General Science . 2022 November 30;3(11):1418-1420. doi: 10.37871/jbres1614.
Comparison Study of Electrooxidation and Photo-Electroxidation for the Treatment of Solutions Containing Acid Blue 9 Dye
Zejli H1*, El Brychy R1, Rguiti MM1 and Groenen Serrano K2*
2Laboratoire de Génie Chimique, Université de Toulouse, CNRS, INPT, UPS, Toulouse, France
The objective of this study is to investigate the contribution of light energy in the electrochemical oxidation treatment of a solution containing a model molecule, Acid Blue 9 dye (AB 9) using a low-cost electrode elaborated by electrodeposition from a lead salt. The elaboration procedure and the characteristics of the obtained Ti/b-PbO2 electrode are given in the Supplementary Information. First, electrolysis and I-E curves were performed in order to determine the best conditions for dye removal and mineralization. Then the effect of illumination is studied.
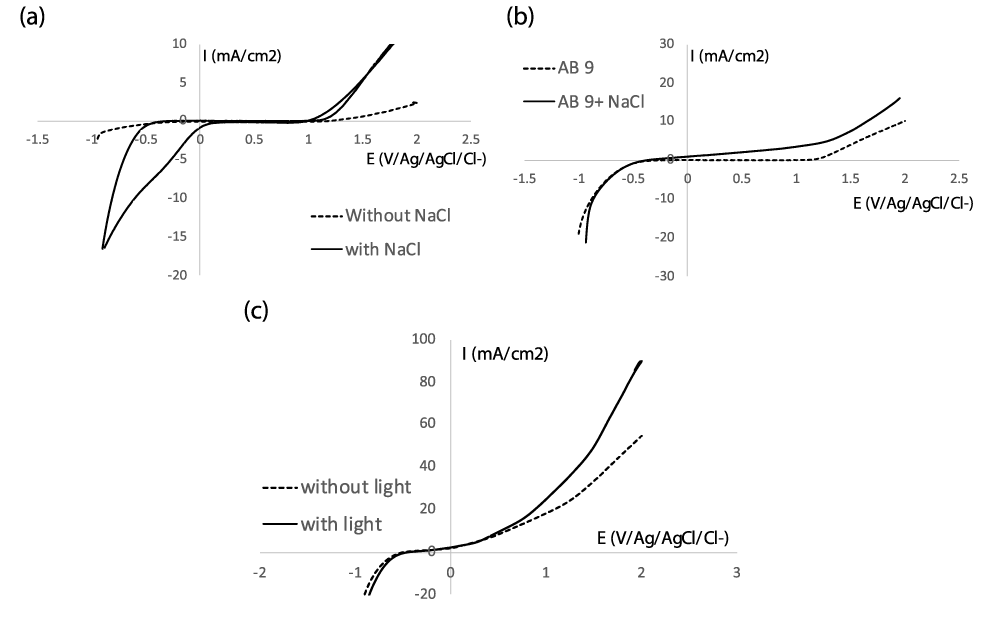
The voltammogram in figure 1a highlights the wide electrochemical window that PbO2 provides and so demonstrates the potentialities of this anode material for the oxidation of refractory compounds. According to figure 1b, no oxidation peak due to the presence of AB 9 appears on PbO2 electrode. This result provides information on the reactional process underlying the oxidation of the dye, which occurs indirectly through the hydroxyl radicals produced during the oxidation of water or any other oxidant produced electrochemically. Additionally, the type of electrolyte can significantly affect the dye oxidation.
