Biology Group . 2022 November 24;3(11):1382-1388. doi: 10.37871/jbres1607.
Sustainable Cell Sources for Cultivated Meat
Derya Ozhava1, Mohit Bhatia2, Joseph Freman3 and Yong Mao1*
2Atelier Meats, 666 Burrard Street, Suite 500, Vancouver, British Columbia, V6C 3P6, Canada
3Department of Biomedical Engineering, Rutgers University, 599 Taylor Rd, Piscataway, NJ 08854, USA
- Cultivated meat
- Clean meat
- Stem cells
- Muscle
- Adipose
- Sustainable cell source
Abstract
Cultivated meat (clean meat) is an emerging yet fast growing research field and industry with a great potential to overcome the limitations of traditional cattle meat production. Cultivated meat leverages the technologies of cell biology and tissue engineering, culturing multiple types of cells and assembling them into a tissue structure construct mimicking the muscle tissues of livestock animals. A sustainable cell source is the first and the utmost important component of cultivated meat technology. In this mini review, cell sources for the main cell types in cultivated meat (muscle cells and fat cells) are described. Stem cells with self-renewal and differentiation potential are the most prominent candidates. Progenitor stem cells from muscle tissues, mesenchymal stem cells isolated from many other tissues and induced Pluripotent Stem Cells (iPSCs) created from terminally differentiated cells have been used as cell sources for cultivated meat. To become a sustainable cell source, which can generate high quantity (extensive in vitro expansion) and high quality (stemness) cells for the making of cultivated meat, these cells still face the challenges and limitation intrinsically associated with in vitro culturing. The efforts and strategies to circumvent such limitations are also discussed.
Introduction
Cultivated meat is synonymous with cell-based meat, clean meat, textured meat or in-vitro meat, is one type of cellular agriculture. Due to the environmental and animal welfare concerns, along with continued demands for more protein based food by the increase of the global population, the need for the development of alternatives to the conventional livestock farming has become inevitable [1]. Cultivated meat employs the conventional cell culture techniques and tissue engineering methodologies to culture animal cells in vitro to form edible structures resembling conventional animal meat cellularly and organoleptically. In 2002, two different organizations made the first attempts at cultivated meat and showed the viability and sustainability of cultivated meat as a food source for the future [2]. One decade later, the first world’s cultured burger, manufactured from cultured cells, was presented by Professor Mark J. Post. This was certainly one of the milestones in the cultivated meat field [3,4]. In recent years, cellular agriculture has attracted tremendous attentions from both academia and industry, the field of developing cultivated meat has been making progresses even leaps towards a unique research interest and a sustainable industry. Many cultivated meat companies all over the world have emerged since 2016. Moreover, the successful commercialization of lab grown chicken nuggets in Singapore in 2020 promised an even faster paced development of cultivated meat in the near future.
The technologies and biomaterials developed for tissue engineering are used to carry out the process of producing cultivated meat which is mainly composed of three primary technical foci, sustainable cell sources, scaffold biomaterials and bioprocessing. In this mini review, recent advancement, and challenges in developing sustainable cell sources will be reviewed.
Meats for human consumption are mostly animal muscle tissues, which comprised of muscle fibers (average about 90%), fat, connective tissue and blood (10%) [5,6]. In the muscle tissues, skeletal myocytes, satellite cells, adipocytes, fibroblasts, endothelial cells and hematopoietic cells are the resident cells. Due to the predominance of muscle and fat tissue in meat, cultured muscle cells and fat cells become the building blocks for cultivated meat [7].
The first step of a common protocol to fabricate cultivated meat is to obtain starter cells from an animal by performing a biopsy [8]. Starter cells must be able to self-renew and differentiate into mature cell types that form the meat. Stem cells are of interest and promising candidates to be used as a starting cell source. Huge numbers of cells are needed to make meats in culture. Based on a rough calculation, ~ 2.9x1011 of muscle cells are needed for 1 kg of wet meat [9]. To achieve such a quantity, it is essential to have sustainable cell sources, which can proliferate/double significantly in vitro while preserving the functionality of the cells.
