Medicine Group . 2022 November 08;3(11):1302-1306. doi: 10.37871/jbres1597.
An Alternative Therapy for Membranous Glomerulonephritis: A Case Report
Rodolfo J Gordillo1* and Rita Dorantes2
2Pathologist at the Hospital Media Sur, México
Abstract
Membranous Glomerulonephritis (GMN) is an autoimmune disease whose etiology has not been defined with precisión, When there is no association with another disease which ocurrs in two thirds of the cases is considered to be idiopathic. It is the most common cause of Nephrotic Syndrome in Adults, primary caucasic and over 60 years of age. It is regarded as a non inflammatory Glomerulonephritis, concept with which I am not completely in agreement because I think as other researchers have taught than in GMN [1] the Complement system contributes to various Glomerulophaties by causing cellular damage by both an inflammation-dependant and inflammation-independent ways. Here I discuss a case of a woman who developed an idiopathic GMN presented with a nephrotic síndrome with severe protracted proteinuria also I describe her initial treatment and the changes that I made and discuss her follow up for 7 years, and current medical status.
Background
GMN is genetically associated in caucasic Europeans with HLA-DR3 and HLA-DQA1and 2 alleles of HLA-DQA1 (allele 0501) located at The Mayor Histocompatibility Complex (MHC) in the short arm of Chromosome 6 [2,3] and the gene PLA2R1 is present in chromosome 2 [4] It can be associated with autoimmune diseases as systemic lupus erythematosus and diabetes mellitus type 1. It is also associated with viral diseases like Hepatitis B, it may be secondary to Drugs and Toxins like gold, penicillamine and non steroidal anti-inflammatory agents and also associated with other diseases like Sarcoidosis, Sickle cell disease, tumors, renal transplantation among others [4]. It is caused by immune complexes formed in subephitelial side of the Glomerulos Membrane (GM) by the deposition binding circulating antibodies to an antigenic target located in the podocytes. In the last 10 years a lot of research has taken place the first of them following the experimental model of Heymann Nephritis described in 1959 by a Pediatrician and his team in Cleveland [5]. Since then sevaral studies have been carried one to try to define the podocyte antigen in humans with the identification of PLA2 [6] being the Target Podocyte Antigen, THSD7A [4] which is a glomerular transmembrane glycoprotein, finally in cases of GMN (-) both to PLA2R and THSD7A it have been isolated two proteins Exostosin 1 and 2 that accumulated in the subepithelial deposits [5] about 40% of the patients will undergo an spontaneous remission and another 30% a poor o no response to immuno- suppressive therapy and will progress to End Stage Kidney Disease (ESKD) and will require Renal Function Replacement Therapy (RFRT) [7].
Case Report
I report the case of a female that initiated her current disease in 2014 at 38 years of age, started with swollen eyelids and maleolar edema, and later anasarca and she developed a full nephrotic síndrome with a normal serum creatinine of 53 µmol/l (0.6 mg/dl) serum colesterol 7.77 mmol/l (301 mg /dl) a creatinine clearence of 117 ml/min/1.73 m2 total Cholesterol 5.66 mmol/l (219 mg/dl), Triglyceride 1.48 mmol/l (131 mg/dl) Vitamin D 24.7 ng/ml, PTH 36.3 pg/ml, proteinuria 3.96 g/day. She suffered in the past from kidney stones and underwent endoscopic surgeries in 1998, 2002 and 2011, no metabolic studies where done at that time; she was reciving only Clortalidona 25 mg 1 qd every day in the mornings as her only treatment for 2 years, without any improvement of her nephrotic síndrome or the masive proteinuria.
I saw her for the 1st.time on 10/03/2016 she was 40 years of age and she only compalained for swollen eyelids and bilateral swollen maleolars, her weigth 62.8 Kg height 162 cm BMI 23.9 BP 100/60 HR 80x´, RR 12x´, T36°C HEENT normal. Heart with normal rhytm an normal sounds, Lungs were well ventilated and without rales, Abdomen was soft without pain on bilateral palpation and with normal bowel sounds, peripheral pulses were present and normal. Neurologic examination was normal.
