other substances. Ulva fasciata could completely remove calcium and alumina from concrete under laboratory conditions, while Chaetomorpha antennina removed some crystalline phases such as portlandite (calcium hydroxide) [25,26] (Figure 4).
Fungi
Fungi are eukaryotic organisms that were previously classified as plants, but unlike plants, they are not capable of photosynthesis, which means they must feed heterotrophically (like animals), and they differ in cell structure, for example they use chitin rather than cellulose to build their cell walls. They have other features in common with animals, for example they have lysosomes in their cells, they use glycogen as a storage substance and the end product of their metabolism is urea. Moreover, their poisons are similar to those of animals. They share with plants, for example, the presence of a cell wall. As with algae, there are unicellular and multicellular species. In nature, they usually occur as decaying organisms, parasites (of both plants and animals) or live in mutualistic relationships with higher organisms (mycorrhiza). Some of them live in a specific symbiosis with algae or cyanobacteria; such a community is called a lichen [27,28].
Considerable moisture makes it easier for fungi, as well as bacteria and algae, to colonise the concrete surface. They are dangerous to concrete for several reasons. Firstly, because of their ability to produce organic acids such as acetic acid, oxalic acid and glucuronic acid, and the calcium present in concrete increases the production of these acids. These acids can dissolve concrete or alter its composition; for example, oxalic acid reacts with calcium compounds to form insoluble calcium oxalate. Another threat is the ability of some fungi to leach calcium, silicon, aluminium and iron from concrete, leading to a measurable reduction in total mass and a change in mechanical properties. A third threat is the ability of fungi to grow through concrete, resulting in larger scale degradation [29,30].
Due to these properties, concrete degradation by fungi can be faster than by bacteria, which also induces it by forming biogenic acids. For example, the bacterium Thiomonas intermedia has been found to degrade concrete at a much slower rate than fungi of the genus Fusarium (sickle cell), even though this bacterial species is very aggressive towards concrete and degrades it rapidly (Figure 5). Other species that cause concrete degradation include Penicillium oxalicum, Aspergillus niger and the genera Mucor, Alternaria and Exophiala [15,30].
Lichens
Lichens, also called lichenised fungi, are symbiotic organisms consisting of a mycobiont (usually an algal fungus) and a photobiont (a green alga or cyanobacterium), with the mycobiont usually forming the majority of the stem. The mycobiont supplies the whole organism with inorganic matter and water and provides protection, while the photobiont produces organic matter through photosynthesis. Algae can survive in dry environments without drying out, thanks to the protection provided by lichens. Lichens are extremely hardy and can survive in habitats with extreme living conditions, e.g. in high or low temperatures or the absence of nutrients (e.g. on bare rocks, tree trunks or walls). However, they are sensitive to environmental pollutants (e.g. the presence of sulphur dioxide in the atmosphere) and can be used as bioindicators [32].
The ability of lichens to biodegrade is the responsibility of the mycobiont, which is in direct contact with the substrate. Biodegradation is usually the result of a combination of physical (pressure exerted by the litter, penetration into the structure, etc.) and chemical factors, such as its own production of organic acids or substances with complexing properties. One possible mechanism is the production of oxalic acid, which is able to react with ions in the substrate to form oxalates (calcium, iron, etc.), which can change the appearance of the surface but, unlike sulphates, do not lead to gradual saponification as they are poorly soluble. At the same time, there is a risk of delamination due to changes in physical properties combined with mechanical interactions of the lichen. Therefore, lichens generally pose less of a threat to building materials, especially their epilithic species, which only form a crust on the surface without penetrating the structure. However, there are also dangerous species, such as Dirina massiliensis f. sorediata, found in coastal areas, although it is spreading rapidly in Europe due to atmospheric pollution, destroying other lichen species. This species can penetrate the substrate up to 20 mm and, with oxalic acid, form calcium oxalate, which can corrode the surface and lead to biodegradation (Figure 6). Another problem is that the effect of lichens on building materials is often underestimated, due to their slow growth, not only on ordinary building materials but also on monuments and sculptures [34].
