Medicine Group . 2022 September 30;3(9):1118-1124. doi: 10.37871/jbres1564.
Vernonia amygdalina Delile Induces Apoptotic Effects of PC3 Cells: Implication in the Prevention of Prostate Cancer
Clement G Yedjou1*, William Johnson2, Solange S Tchounwou3, Shaloam Dasari2, Sylvianne Njiki2 and Paul B Tchounwou2
2Department of Biology, College of Science, Engineering and Technology, Jackson State University, 1400 Lynch Street, Box 18750, Jackson, MS 39217, United States
3Department of Pathology and Laboratory Medicine. School of Medicine, Tulane University, 1430 Tulane Avenue, New Orleans, LA, 70112, United States
- Vernonia amygdalina Delile
- Prostate cancer
- Cell cycle arrest
- Apoptosis
- DNA fragmentation
Abstract
Background: Prostate Cancer (PCa) is one of the common cancers in males and its incidence keeps increasing globally. Approximately 81% of PCa is diagnosed during the early stage of the disease. The treatment options for prostate care include surgery, radiotherapy, and chemotherapy, but these treatments often have side effects that may lead to issues such as impotence or decreased bowel function. Our central goal is to test the apoptotic effects of Vernonia amygdalina Delile (an edible medicinal plant that is relatively inexpensive, nontoxic, and virtually without side effects) for the prevention of PCa using human adenocarcinoma (PC-3) cells as a test model.
Methods: To address our central goal, PC-3 cells were treated with Vernonia amygdalina Delile (VAD). Cell cycle arrest and cell apoptosis were evaluated by Flow Cytometry assessment. Nucleosomal DNA fragmentation was detected by agarose gel electrophoresis.
Results: Flow cytometry data showed that VAD induced cell cycle arrest at the G0/G1 checkpoint and significantly upregulated caspase-3 in treated cells compared to the control cells. Agarose gel electrophoresis resulted in the formation of DNA ladders in VAD-treated cells.
Conclusion: These results suggest that inhibition of cancer cell growth, induction of cell cycle arrest, and apoptosis through caspase-3 activation and nucleosomal DNA fragmentation are involved in the therapeutic mechanisms of VAD as a candidate drug towards the prevention and/or treatment of PCa.
Abbreviations
AO: Acridine orange; Bcl-2: B-cell Lymphoma 2; Bcl-xl: B-cell lymphoma extra large; BID: BH3 Interacting Death Domain Agonist; DNA: Deoxyribonucleic Acid; MAPK: Mitogen-Activated Protein Kinase; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide; PARP: Poly (ADP-ribose) Polymerase; PC-3: Human Prostate Cancer Cells; PCa: Prostate Cancer ; PI: Propidium Iodide; µg/mL: Microgram Per Milliliter; VAD: Vernonia amygdalina Delile
Background
Prostate Cancer (PCa) is the most common noncutaneous cancer and the second leading cause of cancer-related deaths in men [1]. The average age of PCa diagnosis is 66 and it rarely occurs before the age of 40. Due to early diagnostic and therapeutic advances, the number of deaths from PCa continues to decline among all men. However, the death rate remains twice as high among African American men than any other group [2]. In 2015, an estimated 164,690 men were diagnosed with PCa and approximately 29,430 men died from this disease in the United States [3]. In addition, most deaths from PCa are due to metastases that are highly resistant to current conventional therapies [4,5]. Even when it is treated, PCa is more likely to return, especially in the first few years post-treatment because cancer cells can reemerge and develop a fresh tumor, which in many cases can manifest in a different organ due to metastasis. Among men alive today, it is estimated that 1 in 6 White men and 1 in 5 African American men will be diagnosed with the PC in their lifetime and approximately 1 in 33 will die of it [6]. In general, White and African American men have relative a high rate of PCa compared to Asian populations who regularly consume medicinal plants [7]. The treatment options for PCa are surgery, radiotherapy, and chemotherapy. However, these treatments are ineffective once the tumor has metastasized and PCa is currently responsible for about 20% of cancer-related deaths in men in the United States. Therefore, there is an urgent need for the discovery and development of agent(s) efficacious against PCa. Studies have shown that there are alternative treatments other than current therapeutics that might be effective in the management of PCa. One known alternative treatment is the use of medicinal plants that possess antitumor activity against PCa. Vernonia amygdalina Delile (VAD) is a valuable edible medicinal plant that is widespread in West Africa and it is well appreciated in Cameroon for its nutritional and medicinal properties [8,9]. Many herbalists and native doctors in West Africa recommend aqueous extracts of VAD to patients for the treatment of human diseases [10,11]. A 2018 report from our research laboratory demonstrated the antiproliferative effect of VAD in PC-3 and kidney cells [12]. The advantage of using edible medicinal plants over synthetic agents for preventing/treating cancer is that they promote human health without recognizable side effects. In this regard, preliminary data in our laboratory have shown that VAD crude extract inhibits the growth of cancer cells (HL-60, MCF-7, and PC-3 cells), while not affecting normal cells (HEMC) [9,12]. VAD possesses many micronutrients important to the maintenance of human health and prevention of various diseases. However, there are limited studies in the literature about the benefit of medicinal property of VAD towards the prevention and/or treatment of patients with PCa. Therefore, the present work was designed to evaluate the apoptotic mechanisms of VAD towards the prevention and/or treatment of PCa.