In those 2 previous years none study was done to try to clarify the possible etiology and neither a renal biopsy was done. After the first consultation I ordered from the lab a complete inmune Profile por hepatitis virus A,B,C,D, anti HIV type 1,2; seric inmmunoglobulins, Hemolityc Complement CH50 a CBC and a general chemistry, seric Inmunoglobulins were normal, Viral Hepatitis Profile was (-) as well as anti HIV1&2, Anti native DNA were negative, her serum glucose 5.17 mmol/l (93 mg/dl) Urea 3.8mmol/l (22.8 mg/dl) serum Creatinine 58.3 µmol/l (0.66 mg/dl), Complement CH50 49 U/ml was low, Serum albumin 27 g/l (2.7 g/dl) and her urine proteinuria was 3.964 g/day (1.65 g/l). I aso ordered a renal ultrasound 10/12/16 wich showed both kidneys of normal size an localization with chronic inflamatory changes bilateraly with normal cortico:medular relation and absence of focal or diffuse pathology.
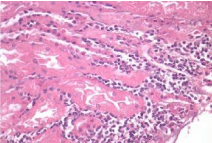
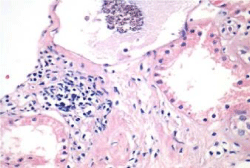
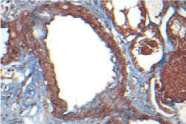
The metabolic study showed a normal PTH 16.22 pg/ml, Oxalate secretion 46.16 µmol/day (5.82 mg/day), normal Citrate exretion of 671 mg/day, and Creatinine clearence was reported 113 ml/min/1.73 m2. Coagulation studies were normal and a percutaneus biopsy was done on 01/12/17 the biopsy was usefull specially the silver-metenamine Jones stain showing irregularities and some widenes and spikes of the Glomerular Basment Membrane, while the Masson stain showed light interstitial fibrosis and light tubular atrophy. Inmuno florescence reveled a deposition with a granular pattern of light epimembranous positive graded 2 for IgG, Kappa and Lambda, scarce C3c while the rest of inmune reactants were negative, a diagnosis of Idiophatic Membranous Glomerunonephritis was established asociated to mild interstitial fibrosis and tubular atrophy (15%). Therapies of Membranous Nephropathy have change with the time [7].
With all the above findings I started on 01/19/17 Micophenolic acid 500 mg tid and due to the presence of fibrosis and atrophy I placed on an anti-inflammatory protocol that I reported been usefull in a previous article [8] and started on pioglitazone 45 mg 1qd, febuxostat 80 mg 1 qd, Vit D 4000 U/day, Atorvastatin 20 mg 1 qd pentoxifiline 400 mg 1 qd. On 02/16/17 She refered to be felling better with dissaperance of the edema, stable blood pressure with a decrease in her proteinuria to 0.604 g/l, Complement C3 was normal at 122 mg/dl with a Glomerular Filtration Rate (GFR) by Cistatine calculated was 111 ml/min/1.73 m2. On 03/30/17 she refered that the swollen eyelids comes and goes as well as the maleolar edema, she has no other complains the physical examination was normal. She had a proteinuria of 1.43 g/l and a GFR by Cistatine C of 105.3 ml/min/1.73 m2 for the proteinuria I increase the dosage of Cell Cept to 500 mg qid. On 04/20/17 she was without symptoms her serum creatinine 58.3 µmol/l (0.65 mg/dl), Urea 3.49 mmol/l (20.9 mg/dl) GFR by Cistatine C 108 ml/min/1.73 and proteinuria continued high at 1.43 g/l. On 05/04/17 I saw her in the office she had swollen eyelids and swollen maleolar regions, her blood pressure was normal at 90/60 mmHg her proteinuria was down to 0.60 g/l her serum creatinine 57.46 µmol/l (0.65 g/dl)and her serum Urea was 3.99 mmmol/l (24 mg/dl), 0n 08/16/17 Antinative DNA was (-) and Anti ENA antibodies were also (-) at the same time her blood hemoglobin was on the lower limit 0f 12.2 g/dl, her Hematocrit was sligthly below normal at 36.8% her serum creatinine was 64.5 µmol/l (0.73 mg/dl). On 09/22/17 a renal gamagram (tecnecium 99) DTPA was done with a GFR of 80/ml/min/1.73 m2 a decrease of 26% from the previous report, with final retention of radioiodine on the right kidney. On 10/04/17 Vesico-ureteral reflux was ruled out with a Vesico-uretro-cystogram. On 10/26/17 her serum albumin went down from 37 g/l to 34 g/l (3.4 g/dl) and her serum creatinine went up to 69.84 µmol/l (0.79 mg/dl) her proteinuria decreased to 0.50 g/l. 0n 11/03/17 because her serum cratinine went down to 61.8 mol/l (0.7 mg/dl) with a normal Urea at 8.47 mmol/l (23.7 mg/dl) and albuminuria of 0.0009 g/l (0.30 mg/dl) I decided to low down on her dosis of Cell Cept to 500 mg 1 tid and kept her on pioglitazone at the same dosage 45 mg 1 qd as well as the rest of anti-inflammatory therapy. Her mineral metabolism as well as her Parathyroid hormone were normal.