Anti-microbial protection of building materials
Since micro-organisms capable of colonising the surface create, among other things, suitable conditions for other micro-organisms by lowering the pH, it is necessary to prevent any contamination of the surface and prevent its colonisation, for example, by applying a protective layer or by strengthening the natural anti-microbial properties of concrete and geopolymers (due to their alkalinity). The protection of building materials, for example, cleaning, is not worth considering since it may not be able to remove micro-organisms that have already penetrated the internal structure. Nevertheless, it could help to degrade them, especially if the building material is infested with micro-organisms that produce quickly soluble compounds employing their biogenic acids, such as the bacteria of the thiobacillus, which make easily soluble calcium sulphate through sulphuric acid. Moreover, cleaning does not solve the problem of the need for prevention.
Directions
The first way to protect the building material is to add the protective agent to one of the components (powder or a liquid mix - for geopolymers) or to add it to the liquid mix during the manufacturing process. The aim is to ensure that the protective additives are evenly distributed throughout the building material's structure, ensuring that micro-organisms cannot penetrate and that the building material retains its anti-microbial properties even if the surface is damaged. Several inorganic and organic substances can be used as anti-microbial agents. Many of the substances used in manufacturing building materials for their anti-microbial properties can also be used to produce special mortars, which are then used only to treat the surface [10].
Among inorganic substances, metals and their oxides (e.g. silver, copper or nickel) are mainly used as anti-microbial substances, both in pure form and in the form of oxides. Other metal compounds (silver molybdate, sodium tungstate and bromide) or special commercial products are also used. The relative anti-bacterial activity of metals and their nanoparticles are in the following order: Ag > Hg > Cu > Cd > Cr > Ni > Pb > Co > Zn > Fe [35].
Among organic substances, phthalocyanines (for example, the copper-phthalocyanine as mentioned earlier - phthalocyanine blue BN), calcium formate, quaternary ammonium compounds or various commercial products (for example, ConBlock MIC or ConShield) are used [35].
Generally, inorganic anti-microbial agents have higher environmental and temperature resistance, which provides them with higher durability and the building materials treated with them retain their anti-microbial properties longer. On the other hand, there is a risk of toxicity and the release of toxic substances into their surroundings. Organic anti-microbial agents have little resistance to higher temperatures, and micro-organisms can become resistant to them, which does not make them suitable candidates for anti-microbial treatment of building materials [35].
Nanoparticles
Nanoparticles are particles with dimensions in the range of 1-100 nm, with a high specific surface area (Surface area per unit mass), giving them unique physical, optical and chemical properties [36].
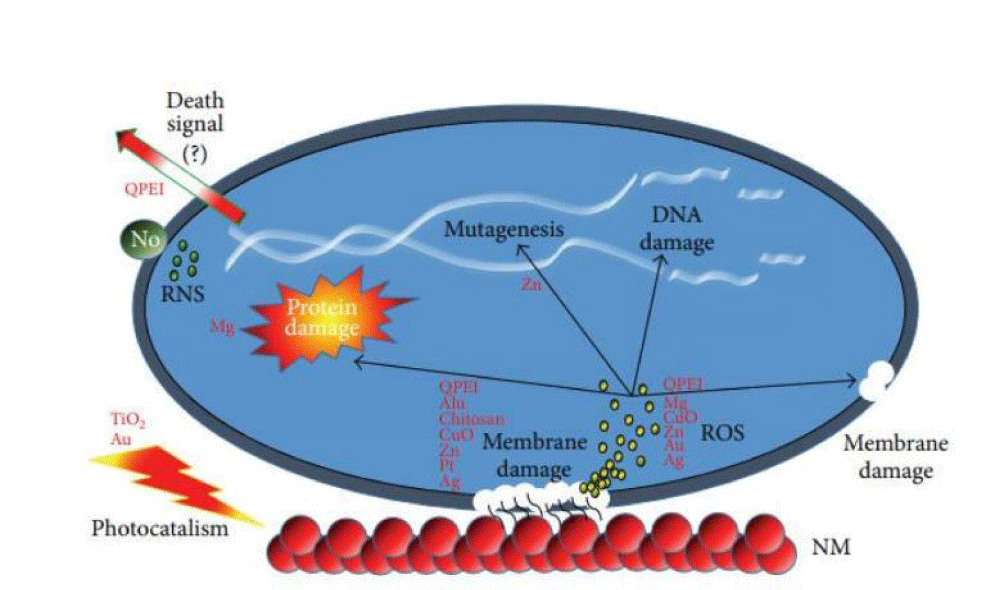
For anti-microbial applications, nanoparticles made of metals and their oxides are mainly used, which, when in contact with cells (e.g. bacterial), can disrupt the electrostatic potential and integrity of their membranes and generate highly Reactive Oxygen Species (ROS) (e.g. oxygen radicals or hydrogen peroxide) that damage DNA and proteins. However, this effect also works on non-microbial cells, making nanoparticles potentially dangerous to the environment, human life, and health. For example, silver nanoparticles (or colloidal silver) can cause a disease called argyria, accumulating under the skin and causing it to turn irreversibly grey-blue [37] (Figure 7).