Cell sources for muscle cells
Cells from muscle tissues: Muscle fibers are made of mature muscle cells (myocytes) and myogenic progenitor stem cells (satellite cells). The formation of muscle fiber or myogenesis, starts with the fusion of activated satellite cells (myoblasts) either with one another to generate new multi-nucleated myofibers or with an existing myofiber to increase the pool of myonuclei and allow muscle growth [10]. Satellite cells are the cell source for initial muscle formation and muscle growth/regeneration [11]. Due to their self-renewal and differentiation ability, satellite cells are the most prominent cell type explored for cultivated meat [12]. Satellite cells are characterized by the expression of the paired type homeobox transcription factor 7 (Pax7) and the absence of the expression of Myoblast Determination Protein 1 (MyoD) (Pax7+MyoD-) [13]. The isolation of satellite cells from biopsies of muscle tissue can be achieved by enzymatic digestion and multiple selection steps including fluorescence-activated cell sorting [12,14]. Since the microenvironment “niche” plays a crucial role in maintenance of the stemness of satellite cells, the expansion of Pax7+MyoD- satellite cells under the standard in vitro culture conditions has been challenging. For example, Pax7+ satellite cells reduced from 100% to 30-50% in only three passages [15]. Currently, many approaches have been explored to overcome or reduce the loss of phenotype of satellite cells. It has been shown that P38-MAPK signaling pathway is involved in the loss of Pax7+ satellite cells during in vitro culturing. With P38 inhibitors, bovine satellite cells showed enhanced proliferation and expression of Pax7+ [16]. Inhibitors against Methyltransferase Setd7 [17], JAK-STAT pathway [18] and activators of P53 pathway [19] have been shown to positively promote the proliferation and maintain Pax7+ phenotype in murine muscle satellite cells. It is still unknown if these small molecules similarly regulate the growth of satellite cells isolated from larger livestock animals remain to be tested.
The Extra Cellular Matrix (ECM) components have been shown directly regulate the proliferation of bovine satellite cells [20,21]. The effects of ECM components such as collagen type I, fibronectin and gelatin, laminin and Matrigel were tested on the proliferation and stemness of porcine muscle satellite cells. Among these ECM components, coating of laminin or Matrigel sustained the proliferation and myogenic differentiation capacity of the porcine satellite cells [22].
Satellite cells isolated from muscle tissues have a high efficiency to differentiated into myoblasts and then form mature myocytes, therefore, muscle satellite cells remain to be the most explored cell sources for cultivated meats. To overcome the limitation of cell expansion, immortalization of these cells may be an important venue to explore. Cell lines developed via spontaneous or intentional (transfection of genes) immortalization have been considered and successfully used for cultivated meats [23]. Such advancement warrants opportunities for developing immortalized satellite cells or myoblasts.
Cells from non-muscle tissues: In addition to cells isolated from muscle tissues, cells from non-muscle tissues are also considered for making muscle fiber in cultivated meats. Bovine Mesenchymal Stem Cells (MSC) can be readily isolated from non-muscle tissues such as bone marrow [24,25], adipose tissues[26], and placental tissues[27-29]. These cells can self-renew and undergo multiple lineage specific differentiation [25,30,31]. While myogenic differentiation of MSC have been achieved, but the differentiation efficiency is low [27,32]. Adding growth factors seem to increase the myogenic differentiation of MSC cultured in ECM 3-dimensional constructs [33].
Even though MSCs have self-renewal ability, to reach the scale of cultivated meat, cells have to be expanded in vitro extensively. MSCs show reduced proliferation and stemness over long term culture [34,35]. Therefore, studies aiming to overcome this limitation are critical. The inclusion of growth factors such as Fibroblast Growth Factor (FGF), Hepatocyte Growth Factor (HGF) in culture showed differential effects on the growth and stemness of human MSCs. FGF promoted significant proliferation with compromised differentiation potential. But HGF promoted cell proliferation mildly but maintained the stemness of cells [36]. Extracellular matrix assembled by cultured cells have also shown promising effects on the proliferation and stemness of human MSCs [37,38]. It is expected that more efforts will be put into improving the quantity while maintaining the quality of bovine MSCs during in vitro expansion in the foreseeable future.
Besides using adult stem cells, re-programed cells are also considered as alternative cells sources. Re-programmed cells such as induced Pluripotent Stem Cells (iPSCs) and trans-differentiated cells overcome the limitation on cell expansion. iPSCs can be induced from terminal different adult cells such as skin fibroblasts by overexpressing transcription factor genes Oct4, Klf4, c-Myc, and Sox2 [39]. Successfully differentiation of iPSCs to functional satellite like cells has been achieved with human iPSCs [40]. However, reports on using animal iPSCs as a source for myoblasts are limited [41]. Another type of re-programed cells that may be used as cell source for making muscle cells is transdifferentiated cells. Directly re-programing of fibroblasts into muscle progenitor cells has been achieved in small animals [42]. While the trans differentiation skipping creating iPSC cells is very attractive but its efficacy and safety for cultivated meat must be evaluated [43].