On 12/28/17 with a serum uric acid 125 µmol/l (2.1 mg/dl) I decreased Turazive from 1 qd to 1/2 qd. The more difficult part of her management was the heavy proteinuria she presented but from december 2017 to january 2018 her proteinuria was reduced from 3.5 g/l to 1,68 g/l and on february to 1,39 g/l her vital signs, her renal function remained normal and there was no edema. Her measured by creatinine clearence was 72.2 ml/min/1,73 m2 on 03/02/18 and stabilized above 95 ml/miun/1,73 m2 for the next 4 months with a serum creatinine of 69.8 mol/l (0.79 mg/dl) quite normal. As she was so stable we continue tappering the dosage of cell cept from 1 tid to 1 bid. On 10/26/18 se has doing pretty good with normal vital signs normal physical exam and her serum creatinine went down again at 66.3 µmol/l (0.75 mg/dl) with a GFR by Cistatine of 99 ml/min/1.73 m2, so we decided in accordance to the patient to decrease Cell Cept to 1 qd and the rest of the therapy unchanged. On the next year 2019 her medical evolution kept fine with a normal serum creatinine of 66 µmol/l (0.75 mg/dl) with the microalbuminuria completely normal to 0.5 mg/dl, Hormonal female profile normal as well as thyroid function tests, her lipid profile was also normal with no cardiac risk. Her antihypertensive therapy with telmisartan originally at 40 mg one at bedtime was reduced to 20 mg1qd also the treatment for her cholesterol and triglycerides was stopped, Cell Cept was discontinue in 2019. She was kept on Vitamin D 4000 IU a day and pioglitazone was decreased from 30 mg to 15 mg 1 qd, bezadfibrate 200 mg 1 qd, pentoxiphylline 400 mg 1 qd, Ezetimiba/simvastatina 10/20 mg 1 qd. With these evolution I decided to perform another renal biopsy to see if there was an improvement in the histopathpology findings the patient was in agreement so the biopsy was performed on 10/22/19 it was succesfull and we obtained 43 glomeruli, the microscopic findings on light and inmunoflurescence stains was analized and the comparation between the two biopsys was analized by the collaboration of our pathologist. Last time I saw her on 30/03/22 she was doing very well without any symptom, her vital signs were normal as well as her physical examination and her renal function.