Methods
Chemicals and media
Culture plates, flasks, test tubes, Acridine Orange and Propidium Iodide (AO/PI) were purchased from Sigma-Aldrich INC (St. Louis, MO). Caspase-3, and cell cycle assay kits were obtained from BD Biosciences (Pharmingen, Becton Dickinson Co., San Diego, CA, USA).
Vernonia amygdalina Delile Preparation: The preparation of Vernonia amygdalina Delile was conducted as previously described [9].
Tissue/cell culture
The human Prostate Cancer (PC-3) cells obtained from the American Type Culture Collection (Manassas, VA) were cultured in Kaign’s Modification of Ham’s F-12K medium, supplemented with 10% Fetal Bovine Serum (FBS), and 0.1% penicillin/streptomycin solution (Sigma-Aldrich, Inc., St. Louis, MO) and grown in an incubator at 37°C in 5% CO2.
Cell cycle distribution determination
Control (untreated) and VAD-treated cells were harvested and washed twice with phosphate-buffered saline. Cell pellets were collected and fixed in 70% cold methanol for 10 minutes. After fixation, cells were stained with 1 mg/mL propiduim iodide and then treated with DNase free-RNase (100 µg/mL) for 15 minutes as previously described [13].
Caspase-3 activity determination
Human Prostate Cancer (PC-3) cells were plated in 6-well plates at a density of 1x106 cells/well and grown to reach 85% confluence. The cells were treated with various doses of VAD. Finally, cells were harvested and lysed to determine caspase-3 activity, as previously described in our laboratory [14].
DNA fragmentation assessment
Control (untreated) and VAD-treated cells were seeded in 6 well plates for 48 hours. After treatment, cells were gently trypsinized, harvested, washed with phosphate-buffered saline. The oligonucleosomal DNA fragments were isolated and analysed as previously described in our laboratory [14]. DNA in the gels was visualized under ultraviolet light after staining with ethidium bromide and photographed.
Statistical analysis
The data are presented as means ± SEMs of three independent experiments. The data were analyzed when possible, using t-test or one-way ANOVA, followed by Turkey pair wise comparisons. p < 0.05 was considered statistically significant.