Cell sources for fat cells
Even though meat is predominantly composed of muscle fibers, fat occupies approximately 30% of the biomass, contributing to better flavor, taste, texture, appearance, and nutrition [44-46]. More positive organoleptic consideration has been declared by consumers for a meat consisting of the higher fat levels [47]. Lipids (fat molecules) and adipocytes (fat cells) are two components of food, having many differences in terms of biological and food properties. A large amount of lipids is stored inside of the adipocytes, representing more natural component of animal meat fat [48,49]. Adipocytes are the predominant component of White Adipose Tissue (WAT) dispersed in different deposits throughout the body, including subcutaneous and visceral fat. Progenitor or MSCs are the origin of the adipocytes, developing unilocular lipid droplets after the differentiation into mature adipocytes. To isolate cells from a biopsied tissue, collagenase type I is used in each species such as bovine, porcine, and other livestock species (duck, goose, turkey, goat, and sheep). Although subcutaneous WAT is the predominant source of cells, visceral WAT could be obtained mainly from chicken and fish for which collagenase type II is used to collect the cells. Adipogenic cells can also be harvested from muscle or intramuscular fat. Fibroadipogenic progenitor cells obtained from muscle can differentiate into mature adipocytes [50,51]. This adipogenic differentiation process may be coordinated by miRNA targeting Runx1 [52]. In the literature several studies have shown the results of adipogenic differentiation of myosatellite cells into fat cells [53-55]. Adipogenic differentiation of MSCs is induced by using a differentiation cocktail basically composed of insulin, dexamethasone, and Iso Butyl Methyl Xanthine (IBMX), and rosiglitazone. A wide variety of differentiation cocktails have been examined to promote adipogenesis [44,56]. Due to safety and cost-effectiveness concerns, optimization of the induction cocktails has become important. For example, using combinations of free fatty acids for bovine preadipocytes appears as a promising solution for differentiation and maturation of adipocytes [57].
The major cell types in WAT are mature adipocytes with different minor cell types known as Stromal Vascular Fraction (SVF) or Stromal Vascular Cells (SVCs) which consist of the mix of preadipocytes, mesenchymal stem cells (adipose-derived stem cells, ASCs), endothelial cells, immune cells, and smooth muscle cells [58,59]. Although ASCs in SVCs have ability to differentiate into other cell types such as osteoblasts, preadipocytes in SVCs the same are settled down to become mature adipocytes. Therefore, isolated cells may show differences in terms of cell types and differentiation pathways. While most of the cell types used were somatic cells from adult animals, some studies utilized immortalized embryonic sources [60]. Pluripotent stem cells such as embryonic stem cells or induced Pluripotent Stem Cells (iPSCs) are another promising cell type that could be applicable for scalable production of adipocytes in the wide range of animal species [61,62]. While iPSCs feature exceptional growth properties, they are formed by including reprogramming genes in somatic cells [63]. There are many reports showing the derivation of iPSCs from chicken, bovine, fish and porcine however, there has been no reports in the literature of successful differentiation of farm-animal derived iPSCs into mature adipocytes [46].
The expansion stem cell sources for adipocytes faces the same challenges as the expression of cell sources for myocytes does [64]. The proliferation and stemness decrease over a long period of in vitro culturing. The research efforts to overcome the limitation on expansion of stem cells for myogenesis as discussed in muscle cells may very well applicable to stem cells identified as cell sources for adipocytes.
Summary
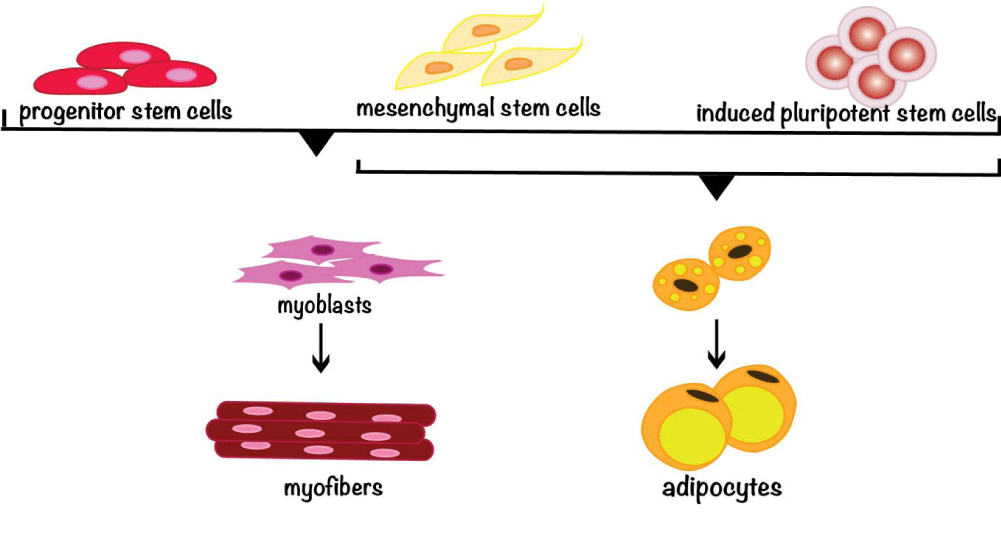
The importance of developing cultivated meat has been widely recognized in recent years. Efforts from both academia and industry have contributed to the tremendous progresses in making cultivated meat. Currently, the most commonly used cell sources for making cultivated meat (Figure 1) are stem cells isolated from the tissues of livestock animals. This dependence on biopsies from live animals or carcass tissues undermines the mission of cultivated meat. On the other hand, birth tissues from livestock are often discarded. Therefore, the stem cells from these tissues warrant additional research and development efforts. The sustainability of stem cells as a cell source depends on the proliferative ability of stem cells and their differentiation ability (stemness) to become muscle or fat cells by demand. Both abilities are limited by extensive in vitro culturing. Efforts to overcome such limitation have shown encouraging results but more advancement in this aspect is called for. The advantages and disadvantages of different cell sources are highlighted (Table 1).