Pathology
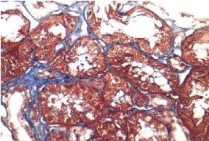
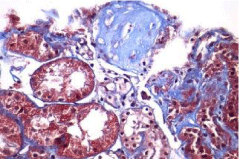
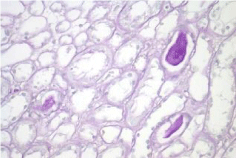
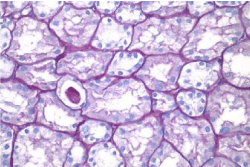
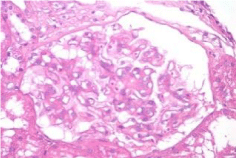
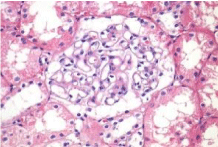
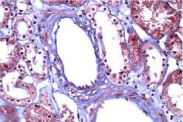
Activity and chronicity scores were calculated based on the scales used for lupus nephropathy (Austin and Balow). For the activity index, the following parameters were taken into account: endocapillary hypercellularity, leukocyte infiltration, subendothelial hyaline deposits, fibrinoid necrosis/karyorrhexis, cellular crescents, and interstitial inflammation. Likewise, the following histological data were included for the chronicity index: glomerular sclerosis, fibrous crescents, tubular atrophy and interstitial fibrosis. In the 2017 biopsy, 13 glomeruli were identified, without sclerosis, the capillaries were open with focal hypercellularity. Tubules with intraluminal PAS positive material. There was mild interstitial fibrosis, moderate plasmocytic inflammation vessels did not present alterations. The activity index was 5 and chronicity was 2. In the 2019 biopsy, 43 glomeruli were observed, 1 sclerosed, which represents 2.3% of the total glomerular population. The capillaries were open and presented normal appearance. However, there were intratubular calcifications. Moderate fibrosis and mild lymphocytic inflammation were observed in the interstitium. The vessels presented normal appearance. The activity index was 3 and the chronicity index was 4 (Table 1).
In conclusion, the microscopic findings show that in two years of disease evolution, cellularity improved, however, there is more interstitial fibrosis. The intratubular calcifications observed in the last biopsy could be transitory and related to other factors such as eating habits, etc. The most important thing about the second biopsy is that it allows us to assess the patient's short- and long-term prognosis. Chronicity signs could be reversible with sustained treatment.
Discussion
As I mentioned early I belive that independently of the absence of inflammatory cells in the kidney the presernce of autoantibodies in 70% of the patients mainly directed to M type phospholipase A2 receptor (that PLA2R) [6] and the complement complex C5-9 has several ways to actívate the innate inmune system and rise the production and secretion of inflammatory Limphokines and Cytokines that produce fibrosis cell proliferation, tubularv atrophy and damage to the kidney [7,8]. I took me one year to chose the drugs to use becauase were mediccines directed to other target but that because of their pluripotential effects can supress the secretion of Lympokines and cytokines, Pioglitazone blockade the production of IL1, IL6, NFKB induces the production of nephrin and protects the podocytes [9], also the Bloquers of the Angiotensin recptor decrease the proteinuria, statins wich boud to PPAR α receptors have also as Vitamin D an inmunomodulatory effects that reduce inflammation [10] and Febuxstat reduces oxidative stress finally pentoxifylline inhibits both the transformig growth factor β and decrease the fibrosis [11]. In this paticular patient despite the tremendous and protracted proteinuria that lasted from 2014 to 2018 with full nephrotic síndrome the combined use of Imunosuppresion and Anti-inflammatory therapy were the key for her treatment also reflected in the improvement of renal function evaluated by serum creatinine and BUN and by Glomerular Filtration Rate calculated by Cistatine C, Creatinine clearance and Renal gamagraphy with Tecnecium 99-DTPA and by de derivate formula by Cockcroft and Gault.
Conclusion
The tratment of Membranous Glomerulonephritis is controversial. Therapy with steroids alone has been used with controversial results, later combined therapies of steroids with cytotoxic drugs had been used including chlorambucil (.2 mg/kg/day) and Methylprednisolone 1g iv in 3 consecutives days with variable ad non clonclusive results. This patient that despite the prolonged active nephrotic syndrome and masive protacted proteinuria went into remission with the combined immunosupresion therapy plus the anti-inflammatory therapy is an example not of patients with sporadic remission thet are also caracterized at this patiente for a lack of response to control nephrotic syndrome or masive proteinuria. Up to now she has beeen with an inactive membranous nephrophaty for 5 consecutive years and without heavy proteinuria and with a normal renal function serum creatinine 68 µmol/l (0.77 mg/dl) the last GFR of 98 ml/min/1.73 m2 and performing normal activities withou any restricción and the complete absence of edema, now wishing to know if in the renal tissue the initial inflamation with intrestitial fibrosis and tubular atrophy a second renal biopsy was performed with the inform conset of the patient and here our colaborator Rita Dorantes MD and Pathologist studied and compared both biopsies to evalute if there was any histopatological improvement, here appear the biopsy imagens and her conclusions. Further prospective longitudinal and radomized studies with a larger number of patients are requiered to validate our finndings. New treatments protocols have been added including rituximab [10]. Last time I saw her was on 09.28.22 her Blood Pressure 110/70 HR 80x’ RR 12x’ se was alert ad well oriented the rest of her examination was norml without pehripherial edema; her last serum Urea 4,6 mmol/l (27.8 mg/dl) last serum creatinine 52.1 µmmol/l (0.59 mg/dl) microalumiuria 20 mcg/ml, GFR y Cistatie C 89.33 ml/mi/1.73 m2, Se is completely asiyntomatic and preforming her work without problems.