Results
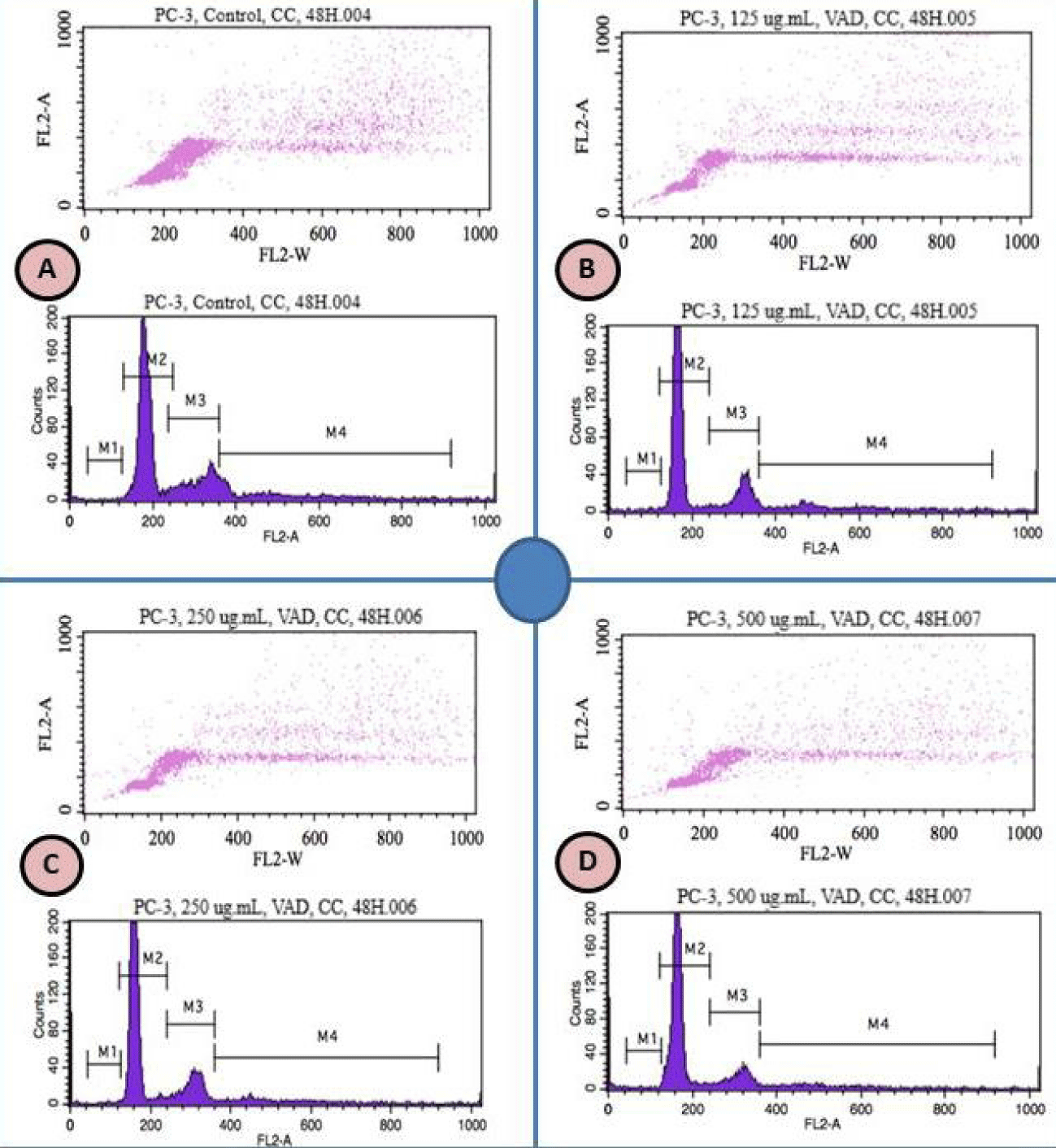
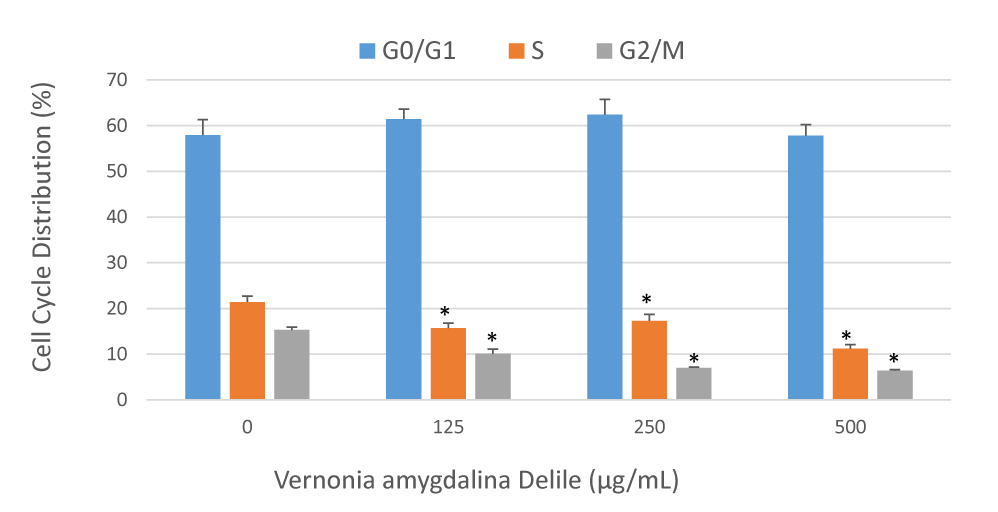
Vernonia amygdalina Delile induced cell cycle arrest in PC-3 cells
A recent study in our laboratory has indicated that VAD inhibits cell growth and induces DNA damage of PC-3 cells [9]. Based on this indication, we investigated whether VAD induced DNA damage in PC-3 cells is mediated through cell cycle arrest. We treated PC-3 cells with increased concentrations of VAD and identified the percentages of cells in different phases of the cell cycle by flow cytometry. We found that VAD induced a concentration-dependent cell cycle arrest at the G0/G1 checkpoint after 48 hours of treatment (Figure 1). We concluded that VAD induced DNA damage is mediated through cell cycle arrest at the G0/G1 checkpoint (Figure 2).