Nonetheless, silver nanoparticles are also often investigated as a possible anti-microbial admixture in building materials, as they exhibit the highest anti-microbial properties of all metals, which they retain even at low concentrations. However, their main disadvantage is their high cost. In a study investigating the properties of a geopolymer admixed with silver nanoparticles prepared by bioreduction of silver nitrate, it was found that, regardless of the concentrations used, such a geopolymer was able to effectively inhibit the proliferation of various bacterial species (no specific strain was tested, the samples were only exposed to a typical environment) on its surface. However, it did not completely destroy them. It was also found that the admixture of silver nanoparticles did not change the thermal conductivity of geopolymers (silver as a metal has high thermal conductivity, while geopolymers have a very low one), thanks to which geopolymers treated in this way can be easily used as thermal insulation (one of the common uses of geopolymers) [39].
Titanium dioxide particles are also often used and studied mainly for their photocatalytic properties; irradiating with UV light produces hydroxyl radicals and oxygen radicals, increasing their anti-bacterial properties and allowing self-cleaning surfaces, as they can also act as a catalyst for the decomposition of organic substances. In testing the effect of titanium dioxide nanoparticles on the growth of green algae and fungi of the species Aspergillus niger, a 54% reduction in algal growth and 24% reduction in fungal growth were measured when 5 wt.% of titanium dioxide nanoparticles were used. Only a slight deterioration in mechanical properties (mainly compressive strength) was also measured [40].
However, in another study on the changes in mechanical properties of geopolymers with the addition of titanium dioxide nanoparticles, it was observed that a higher content of this admixture could also improve the mechanical properties. Specifically, at 10 wt% of titanium dioxide nanoparticles, an increase in tensile strength of 41% was observed compared to the control sample [41].
These studies differed in the geopolymer base used. In the first study (decrease in compressive strength), samples of fly ash geopolymer were tested, while in the second one (increase in compressive strength), samples of metakaolin geopolymer were tested. There is also no deterioration in mechanical properties when used in concrete production [42].
In addition to nanoparticles or other nanostructures, nanohybrids, i.e. nanomaterials consisting of different components, can also be used. In a study dealing with the increase of the durability of geopolymer, the addition of zinc oxide nanotubes on the surface of which silica (silicon) nanoparticles were bonded was tested. The geopolymer thus modified exhibits resistance to biodegradation (by forming reactive oxygen species, disrupting cell membranes and damaging DNA), and the presence of the nanotubes resulted in improved compressive strength compared to samples with the addition of silica alone [43].