| Table 1: Comparison of different types of cells used as cell sources for cultivated meat. | |||
| Cell Types | Advantages | Disadvantages | Sources of cells |
| Progenitor stem cells (muscle satellite cells) | Easy to differentiate to the desired cell type | Limited proliferation potential; harvest cells from animal tissues | Biopsy of muscle tissue from live animal or carcass |
| Mesenchymal stem cells | Self-renewal (proliferation) Multiple tissue sources Differentiation to multiple lineages |
The loss of proliferation ability and stemness during extensive in vitro expansion; require efficient and accurate lineage specific differentiation | Bone marrow, adipose tissues from live animal or carcass Placental tissue from birth |
| iPSC | Potential to be differentiated to muscle cells and fat cells from the same cell source | Creation of iPSC cells; rely on efficient differentiation, maturation | Multiple cell sources including terminally differentiated cells |
| Immortalized primary or stem cell lines | Unlimited cell expansion | Low efficiency of spontaneous immortalization; off-target effect of gene transfection; loss of the functionality or stemness of cells |
Muscle satellite cells, mesenchymal stem cells |
Perspectives
While only the cell sources are discussed here, the development and optimization of the scaffold materials for making the textured meats, the bioprocesses such as the vast expansion of cells by bioreactors, marbling the cultivated meat by additive manufacturing are all crucial for making the cultivated meat. A few comprehensive reviews on those topics are listed [9,12,65-67]. Even if cultivated meat is feasible and promising to evolve into a successful industry, it still has to overcome many challenges such as;
- Developing effective and cost-efficient technologies.
- Understanding and mimicking the textures and flavors of livestock meat.
- Seeking governmental regulatory approvals.
- Educating and improving customer acceptance of cultivated meat.
References
- Choudhury D, Tseng TW, Swartz E. The Business of Cultured Meat. Trends Biotechnol. 2020 Jun;38(6):573-577. doi: 10.1016/j.tibtech.2020.02.012. PMID: 32407686.
- Catts O, Zurr I, The ethics of experiential engagement with the manipulation of life. Tactical Biopolitics: Art, Activism, and Technoscience. 2008;125-142. doi: 10.7551/mitpress/9780262042499.003.0008.
- Stephens N, Ruivenkamp M. Promise and Ontological Ambiguity in the in vitro Meat Imagescape: From Laboratory Myotubes to the Cultured Burger. Sci Cult (Lond). 2016 Jul 2;25(3):327-355. doi: 10.1080/09505431.2016.1171836. Epub 2016 Jul 8. PMID: 27695202; PMCID: PMC5022697.
- Ng ET, Singh S, Yap WS, Tay SH, Choudhury D. Cultured meat - a patentometric analysis. Crit Rev Food Sci Nutr. 2021 Oct 19:1-11. doi: 10.1080/10408398.2021.1980760. Epub ahead of print. PMID: 34664530.
- Reiss J, Robertson S, Suzuki M. Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. Int J Mol Sci. 2021 Jul 13;22(14):7513. doi: 10.3390/ijms22147513. PMID: 34299132; PMCID: PMC8307620.
- Listrat A, Lebret B, Louveau I, Astruc T, Bonnet M, Lefaucheur L, Picard B, Bugeon J. How Muscle Structure and Composition Influence Meat and Flesh Quality. ScientificWorldJournal. 2016;2016:3182746. doi: 10.1155/2016/3182746. Epub 2016 Feb 28. PMID: 27022618; PMCID: PMC4789028.
- Zagury Y, Ianovici I, Landau S, Lavon N, Levenberg S. Engineered marble-like bovine fat tissue for cultured meat. Commun Biol. 2022 Sep 8;5(1):927. doi: 10.1038/s42003-022-03852-5. PMID: 36071206; PMCID: PMC9452530.
- Seah JSH, Singh S, Tan LP, Choudhury D. Scaffolds for the manufacture of cultured meat. Crit Rev Biotechnol. 2022 Mar;42(2):311-323. doi: 10.1080/07388551.2021.1931803. Epub 2021 Jun 20. PMID: 34151657.
- Allan SJ, De Bank PA, Ellis MJ. Bioprocess design considerations for cultured meat production with a focus on the expansion bioreactor. Frontiers in Sustainable Food Systems. 2019. doi: 10.3389/fsufs.2019.00044.
- Sampath SC, Sampath SC, Millay DP. Myoblast fusion confusion: the resolution begins. Skelet Muscle. 2018 Jan 31;8(1):3. doi: 10.1186/s13395-017-0149-3. PMID: 29386054; PMCID: PMC5793351.
- Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007 Dec;19(6):628-33. doi: 10.1016/j.ceb.2007.09.012. Epub 2007 Nov 8. PMID: 17996437; PMCID: PMC2215059.
- Guan X, Zhou J, Du G, Chen J. Bioprocessing technology of muscle stem cells: implications for cultured meat. Trends Biotechnol. 2022 Jun;40(6):721-734. doi: 10.1016/j.tibtech.2021.11.004. Epub 2021 Dec 6. PMID: 34887105.
- Rodriguez-Outeiriño L, Hernandez-Torres F, Ramírez-de Acuña F, Matías-Valiente L, Sanchez-Fernandez C, Franco D, Aranega AE. Muscle Satellite Cell Heterogeneity: Does Embryonic Origin Matter? Front Cell Dev Biol. 2021 Oct 15;9:750534. doi: 10.3389/fcell.2021.750534. PMID: 34722534; PMCID: PMC8554119.
- Syverud BC, Lee JD, VanDusen KW, Larkin LM. Isolation and Purification of Satellite Cells for Skeletal Muscle Tissue Engineering. J Regen Med. 2014;3(2):117. doi: 10.4172/2325-9620.1000117. PMID: 26413555; PMCID: PMC4582791.
- Garcia SM, Tamaki S, Lee S, Wong A, Jose A, Dreux J, Kouklis G, Sbitany H, Seth R, Knott PD, Heaton C, Ryan WR, Kim EA, Hansen SL, Hoffman WY, Pomerantz JH. High-Yield Purification, Preservation, and Serial Transplantation of Human Satellite Cells. Stem Cell Reports. 2018 Mar 13;10(3):1160-1174. doi: 10.1016/j.stemcr.2018.01.022. Epub 2018 Mar 1. PMID: 29478895; PMCID: PMC5918346.
- Ding S, Swennen GNM, Messmer T, Gagliardi M, Molin DGM, Li C, Zhou G, Post MJ. Maintaining bovine satellite cells stemness through p38 pathway. Sci Rep. 2018 Jul 17;8(1):10808. doi: 10.1038/s41598-018-28746-7. PMID: 30018348; PMCID: PMC6050236.
- Judson RN, Quarta M, Oudhoff MJ, Soliman H, Yi L, Chang CK, Loi G, Vander Werff R, Cait A, Hamer M, Blonigan J, Paine P, Doan LTN, Groppa E, He W, Su L, Zhang RH, Xu P, Eisner C, Low M, Barta I, Lewis CB, Zaph C, Karimi MM, Rando TA, Rossi FM. Inhibition of Methyltransferase Setd7 Allows the In Vitro Expansion of Myogenic Stem Cells with Improved Therapeutic Potential. Cell Stem Cell. 2018 Feb 1;22(2):177-190.e7. doi: 10.1016/j.stem.2017.12.010. Epub 2018 Jan 25. PMID: 29395054; PMCID: PMC6031334.
- Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, Rudnicki MA. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med. 2014 Oct;20(10):1174-81. doi: 10.1038/nm.3655. Epub 2014 Sep 7. Erratum in: Nat Med. 2014 Oct;(10):1217. Erratum in: Nat Med. 2015 Apr;21(4):414. PMID: 25194569; PMCID: PMC4191983.
- Flamini V, Ghadiali RS, Antczak P, Rothwell A, Turnbull JE, Pisconti A. The Satellite Cell Niche Regulates the Balance between Myoblast Differentiation and Self-Renewal via p53. Stem Cell Reports. 2018 Mar 13;10(3):970-983. doi: 10.1016/j.stemcr.2018.01.007. Epub 2018 Feb 8. PMID: 29429962; PMCID: PMC5918193.
- Thomas K, Engler AJ, Meyer GA. Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res. 2015 Feb;56(1):1-8. doi: 10.3109/03008207.2014.947369. Epub 2014 Aug 12. PMID: 25047058; PMCID: PMC4464813.
- Li X, Wang Z, Tong H, Yan Y, Li S. Effects of COL8A1 on the proliferation of muscle-derived satellite cells. Cell Biol Int. 2018 Sep;42(9):1132-1140. doi: 10.1002/cbin.10979. Epub 2018 Jul 23. PMID: 29696735.
- Wilschut KJ, Haagsman HP, Roelen BA. Extracellular matrix components direct porcine muscle stem cell behavior. Exp Cell Res. 2010 Feb 1;316(3):341-52. doi: 10.1016/j.yexcr.2009.10.014. Epub 2009 Oct 22. PMID: 19853598.
- Soice E, Johnston J. Immortalizing Cells for Human Consumption. Int J Mol Sci. 2021 Oct 28;22(21):11660. doi: 10.3390/ijms222111660. PMID: 34769088; PMCID: PMC8584139.