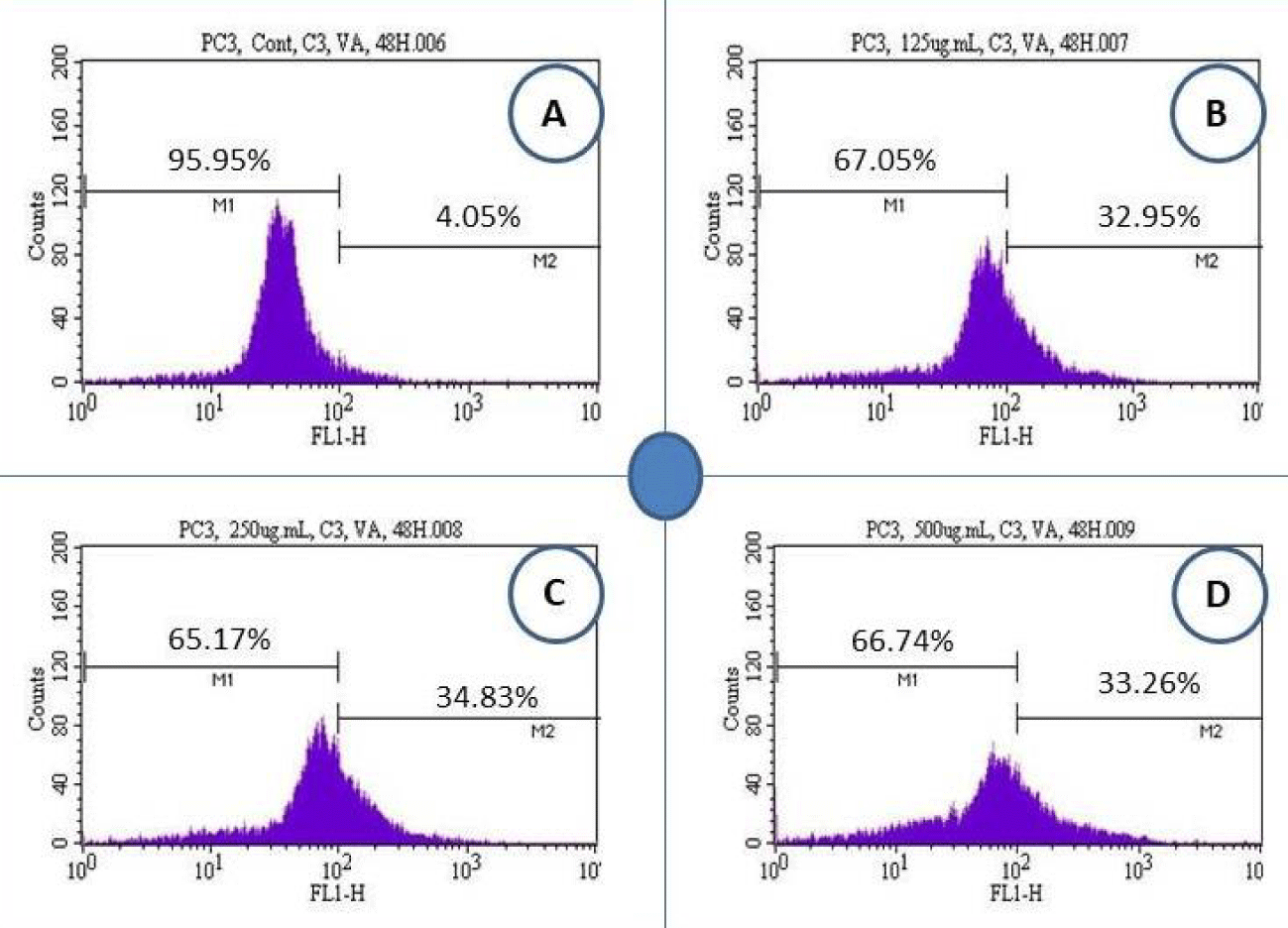
Vernonia amygdalina Delile induced caspase-3 activity in PC-3 cells
We analyzed PC-3 cells for caspase-3 activity upon 48 hours’ treatment with VAD crude extract. We detected marked caspase-3 activation in VAD crude extract-treated cells compared to the control (Figure 3).