Surface protection
It is possible to cover only the surface with an anti-bacterial substance to protect the building material or at least resist the influence of biogenic acids. Commonly used materials are mortars, i.e. hardening mixtures consisting of water, sand, lime, cement or other additives, and varnishes. Mortars have a similar or identical composition to concrete or geopolymer cement, which makes them susceptible to microbially induced degradation, but they can be modified with anti-microbial admixtures to prevent surface colonisation. Assuming that the admixture used is expensive, using a mortar or paint can significantly reduce the cost of protecting the building material. However, if the paint or mortar is washed off or delaminates, the building material is left without protection, which means additional costs for reapplying the protective layer. Epoxy varnishes (e.g. Sikagard 62 or Ameron), epoxy-modified mortars (e.g. Sikagard 75 Epocem), a latex-modified mortar was prepared for this study, and a commercial mortar designed for use in the sewer system (SewperCoat), consisting of calcium aluminate cement and calcium aluminate aggregate, branded as Alag. Concrete samples modified with these substances were subsequently exposed to Thiobacillus ferrooxidans, and the durability of each protective layer was evaluated. The epoxy varnishes and calcium aluminate mortar showed the highest resistance, with no biofilm formation even after 60 days. However, some showed discolouration or blistering, which may indicate a possible disruption of the protective layer with prolonged exposure to the bacteria. Epoxies or latex-modified mortars also increased the resistance of the concrete to bacterial attack compared to control concrete samples of different compositions without surface treatment (of which the samples with blast furnace slag and silica fume (ultrafine silica fume) were the least susceptible to biodegradation). However, these layers did form biofilms and degraded them [44].
In another study, other surface treatment options were tested to resist biodegradation in wastewater. Epoxy varnish with coal tar admixture, mortar modified with CCCWC (cement-based capillary crystalline waterproofing coating) - a mixture consisting of alkali metal salts, complex compounds and other substances - was used to coat Portland cement samples, which is designed to provide waterproofing and to fill and seal cracks and pores in concrete, which can also prevent bacteria from penetrating) Furthermore, mortar was modified with bactericidal agents (BN phthalocyanine blue - copper phthalocyanine, copper oxide and potassium nitrate in a ratio of 1:1:1). These samples were then exposed to an artificially generated wastewater concentrate for 60 days, and weight loss and other parameters were measured. It was found that of the three surface treatments, the epoxy varnish with coal tar admixture provided the highest resistance to biodegradation, with the lowest weight loss, but blistering was also observed on its surface. Mortar with bactericidal admixtures was also evaluated as a good way to limit concrete biodegradation. However, the waterproof mortar increased the resistance of the concretes to biodegradation only slightly compared to the control sample [45].
Metal nanoparticles or hybrid nanomaterials can also be used in surface protection to provide anti-microbial properties. In a study on developing an anti-bacterial geopolymer mortar, silver nanoparticles of 3-7 nm were used, which were adsorbed onto the surface of nanosilica (silica particles of 20-50 nm) and used as an admixture in the production of the geopolymer. Subsequently, their anti-bacterial properties were tested. The mortar exhibited bactericidal properties even at very low concentrations of silver-modified silica. Specifically, its MBC (Minimum Bactericidal Concentration) was determined to be 0.43 µg/ml for the elimination of Staphylococcus aureus, i.e. Golden Staphylococcus (a gram-positive bacterium known for its ability to cause difficult-to-treat infections and resistance to antibiotics) and 0. 32 µg/ml to eliminate Escherichia coli (gram-negative bacteria). The MIC (Minimum Inhibitory Concentration) was set at 0.15 µg/ml for S. aureus and 0.1 µg/ml for E. coli. In addition, it was also found that the use of silver functionalised silica did not alter the mechanical properties of the mortar compared to the use of an equivalent amount of silica alone [46-48].
In another study, the anti-bacterial properties of a geopolymer mortar admixed with nanoparticles of titanium dioxide (10 wt. %) and copper oxide (5 wt. %) were tested on Staphylococcus aureus, Pseudomonas aeruginous and Escherichia coli . It was found that the addition of titanium dioxide nanoparticles inhibited the population growth of these bacterial species; however, the addition of copper oxide nanoparticles (at a given concentration) did not [49].
Conclusion
- Although geopolymeric and ordinary concretes have a highly alkaline structure, micro-organism creates biofilm on their surface as they age.
- In the long term, micro-organisms cause concrete degradation and corrosion, leading to significant defects in the structural matrix.
- Additives of inorganic (silver, copper, nickel and their oxides) or organic compounds as anti-bacterial agents in the mortar mixture provide excellent protection against biological activity on the surface of the concrete.
- Nanoparticles additives such as TiO2 with photocatalytic activity show highly anti-bacterial and self-cleaning properties.
- Another method to prevent biofilm formation on the concrete is surface treatment with epoxy or latex-modified varnish. Nanoparticles can also be used as surface coating materials.