- Lu T, Huang Y, Wang H, Ma Y, Guan W. Multi-lineage potential research of bone marrow-derived stromal cells (BMSCs) from cattle. Appl Biochem Biotechnol. 2014 Jan;172(1):21-35. doi: 10.1007/s12010-013-0458-x. Epub 2013 Sep 18. PMID: 24043451.
- Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res. 2005 Feb;319(2):243-53. doi: 10.1007/s00441-004-1012-5. Epub 2004 Nov 20. PMID: 15654654.
- Ren Y, Wu H, Ma Y, Cang M, Wang R, Liu D. [Isolation, cultivation and identification of adipose-derived stem cell in bovines]. Sheng Wu Gong Cheng Xue Bao. 2010 Dec;26(12):1645-51. Chinese. PMID: 21387826.
- Gang EJ, Jeong JA, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004;22(4):617-24. doi: 10.1634/stemcells.22-4-617. PMID: 15277707.
- Cardoso TC, Okamura LH, Baptistella JC, Gameiro R, Ferreira HL, Marinho M, Flores EF. Isolation, characterization and immunomodulatory-associated gene transcription of Wharton's jelly-derived multipotent mesenchymal stromal cells at different trimesters of cow pregnancy. Cell Tissue Res. 2017 Feb;367(2):243-256. doi: 10.1007/s00441-016-2504-9. Epub 2016 Sep 27. PMID: 27677269.
- Cardoso TC, Ferrari HF, Garcia AF, Novais JB, Silva-Frade C, Ferrarezi MC, Andrade AL, Gameiro R. Isolation and characterization of Wharton's jelly-derived multipotent mesenchymal stromal cells obtained from bovine umbilical cord and maintained in a defined serum-free three-dimensional system. BMC Biotechnol. 2012 May 4;12:18. doi: 10.1186/1472-6750-12-18. PMID: 22559872; PMCID: PMC3443425.
- Hill ABT, Bressan FF, Murphy BD, Garcia JM. Applications of mesenchymal stem cell technology in bovine species. Stem Cell Res Ther. 2019 Jan 24;10(1):44. doi: 10.1186/s13287-019-1145-9. PMID: 30678726; PMCID: PMC6345009.
- Okamura LH, Cordero P, Palomino J, Parraguez VH, Torres CG, Peralta OA. Myogenic Differentiation Potential of Mesenchymal Stem Cells Derived from Fetal Bovine Bone Marrow. Anim Biotechnol. 2018 Jan 2;29(1):1-11. doi: 10.1080/10495398.2016.1276926. Epub 2017 Mar 7. PMID: 28267409.
- Mizuno H, Zuk PA, Zhu M, Lorenz HP, Benhaim P, Hedrick MH. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 2002 Jan;109(1):199-209; discussion 210-1. doi: 10.1097/00006534-200201000-00030. PMID: 11786812.
- Witt R, Weigand A, Boos AM, Cai A, Dippold D, Boccaccini AR, Schubert DW, Hardt M, Lange C, Arkudas A, Horch RE, Beier JP. Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering. BMC Cell Biol. 2017 Feb 28;18(1):15. doi: 10.1186/s12860-017-0131-2. PMID: 28245809; PMCID: PMC5331627.
- Jiang T, Xu G, Wang Q, Yang L, Zheng L, Zhao J, Zhang X. in vitro expansion impaired the stemness of early passage mesenchymal stem cells for treatment of cartilage defects. Cell Death Dis. 2017 Jun 1;8(6):e2851. doi: 10.1038/cddis.2017.215. Erratum in: Cell Death Dis. 2019 Sep 26;10(10):716. PMID: 28569773; PMCID: PMC5520885.
- Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008 Jun 1;68(11):4229-38. doi: 10.1158/0008-5472.CAN-07-5272. PMID: 18519682; PMCID: PMC2713721.
- Eom YW, Oh JE, Lee JI, Baik SK, Rhee KJ, Shin HC, Kim YM, Ahn CM, Kong JH, Kim HS, Shim KY. The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2014 Feb 28;445(1):16-22. doi: 10.1016/j.bbrc.2014.01.084. Epub 2014 Feb 1. PMID: 24491556.
- Rakian R, Block TJ, Johnson SM, Marinkovic M, Wu J, Dai Q, Dean DD, Chen XD. Native extracellular matrix preserves mesenchymal stem cell "stemness" and differentiation potential under serum-free culture conditions. Stem Cell Res Ther. 2015 Dec 1;6:235. doi: 10.1186/s13287-015-0235-6. PMID: 26620283; PMCID: PMC4666167.
- Mao Y, Hoffman T, Wu A, Goyal R, Kohn J. Cell type-specific extracellular matrix guided the differentiation of human mesenchymal stem cells in 3D polymeric scaffolds. J Mater Sci Mater Med. 2017 Jul;28(7):100. doi: 10.1007/s10856-017-5912-9. Epub 2017 May 22. PMID: 28534283; PMCID: PMC5440495.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663-76. doi: 10.1016/j.cell.2006.07.024. Epub 2006 Aug 10. PMID: 16904174.