Table 1 shows the summary data of caspase-3 assay obtained from the flow cytometry analysis. Values are shown as means ± SDs of 3 replicates per experiment. *Significantly different at p < 0.05 to the control group. As seen in table 1, after 48 hours of cells treatment with VAD, the percentages of caspase-3 positive cells (apoptotic cells) were 4.05 ± 0.8%, 32.95 ± 1.4%, 34.87 ± 4.6%, and 33.26 ± 1.7% in 0, 125, 250, and 500 µg/mL of VAD, respectively.
| Table 1: Percentage of Cells and Corresponding Responses in Caspase-3 Activity. | ||
| Doses (µg/mL) | Caspase-3 Negative or Viable Cells (Mean ± SD) % | Caspase-3 Positive or Apoptotic Cells (Mean ± SD) % |
| 0 | 95.95 ± 0.8% | 4.05 ± 0.8% |
| 125 | 67.05 ± 1.4%* | 32.95 ± 1.4%* |
| 250 | 65.13 ± 4.6%* | 34.87 ± 4.6%* |
| 500 | 66.74 ± 1.7%* | 33.26 ± 1.7%* |
| *p < 0.05 compared with the control group | ||
Vernonia amygdalina Delile induced nucleosomal DNA fragmentation in PC-3 cells
To confirm whether the apoptotic effect induced by VAD crude extract in PC-3 cells involves DNA fragmentation, we performed the DNA laddering assay. Our results revealed a presence of typical ladder formation upon 48 hours of VAD treatment. Apoptosis can be visualized as a ladder pattern of 180-200 bp due to DNA cleavage by the activation of a nuclear endonuclease in a standard agarose gel electrophoresis [15]. Here, we showed the formation of the DNA ladder in gel electrophoresis by induction of apoptosis in PC-cells treated with VAD crude extract (Figure 4).
Discussion
Prostate Cancer (PCa) is one of the common cancers that threatens men health and its incidence keeps increasing globally [16]. Modifiable risk factors such as regular physical exercise, healthy diet, weight control, and smoking cessation are more likely to lower the risk of Prostate Cancer development. Approximately 81% of PCa is diagnosed during the early stage of the disease. The treatment options for prostate care include surgery, radiotherapy, and chemotherapy, but these treatments often have side effects that may result in health issues such as impotence or decrease bowel function [17,18]. Several medicinal plants have been demonstrated to have promising chemotherapeutic effects against various cancers including PCa. In addition, medicinal plants have been used for the prevention and/or treatment of cancer for many years and have led to the discovery of effective anticancer drugs such as Paclitaxel (Taxol) derived from the bark of the Taxus brevifolia tree [19]. Therefore, medicinal plants serve as resources for anticancer drug discovery. Worldwide, many people rely on medicinal plants and their preparations to prevent and/or treat cancer. Knowing that the vast majority of clinically approved anticancer drugs are derived from natural medicinal plants [20], we previously tested the activity of VAD towards the treatment of PCa and we observed that VAD crude extract inhibits cell proliferation of PC-3 cells, and induce DNA damage through oxidative stress. Several scientific studies indicated that medicinal plants and their active compounds are useful to fight cancer by enhancing the immune system, decreasing side effects of synthetic antitumor agents, overcoming resistance to chemotherapy, and exerting synergistic drug interactions in combination with other drugs [21-24]. Here, we showed that VAD induced morphological alterations characteristic of apoptosis and necrosis such as cell shrinkage, blebbing of the plasma membrane, chromatin condensation, cytoplasmic swelling, cell membrane damage, organelle breakdown, and formation of apoptotic bodies in treated PC-3 cells compared to the control with intact cell morphology. In agreement with our observations, other studies have observed similar morphological alterations in vitro and in vivo [25,26].