References
- Segawa Y, Hisano S, Matsushita M, Fujita T, Hirose S, Takeshita M, Iwasaki H. IgG subclasses and complement pathway in segmental and global membranous nephropathy. Pediatr Nephrol. 2010 Jun;25(6):1091-9. doi: 10.1007/s00467-009-1439-8. Epub 2010 Feb 12. PMID: 20151159.
- HEYMANN W, HACKEL DB, HARWOOD S, WILSON SG, HUNTER JL. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660-4. doi: 10.3181/00379727-100-24736. PMID: 13645677.
- Ronco P, Debiec H. Molecular Pathogenesis of Membranous Nephropathy. Annu Rev Pathol. 2020 Jan 24;15:287-313. doi: 10.1146/annurev-pathol-020117-043811. Epub 2019 Oct 17. PMID: 31622560.
- Klouda PT, Manos J, Acheson EJ, Dyer PA, Goldby FS, Harris R, Lawler W, Mallick NP, Williams G. Strong association between idiopathic membranous nephropathy and HLA-DRW3. Lancet. 1979 Oct 13;2(8146):770-1. doi: 10.1016/s0140-6736(79)92118-4. PMID: 90863.
- Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011 Feb 17;364(7):616-26. doi: 10.1056/NEJMoa1009742. PMID: 21323541.
- Fresquet M, Jowitt TA, Gummadova J, Collins R, O'Cualain R, McKenzie EA, Lennon R, Brenchley PE. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol. 2015 Feb;26(2):302-13. doi: 10.1681/ASN.2014050502. Epub 2014 Oct 6. PMID: 25288605; PMCID: PMC4310666.
- Scolari F, Alberici F, Mescia F, Delbarba E, Trujillo H, Praga M, Ponticelli C. Therapies for Membranous Nephropathy: A Tale From the Old and New Millennia. Front Immunol. 2022 Mar 1;13:789713. doi: 10.3389/fimmu.2022.789713. PMID: 35300332; PMCID: PMC8921478.
- Gordillo RJ. A novel therapy to reverse end stage kidney renal diseases in its early stages 1 & 2 to a normal renal function or to stabilize and halt ist progression in moreb advances stages. J of Clinical Nephrology and Research. 2021;8(1):1102-1109. doi: 10.47739/2379-0652/1102.
- Panchapakesan U, Chen XM, Pollock CA. Drug insight: thiazolidinediones and diabetic nephropathy--relevance to renoprotection. Nat Clin Pract Nephrol. 2005 Nov;1(1):33-43. doi: 10.1038/ncpneph0029. PMID: 16932362.
- Seitz-Polski B, Dolla G, Payré C, Girard CA, Polidori J, Zorzi K, Birgy-Barelli E, Jullien P, Courivaud C, Krummel T, Benzaken S, Bernard G, Burtey S, Mariat C, Esnault VL, Lambeau G. Epitope Spreading of Autoantibody Response to PLA2R Associates with Poor Prognosis in Membranous Nephropathy. J Am Soc Nephrol. 2016 May;27(5):1517-33. doi: 10.1681/ASN.2014111061. Epub 2015 Nov 13. PMID: 26567246; PMCID: PMC4849812.
- Fiorentino M, Tondolo F, Bruno F, Infante B, Grandaliano G, Gesualdo L, Manno C. Treatment with rituximab in idiopathic membranous nephropathy. Clin Kidney J. 2016 Dec;9(6):788-793. doi: 10.1093/ckj/sfw091. Epub 2016 Oct 13. PMID: 27994855; PMCID: PMC5162414.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.