- van der Wal E, Herrero-Hernandez P, Wan R, Broeders M, In 't Groen SLM, van Gestel TJM, van IJcken WFJ, Cheung TH, van der Ploeg AT, Schaaf GJ, Pijnappel WWMP. Large-Scale Expansion of Human iPSC-Derived Skeletal Muscle Cells for Disease Modeling and Cell-Based Therapeutic Strategies. Stem Cell Reports. 2018 Jun 5;10(6):1975-1990. doi: 10.1016/j.stemcr.2018.04.002. Epub 2018 May 3. PMID: 29731431; PMCID: PMC5993675.
- Rosselló RA, Chen CC, Dai R, Howard JT, Hochgeschwender U, Jarvis ED. Mammalian genes induce partially reprogrammed pluripotent stem cells in non-mammalian vertebrate and invertebrate species. Elife. 2013 Sep 3;2:e00036. doi: 10.7554/eLife.00036. PMID: 24015354; PMCID: PMC3762186.
- Ito N, Kii I, Shimizu N, Tanaka H, Takeda S. Direct reprogramming of fibroblasts into skeletal muscle progenitor cells by transcription factors enriched in undifferentiated subpopulation of satellite cells. Sci Rep. 2017 Aug 14;7(1):8097. doi: 10.1038/s41598-017-08232-2. Erratum in: Sci Rep. 2018 Apr 25;8(1):6733. Erratum in: Sci Rep. 2018 Jun 7;8(1):8991. PMID: 28808339; PMCID: PMC5556026.
- Prasad A, Teh DB, Shah Jahan FR, Manivannan J, Chua SM, All AH. Direct Conversion Through Trans-Differentiation: Efficacy and Safety. Stem Cells Dev. 2017 Feb 1;26(3):154-165. doi: 10.1089/scd.2016.0174. Epub 2016 Dec 19. PMID: 27796171.
- Fish KD, Rubio NR, Stout AJ, Yuen JSK, Kaplan DL. Prospects and challenges for cell-cultured fat as a novel food ingredient. Trends Food Sci Technol. 2020 Apr;98:53-67. doi: 10.1016/j.tifs.2020.02.005. Epub 2020 Feb 11. PMID: 32123465; PMCID: PMC7051019.
- Fraeye I, Kratka M, Vandenburgh H, Thorrez L. Sensorial and Nutritional Aspects of Cultured Meat in Comparison to Traditional Meat: Much to Be Inferred. Front Nutr. 2020 Mar 24;7:35. doi: 10.3389/fnut.2020.00035. PMID: 32266282; PMCID: PMC7105824.
- Sugii S, Wong CYQ, Lwin AKO, Chew LJM. Reassessment of adipocyte technology for cellular agriculture of alternative fat. Compr Rev Food Sci Food Saf. 2022 Sep;21(5):4146-4163. doi: 10.1111/1541-4337.13021. Epub 2022 Aug 26. PMID: 36018497.
- Frank D, Joo ST, Warner R. Consumer Acceptability of Intramuscular Fat. Korean J Food Sci Anim Resour. 2016;36(6):699-708. doi: 10.5851/kosfa.2016.36.6.699. Epub 2016 Dec 31. PMID: 28115880; PMCID: PMC5243953.
- Sugii S, Wong CYQ, Lwin AKO, Chew LJM. Alternative fat: redefining adipocytes for biomanufacturing cultivated meat. Trends Biotechnol. 2022 Sep 15:S0167-7799(22)00223-2. doi: 10.1016/j.tibtech.2022.08.005. Epub ahead of print. PMID: 36117023.
- Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, Hughes SI, Whittington FM. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008 Apr;78(4):343-58. doi: 10.1016/j.meatsci.2007.07.019. Epub 2007 Jul 21. PMID: 22062452.
- Dohmen RGJ, Hubalek S, Melke J, Messmer T, Cantoni F, Mei A, Hueber R, Mitic R, Remmers D, Moutsatsou P, Post MJ, Jackisch L, Flack JE. Muscle-derived fibro-adipogenic progenitor cells for production of cultured bovine adipose tissue. NPJ Sci Food. 2022 Jan 24;6(1):6. doi: 10.1038/s41538-021-00122-2. PMID: 35075125; PMCID: PMC8786866.
- Torii SI, Kawada T, Matsuda K, Matsui T, Ishihara T, Yano H. Thiazolidinedione induces the adipose differentiation of fibroblast-like cells resident within bovine skeletal muscle. Cell Biol Int. 1998;22(6):421-7. doi: 10.1006/cbir.1998.0270. PMID: 10328850.