Medicinal plants are important candidates for potential anticancer chemotherapies that inhibit tumor cell proliferation and control cell cycle [27]. Therefore, understanding how VAD induces cell cycle arrest in vitro represents an important step for translational research and clinical trials. In order to test the effect of VAD on cellular distribution, we performed cell cycle analysis by the flow cytometry after staining the PC-3 cells with propidium iodide. We observed that VAD exerts its anti-proliferative activity on human PCa cells at least in part through cell cycle arrest at G0/G1 phase (Figures 1,2). We detected that VAD treatment increased the proportion of G0/G1 (M2 region) cell population from 57.2% in the control sample to 62.4 in the treated at 250 µg/mL. On the other hand, G2/M (M3 and M4 regions) decreased with increased doses of VAD (Figures 1,2). To the best of our knowledge, this is the first report indicating that VAD induces cell cycle arrest at the G0/G1 checkpoint in human adenocarcinoma (PC-3) cells. Consistent with our results, several previous studies indicated that natural compounds induced cell cycle arrest and apoptosis in vitro and in vivo. Both cell cycle arrest and apoptosis have the potential to eliminate and kill cancer cells. For example, berberine (a natural product) induced G1-phase cell cycle arrest and caspase-3 dependent apoptosis in Prostate Cancer cells [28]. Another study indicated that berberine inhibited the growth of human PCa by inducing G0/G1 or M2/M phase arrest at different doses [29]. Green tea constituent (-)-epigallocatechin-3-gallate induced growth inhibition, cell cycle dysregulation, and apoptosis of androgen-sensitive and androgen-insensitive human PCa cells [30]. Apigenin induced cell cycle arrest and apoptosis in xenograft Prostate Cancer model [31], which is found to be mediated through modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human PCa cells [32].
Medicinal plants have drawn increasing attention as an antitumor agent due to their ability to trigger apoptosis [33,34]. A recent study in our laboratory has demonstrated that VAD induces apoptosis of PC-3 cells through phosphatidylserine externalization [9]. However, further studies are required to establish the molecular mechanism behind the apoptotic effect of VAD on PCa cells. To further understand how VAD induces its apoptotic effect, we performed flow cytometry analysis to assess the activation of caspase-3. Caspases are activated in the apoptotic cell both by extrinsic (death ligand) and intrinsic (mitochondrial) pathways. In the present study, VAD induces caspase-3 activity and thereby stops the growth of PC-3 cells. Consistent with our result, a previous study indicated that medicinal plants such as curcumin activated caspase-8, induced BID cleavage, caused mitochondrial cytochrome c release, and induced caspase-3 activation and PARP cleavage in HL-60 (neo) cells but not in Bcl-2 and Bcl-xl transfected cells [35]. Another study indicated that curcumin treatment caused typical apoptotic morphological alterations, induced phosphatidylserine externalization and caspase-3 activation in HemECs [36]. Given the effectiveness of VAD on the inhibition of cancer cell growth and induction of cell cycle arrest, apoptosis through phosphatidylserine externalization and caspase-3 activation, we confirmed the apoptotic response by performing DNA laddering/fragmentation assay. Our results showed the formation of the DNA ladder in gel electrophoresis by induction of apoptosis in PCa-cells treated with VAD. Nucleosomal DNA fragmentation is a key feature of apoptosis, which is characterized by changes in the nucleus morphology including chromatin condensation and fragmentation, cell shrinkage, cell rounding and formation of apoptotic cell bodies [37,38].
To our knowledge, this is the first time to report that VAD crude extract inhibits cancer cell proliferation and induces cell cycle arrest, apoptosis through phosphatidylserine externalization, caspase-3 activation, and nucleosomal DNA fragmentation in PC-3 cells. Overall, studies in our lab and from other research labs indicated that biologically active compounds from medicinal plants target and kill cancer cells through several mechanisms including (1) inhibition of cell growth, oxidative stress, carcinogenesis, and angiogenesis; and (2) induction of DNA damage, cell cycle arrest, autophagy, extrinsic and extrinsic apoptosis [8,9,21,23,39,40-43].