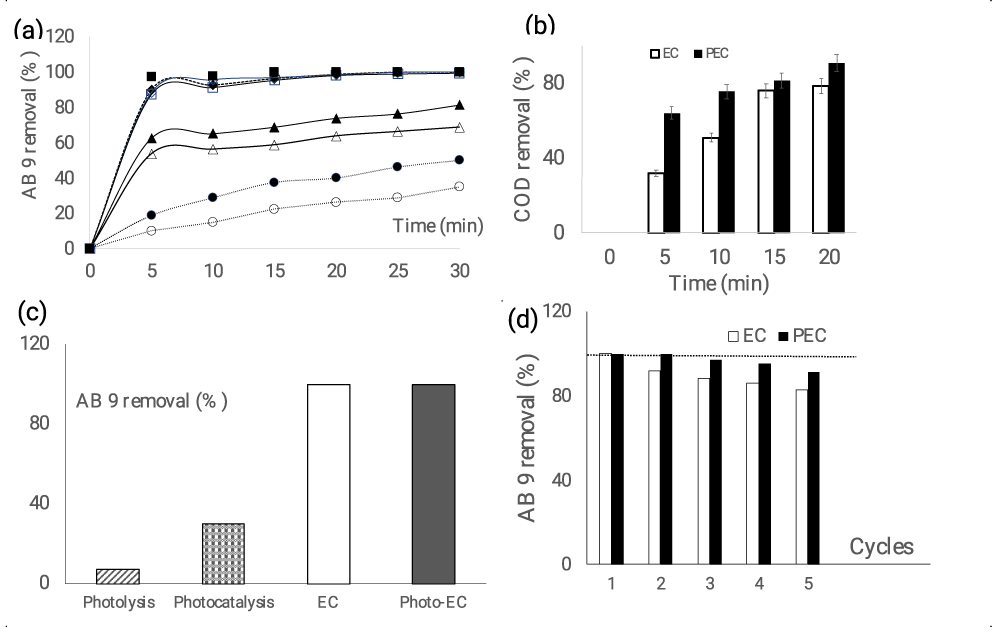
A series of galvanostatic electrolysis were carried out on solutions containing AB 9 using additions of sodium chloride (0.002, 0.005, and 0.01 mol L-1) to a primary electrolyte of Na2SO4 (0.1 mol L-1). Figure 2a demonstrates that after just five minutes of electrolysis, a NaCl concentration of at least 0.005 molL-1 (equivalent to a molar concentration ratio of allows for the removal of more than 90% of the compound, but in the absence of NaCl, only 10% vanished. A ratio of yields an intermediate value of 53%. This outcome emphasizes how important chloride ions were in the process of the solution's decolorization.
According to the voltammograms obtained on figure 1a,b, an oxidation current density resulting from the oxidation of chloride appears at potentials higher than 1.3 V/Ag/AgCl/Cl-, associated with a cathodic peak at -0.3 V/ref. Equation 1 and 2 gives the oxidation reaction of chloride ions into hypochlorous acid which is the strongest oxidizing agent of the chlorine oxyanions. Its oxidizing ability makes it an excellent bleaching agent.
Additional experiments that involved changing the initial pH to three different values—3, 6.8 and 10—showed that abatement of 88% and 82.5 % were achieved after 5 minutes of electrolysis at pH 3 and 6.8 respectively, where the HOCl form predominates, compared to 70% at pH 10, where the OCl- form is the most stable.
Additionally, the Chemical Oxygen Demand (COD) measurements during the electrolysis reveal that after 5 minutes, 68% of the initial COD is still present, and 20 minutes of electrolysis are needed to accomplish 79% abatement (Figure 2b). The role of light energy in treating this solution was investigated concurrently. As seen in figure 2b, the reactor is exposed to UV and visible light, which speeds up decolorization and, in particular, COD abatement. In fact, under illumination, the COD abatement rises from 43 to 65%. This outcome emphasizes the synergistic effect of light assisted electrochemical oxidation. This trend was confirmed by the voltammogram of figure 1c which evidences the increasing current density under illumination.
The impact of each technique on the process of the solution's decolorization was then investigated through an experiment (Figure 2c). Only 7% of the dye can be removed by light alone (photolysis), but this rate can be increased to 30% by submerging the PbO2 deposit in the solution (photocatalysis), 100% by polarizing the sample for 30 minutes with (photoelectrocatalysis), or without adding additional illumination (electrocatalysis). Evaluating the operating cost and the lifetime of the electrodes is crucial for determining whether a process is economically viable.
The EC energy consumption (in kWh m-3) was determined using Equation 3:
where Ucell, j,t, and V are, respectively, the average cell voltage (V), electrical current (A), electrolysis duration (s), and volume of treated solution (m3). When treatment is assisted by lighting, the lamp's (160 W) power usage is also taken into account. After 25 and 15 minutes for EC and PEC, respectively, it is discovered that 0.027 and 0.055 kWhm-3 with I = 5 mAcm-2 are needed to oxidize 90% of the AB9 dye (80 ppm). When light energy is used, electricity consumption increases by twofold. However, employing the anode material over multiple cycles (one cycle equaling 30 minutes of electrolysis at 15 mA cm-2) reveals a 17% reduction in material performance at the fifth cycle, which can be largely compensated by light energy, as shown in figure 2d.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.