Acknowledgement
This work was supported by the Student Grant Competition of the Technical University of Liberec under project no. SGS-2022-5066. In addition, the publication was supported by the project "Antibacterial Coatings Containing Carbon Nanoparticles Obtained by Sol-Gel Method" registration number TH71020001 was obtained with financial support from the Technology Agency of the Czech Republic under the Epsilon programme, in the call M-ERA.Net2.
Funding
This publication was supported by the project "Antibacterial Coatings Containing Carbon Nanoparticles Obtained by Sol-Gel Method" registration number TH71020001 was obtained with financial support from the Technology Agency of the Czech Republic under the Epsilon programme, in the call M-ERA.Net2.
References
- Gillar Vaclav. Geopolymers: Production, properties and use. Ostrava. Bachelor thesis. 2013.
- Ruzek Vojtech. Effect of flue gas additives and plasma treatment on the surface properties of geopolymers. Liberec. 2020.
- The mystery of the durability of Roman concrete. PETR J. 2011.
- Dufkova. Special composite materials for construction. Liberec. 2011.
- Luukkonen T, Heponiemi A, Runtti H. Application of alkali-activated materials for water and wastewater treatment: a review. Rev Environ Sci Biotechnol. 2019;18:271–297.
- Fly Ash Facts for Highway Engineers. Federal highway administration.
- Ling T, John VH, Yuan LL, Mark ES, Jerry CC. Solid-state NMR study of geopolymer pre-pared by sol–gel chemistry. Journal of Solid State Chemistry. 2010;183(12):3017-3022.
- Quyen VT, Gábor M, Thai D, Van HB, Sandor N. The influence of process conditions on ground coal slag and blast furnace slag based geopolymer properties. Rudarsko-geolosko-naftni zbornik. 2020;35:15-20.
- Davidovits PJ. Properties of Geopolymer Cements. Alkaline Cem Concr. 1994;131–149.
- Components of cement. 2020.
- AWANG, Hanizam, Muhammad AH. Influence of kenaf and polypropylene fibres on mechanical and durability properties of fibre reinforced lightweight foamed concrete. Journal of Engineering Science and Technology. 2015.
- Composition of cement. PennState College of Engineering. 2020.
- Le Chi. Study of heat resistance of fire barriers using composites based on geopolymers. Liberec. 2015.
- Geopolymer Valley. Threads go to the Czech Republic. 2007.
- Wei S, Jiang Z, Liu H, Zhou D, Sanchez SM. Microbiologically induced deterioration of concrete: A review. Braz J Microbiol. 2014;44(4):1001-1007.
- Allahverdi, Skvára F. Sulfuric acid attack on hardened paste of geopolymer cements Part 1. Mechanism of corrosion at relatively high concentrations. Ceramics. 2005;49:225-229.
- Cyrill. Microbial induced acid corrosion in sewer environments. 2017.
- Shively, Jessup, Brown, Saunders R. Functional organelles in prokaryotes - polyhedral inclusions (Carboxysomes) of Thiobacillus Neapolitanus. 1973;182:584-586.
- Bacteria. 2020.
- Nitrification. In: Wikipedia: The free encyclopedia. San Francisco (CA): Wikimedia Foundation. 2001.
- Wasserbauer R, Zadák Z, Novotny J. Nitrifying bacteria on the asbestos-cement roofs of stable buildings. International Biodeterioration. 1988;24(3):153–165.
- Hans Christian Gram. San Francisco (CA). Wikimedia Foundation. 2022.
- Tripathi N, Sapra A. Gram Staining. Stat Pearls. Treasure Island (FL): Stat Pearls Publishing. 2022.
- Algae. In: Wikipedia: the free encyclopedia. San Francisco (CA): Wikimedia Foundation. 2001.
- Jayakumar S, Manakula S. Effect of macro algae ulva fasciata on concrete structures. International Journal of Physical Sciences. 2012:7.
- Jayakumar, Sriharibabu, Saravanane, Raman. Biodeterioration of coastal concrete structures by Macro algae - Chaetomorpha antennina. Materials Research. 2009;12(4):465-472.