- Wosczyna MN, Perez Carbajal EE, Wagner MW, Paredes S, Konishi CT, Liu L, Wang TT, Walsh RA, Gan Q, Morrissey CS, Rando TA. Targeting microRNA-mediated gene repression limits adipogenic conversion of skeletal muscle mesenchymal stromal cells. Cell Stem Cell. 2021 Jul 1;28(7):1323-1334.e8. doi: 10.1016/j.stem.2021.04.008. Epub 2021 May 3. PMID: 33945794; PMCID: PMC8254802.
- Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, Abatangelo G, Pozzan T, Pinton P, Rizzuto R. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A. 2008 Jan 29;105(4):1226-31. doi: 10.1073/pnas.0711402105. Epub 2008 Jan 22. PMID: 18212116; PMCID: PMC2234120.
- Liu Y, Jiang B, Fu C, Hao R. Cloning and characterization of adipogenin and its overexpression enhances fat accumulation of bovine myosatellite cells. Gene. 2017 Feb 15;601:27-35. doi: 10.1016/j.gene.2016.11.040. Epub 2016 Dec 1. PMID: 27914980.
- Li XZ, Yan Y, Zhang JF, Sun JF, Sun B, Yan CG, Choi SH, Johnson BJ, Kim JK, Smith SB. Oleic acid in the absence of a PPARγ agonist increases adipogenic gene expression in bovine muscle satellite cells1. J Anim Sci. 2019 Oct 3;97(10):4114-4123. doi: 10.1093/jas/skz269. PMID: 31424542; PMCID: PMC6776314.
- Scott MA, Nguyen VT, Levi B, James AW. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011 Oct;20(10):1793-804. doi: 10.1089/scd.2011.0040. Epub 2011 Jun 20. PMID: 21526925; PMCID: PMC3182038.
- Mehta F, Theunissen R, Post MJ. Adipogenesis from Bovine Precursors. Methods Mol Biol. 2019;1889:111-125. doi: 10.1007/978-1-4939-8897-6_8. PMID: 30367412.
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012 Feb;53(2):227-46. doi: 10.1194/jlr.R021089. Epub 2011 Dec 2. PMID: 22140268; PMCID: PMC3269153.
- Ong WK, Sugii S. Adipose-derived stem cells: fatty potentials for therapy. Int J Biochem Cell Biol. 2013 Jun;45(6):1083-6. doi: 10.1016/j.biocel.2013.02.013. Epub 2013 Mar 1. PMID: 23458962.
- Yin J, Jin X, Beck S, Kang DH, Hong Z, Li Z, Jin Y, Zhang Q, Choi YJ, Kim SC, Kim H. in vitro myogenic and adipogenic differentiation model of genetically engineered bovine embryonic fibroblast cell lines. Biotechnol Lett. 2010 Feb;32(2):195-202. doi: 10.1007/s10529-009-0142-y. Epub 2009 Oct 16. PMID: 19834648.
- Stanton MM, Tzatzalos E, Donne M, Kolundzic N, Helgason I, Ilic D. Prospects for the Use of Induced Pluripotent Stem Cells in Animal Conservation and Environmental Protection. Stem Cells Transl Med. 2019 Jan;8(1):7-13. doi: 10.1002/sctm.18-0047. Epub 2018 Sep 24. PMID: 30251393; PMCID: PMC6312526.
- Yuen JSK Jr, Stout AJ, Kawecki NS, Letcher SM, Theodossiou SK, Cohen JM, Barrick BM, Saad MK, Rubio NR, Pietropinto JA, DiCindio H, Zhang SW, Rowat AC, Kaplan DL. Perspectives on scaling production of adipose tissue for food applications. Biomaterials. 2022 Jan;280:121273. doi: 10.1016/j.biomaterials.2021.121273. Epub 2021 Nov 29. PMID: 34933254; PMCID: PMC8725203.
- Xu W, Li H, Peng L, Pu L, Xiang S, Li Y, Tao L, Liu W, Liu J, Xiao Y, Liu S. Fish Pluripotent Stem-Like Cell Line Induced by Small-Molecule Compounds From Caudal Fin and its Developmental Potentiality. Front Cell Dev Biol. 2022 Jan 20;9:817779. doi: 10.3389/fcell.2021.817779. PMID: 35127728; PMCID: PMC8811452.
- Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008 May 21;3(5):e2213. doi: 10.1371/journal.pone.0002213. PMID: 18493317; PMCID: PMC2374903.
- Ben-Arye T, Levenberg S. Tissue engineering for clean meat production. Frontiers in Sustainable Food Systems. 2019;3.
- Bomkamp C, Skaalure SC, Fernando GF, Ben-Arye T, Swartz EW, Specht EA. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv Sci (Weinh). 2022 Jan;9(3):e2102908. doi: 10.1002/advs.202102908. Epub 2021 Nov 16. PMID: 34786874; PMCID: PMC8787436.
- Post MJ, Levenberg S, Kaplan DL, Genovese N, Fu JA, Bryant CJ, Negowetti N, Verzijden K, Moutsatsou P. Scientific, sustainability and regulatory challenges of cultured meat. Nat Food. 2020;1(7):403-415.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.