Conclusion
The nutritional and medicinal effects of Vernonia amygdalina Delile (VAD) have been known for decades, and our understanding of the therapeutic mechanisms that underlay these effects is steadily increasing. In the present, we have demonstrated that VAD inhibits the growth of PC-3 cells through cell cycle arrest at the G0/G1 checkpoint, activation of caspase-3, and nucleosomal DNA fragmentation as revealed by the flow cytometry assessment and DNA laddering assay. We previously demonstrated that VAD inhibited the growth of PC-3 cells at least through DNA damage, oxidative stress, and modulation of phosphatidylserine externalization in apoptotic cells. Together, our previous and current results support the notion that apoptosis is a potential mechanism by which VAD exerts its anticancer activity in human PCa cells. Based on our findings, we conclude that VAD possesses tumor-fighting properties. However, in vivo studies and clinical trials are needed to validate the effectiveness of VAD for the prevention and/or treatment of PCa.
Acknowledgment
We thank Dr. Richard A. Alo and Dr. Lekan Latinwo at Florida Agricultural and Mechanical University for his technical support.
Funding
This work was funded in part by the Faculty Research Award Program (FRAP) at Florida Agricultural and Mechanical University, Tallahassee, FL, United States; and in part by the National Institutes of Health (NIH), Grant # 1U54MD015929-01 at Jackson State University, Jackson, MS, United States.
Author contributions
Conceptualization, CGY, WJ, SST, and PBT; Methodology, CGY, WJ, SN, SST, and SD; Literature Review, CGY, SST, SD, SN, WJ, and PBT, Supervision and Funding, CGY, and PBT. All the authors read and approved the final manuscript.
Competing interests
The authors declare that they have no conflict of interests.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. TL - 60. CA Cancer J Clin. 2010. doi:10.3322/caac.20073
- Howlader N, Noone AM, Krapcho M, et al. National Cancer Institute SEER Cancer Statistics Review 1975-2012. Natl Cancer Inst. 2015. doi: 10.1103/PhysRevA.69.053616
- Siegel R, Miller K, Jemal A. Cancer statistics, 2015 . CA Cancer J Clin. 2015;65(1):29. doi:10.3322/caac.21254.
- Ferraldeschi R, Pezaro C, Karavasilis V, de Bono J. Abiraterone and Novel Antiandrogens: Overcoming Castration Resistance in Prostate Cancer. Annu Rev Med. 2013;64(1):1-13. doi: 10.1146/annurev-med-121211-091605
- Roudier MP, True LD, Higano CS, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003. doi: 10.1109/INNOVATIONS.2011.5893843
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012. doi: 10.3322/caac.21149
- Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: Progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2193-2203. doi: 10.1158/1055-9965.EPI-06-0942
- Yedjou C, Izevbigie E, Tchounwou P. Preclinical assessment of Vernonia amygdalina leaf extracts as DNA damaging anti-cancer agent in the management of breast cancer. In: International Journal of Environmental Research and Public Health. 2008;5:337-341. doi: 10.3390/ijerph5050337
- Johnson W, Tchounwou PB, Yedjou CG. Therapeutic mechanisms of Vernonia amygdalina delile in the treatment of prostate cancer. Molecules. 2017;22(10). doi: 10.3390/molecules22101594
- Oyedeji KO, Bolarinwa AF, Akintola AM. Effect Of Methanolic Extract of Vernonia amygdalina on Haematological and Plasma Biochemical Parameters in Male Albino Rats. J Dent Med Sci. 2013.
- Yedjou CG. Vernonia amygdalina—Induced Growth Arrest and Apoptosis of Breast Cancer (MCF-7) Cells. Pharmacol Pharm. 2013. doi: 10.4236/pp.2013.41013
- Clement G. Yedjou SSTKW& PBT. Novel Cellular Staining Protocol And Antiproliferative Effect Of Vernonia amygdalina Delile On Lung And Prostate Cancer Cells. August 2018. doi: 10.5281/ZENODO.1403369
- Yedjou CG, Tchounwou HM, Tchounwou PB. DNA damage, cell cycle arrest, and apoptosis induction caused by lead in human leukemia cells. Int J Environ Res Public Health. 2015;13(1). doi: 10.3390/ijerph13010056
- Yedjou C, Tchounwou P, Jenkins J, McMurray R. Basic mechanisms of arsenic trioxide (ATO)-induced apoptosis in human leukemia (HL-60) cells. J Hematol Oncol. 2010. doi: 10.1186/1756-8722-3-28
- Ioannou YA, Chen FW. Quantitation of DNA fragmentation in apoptosis. Nucleic Acids Res. 1996. doi: 10.1093/nar/24.5.992
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-2917. doi: 10.1002/ijc.25516
- Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92(19):1582-1592.
- Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283(3):354-360. doi: 10.1001/jama.283.3.354
- Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2(February):143. doi: 10.1038/nrc723
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007. doi: 10.1021/np068054v
- Singh S, Sharma B, Kanwar SS, Kumar A. Lead Phytochemicals for Anticancer Drug Development. Front Plant Sci. 2016. doi: 10.3389/fpls.2016.01667
- Bachrach Z. Contribution of Selected Medicinal Plants for Cancer Prevention and Therapy. Acta Fac Medicae Naissensis. 2012. doi: 10.2478/v10283-012-0016-4
- Efferth T, Koch E. Complex Interactions between Phytochemicals. The Multi-Target Therapeutic Concept of Phytotherapy. Curr Drug Targets. 2011. doi: 10.2174/138945011793591626
- Zacchino SA, Butassi E, Cordisco E, Svetaz LA. Hybrid combinations containing natural products and antimicrobial drugs that interfere with bacterial and fungal biofilms. Phytomedicine. 2017.
- Gonzalez-Polo R-A. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005. doi: 10.1242/jcs.02447
- Edinger AL, Thompson CB. Death by design: Apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004. doi: 10.1016/j.ceb.2004.09.011
- Amin ARMR, Karpowicz PA, Carey TE, et al. Evasion of anti-growth signaling: A key step in tumorigenesis and potential target for treatment and prophylaxis by natural compounds. Semin Cancer Biol. 2015;35:S55-S77. doi: 10.1016/j.semcancer.2015.02.005
- Mantena SK. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol Cancer Ther. 2006;5(2):296-308. doi: 10.1158/1535-7163.MCT-05-0448
- LU W, DU S, WANG J. Berberine inhibits the proliferation of prostate cancer cells and induces G0/G1 or G2/M phase arrest at different concentrations. Mol Med Rep. 2015;11(5):3920-3924. doi: 10.3892/mmr.2014.3139
- Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287(4):914-920. doi: 10.1006/bbrc.2001.5672
- Shukla S, Gupta S. Molecular targets for apigenin-induced cell cycle arrest and apoptosis in prostate cancer cell xenograft. Mol Cancer Ther. 2006;5(4):843-852. doi: 10.1158/1535-7163.MCT-05-0370
- Shukla S, Gupta S. Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell Cycle. 2007;6(9):1102-1114. doi: 10.4161/cc.6.9.4146
- Fulda S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010. doi: 10.1055/s-0030-1249961
- Bailly C. Ready for a comeback of natural products in oncology. Biochem Pharmacol. 2009. doi: 10.1016/j.bcp.2008.12.013
- Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release : its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002. doi: 10.1093/carcin/23.1.143
- Lou S, Wang Y, Yu Z, Guan K, Kan Q. Curcumin induces apoptosis and inhibits proliferation in infantile hemangioma endothelial cells via downregulation of MCL-1 and HIF-1α. Med (United States). 2018. doi: 10.1097/MD.0000000000009562
- Wong RSY. Apoptosis in cancer: From pathogenesis to treatment. J Exp Clin Cancer Res. 2011. doi: 10.1186/1756-9966-30-87
- Yeung MC. Accelerated apoptotic DNA laddering protocol. Biotechniques. 2002.
- Yedjou CG, Tchounwou PB. in vitro assessment of oxidative stress and apoptotic mechanisms of garlic extract in the treatment of acute promyelocytic leukemia. J Cancer Sci Ther. 2012;2012(Suppl 3):6. doi: 10.4172/1948-5956.S3-006
- Izevbigie EB. Discovery of water-soluble anticancer agents (edotides) from a vegetable found in Benin City, Nigeria. Exp Biol Med (Maywood). 2003;228(3):293-298. doi: 1535-3702/03/2283-0293
- Chen Z, Sun X, Shen S, et al. Wedelolactone, a naturally occurring coumestan, enhances interferon-γ signaling through inhibiting STAT1 protein dephosphorylation. J Biol Chem. 2013. doi: 10.1074/jbc.M112.442970
- Millimouno FM, Dong J, Yang L, Li J, Li X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev Res. 2014. doi: 10.1158/1940-6207.CAPR-14-0136
- Aung TN, Qu Z, Kortschak RD, Adelson DL. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int J Mol Sci. 2017. doi: 10.3390/ijms18030656
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.