- How are fungi different from plants and other organisms? Sodium Media. 2020.
- Fungi vs. Plants. Biology dictionary. 2020.
- Fomina, Marina P, Olishevska, Snizhana, Pisanska, Hillier, Stephen. Fungal deterioration of barrier concrete used in nuclear waste disposal. Geomicrobiology Journal. 2007;24:643-653.
- Dong G, Tim F, Neal B, Ralph M. Biodeterioration of concrete by the fungus fusarium. International Biodeterioration & Biodegradation. 1998;41(2):101-109.
- What does black mold look like in various surfaces? Clean Water Partners. 2020.
- Lisejniky. Educanet ostrava. 2020.
- Dirina massiliensis f sorediata. In: Dorset Nature. 2020.
- Salvadori, Ornella, Casanova, Annalaura. The role of fungi and lichens in the biodeterioration of stone monuments. The Open Conference Proceedings Journal. 2016;7:39-54.
- Qiu, Liangsheng, Dong, Sufen, Ashour, Ashraf, Baoguo. Anti-microbial concrete for smart and durable infrastructures: A review. Construction and Building Materials. 2020;260:120456.
- What Are Nanoparticles? Definition, size, uses and properties. Twi Global. 2017.
- Ruzek, Vojtech. Antibacterial effect of nanoparticles, positive and negative effects. Dspace TUL. 2020.
- Beyth N, Haddad Y, Domb A, Khan W, Hazan R. Alternative anti-microbial approach: Nano-antimicrobial materials. Evid Based Complement Alternat Med. 2015;2015:246012. doi: 10.1155/2015/246012. Epub 2015 Mar 16. PMID: 25861355; PMCID: PMC4378595.
- Armayani M, Pratama, Muhammad, Subaer. The properties of nano silver (Ag)-geopolymer as antibacterial composite for functional surface materials. Matec Web of Conferences. 2017;97:01010.
- Tuntachon, Soebpong, Kamwilaisak, Khanita, Somdee, Theerasak, Mongkoltanaruk, Wiyada S, Vanchai, Kornkanok, Ampol, Prinya. Resistance to algae and fungi formation of high calcium fly ash geopolymer paste containing TiO2. Journal of Building Engineering. 2019;25:100817.
- Ambikakumari S, Krishnan U, Yang. Self-cleaning performance of nano-TiO2 modified metakaolin-based geopolymers. Cement and Concrete Composites. 2021;115:103847.
- Bibova L, Hana S, Pližingrová E, Jakubíčková M, Sázavská T, Dohnalek P, Lenka, Jirkovský J. Photocatalytic concrete screeds with self-cleaning and antimicrobial function. Open Access Conference Proceedings. 2020;157-162. 10.37904/nanocon.2019.8515.
- Sarkar, Manas, Maiti, Moumita, Soumen, Shilang, Qinghua. ZnO-SiO2 nanohybrid decorated sustainable geopolymer retaining anti-biodeterioration activity with improved durability. Materials Science and Engineering. 2018;92.
- Berndt, Marita. Evaluation of coatings, mortars and mix design for protection of concrete against sulphur oxidising bacteria. Construction and Building Materials. 2011;25:3893-3902.
- Kong, Lijuan, Jun Z, Bei. Effectiveness of surface coatings against intensified sewage corrosion of concrete. Journal of Wuhan University of Technology-Mater. 2019;34:1177-1186.
- Adak, Dibyendu, Manas, Moumita, Abiral, Saroj, Brajadulal. Anti-microbial efficiency of nano silver–silica modified geopolymer mortar for eco-friendly green construction technology. RSC Advances. 2015:64037-64045.
- Láník Pavel. Geopolymer composite systems and their resistance to mechanical stress. Liberec. 2014.
- Topinkova M. Possibilities of modification of hydration processes and alkaline activated binders. Ostrava. 2011.
- Gutierrez, Villaquiran, Monica, Ramirez B, Astudillo, Mejia, Ruby. Evaluation of the Antibacterial Activity of a Geopolymer Mortar Based on Metakaolin Supplemented with TiO2 and CuO Particles Using Glass Waste as Fine Aggregate. Coatings. 2020;10:157.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.