Biology Group . 2023 January 27;4(1):117-125. doi: 10.37871/jbres1655.
-
Subject area(s):
- Zosteran pectin
- Adhesion
- Bioactivities
- Cryoprotectants
- Leukocytes
- Cell viability
New Possibilities of Using Zosteran Pectin from the Marine Plant Zostera marina (L.)
Marta Sergushkina1*, Oksana Zaitseva1, Andrey Khudyakov1, Tatyana Polezhaeva1, Olga Solomina1 and Inna Paturova2
2Kirov State Medical University, Kirov, Russia
- Zosteran pectin
- Adhesion
- Bioactivities
- Cryoprotectants
- Leukocytes
- Cell viability
Abstract
Zosteran pectin isolated from the perennial marine plant Zostera marina (L.) has a complex molecular structure and a wide range of biological activity.
Aim Study: This study aims to identify new properties (adhesive, cryoprotective) of zosteran pectin.
Materials and Methods: The adhesive properties of zosteran were investigated by evaluating the microorganism adhesiveness index and the erythrocyte participation rate. To assess the cryoprotective properties of zosteran, the cryoscopic method and various methods for assessing the viability of leukocytes (cryoresistance, cell membrane integrity, and phagocytosis) were used.
Results: Zosteran pectin in each assay consistently revealed more adhesive and cryoprotective properties compared to other substances. The results revealed that zosteran at a concentration of 0.3% increase the percentage of red blood cells with lactobacilli attached to their surface. The addition of zosteran to various cryoprotectants changed the osmolarity and freezing point of only the mixture of pectin with DMSO. Zosteran/DMSO cryosolution significantly better retained the viability of leukocytes after exposure to negative temperatures (-80°C), compared with a solution containing only DMSO.
Conclusion: Our data reveal new properties of zosteran, due to which pectin changes the conditions of bacterial adhesive activity. This can be used to enhance the perception of microorganisms by intestinal cells in representatives of normal microflora or to reduce it in pathogens of infectious diseases. The obtained cryoprotective effect of the solution with zosteran can be used for further research on the preservation of biological objects (spermatozoa, platelets, etc.) at low temperatures.
Introduction
It is known that marine flora plants have a pronounced biological activity, which is associated with a number of features of their biochemistry. These features, in all likelihood, were formed as a result of the adaptation of plants to life in the marine environment (water salinity, temperature fluctuations, an increase in water viscosity, light regime and nutrient content) [1]. One of the most promising marine flora pectins is zosteran pectin. It is obtained from the perennial marine flowering plant Zostera marina (L.) fam. Zosteraceae [2]. Zosteran has a high gel-forming ability, plays an important role in the growth and development of a plant, maintains its turgor and regulates water-salt exchange with the environment, causes resistance to natural processes of decay and destruction.
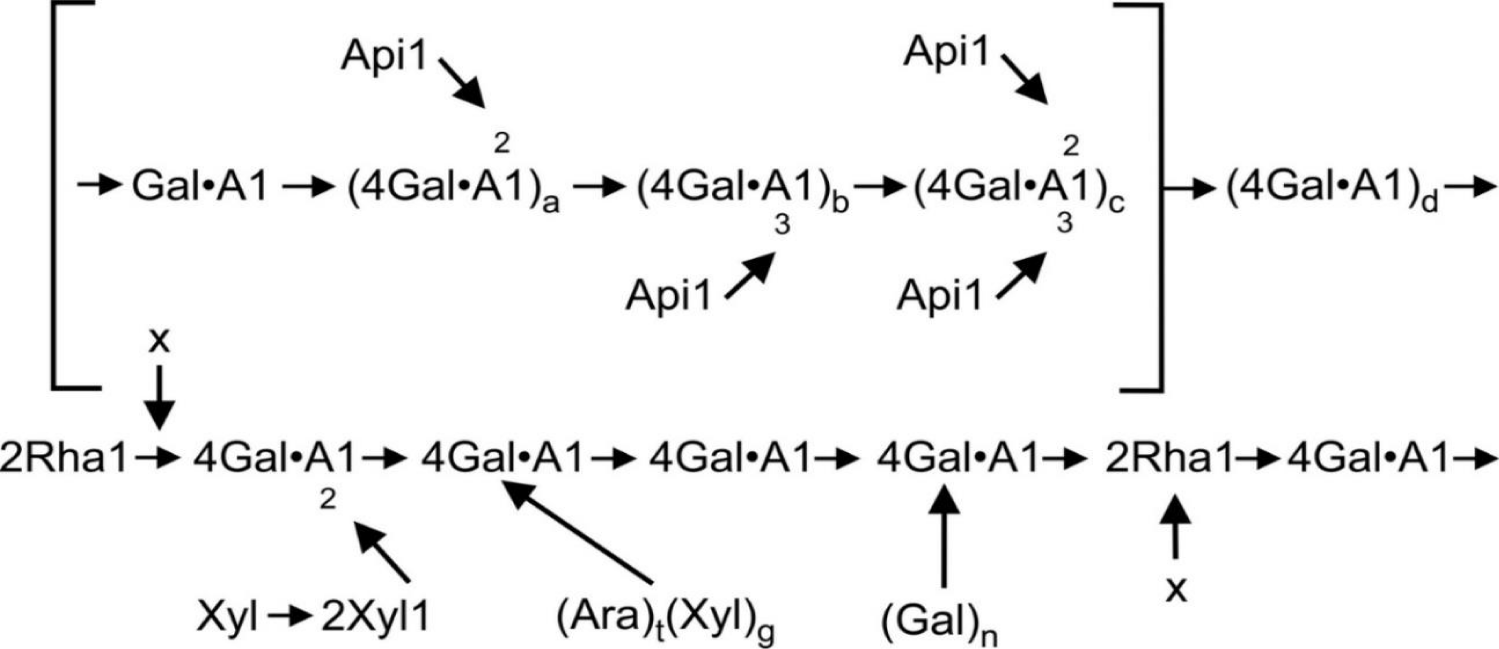
Zosteran is a carbohydrate polymer of galacturonic acid and has a more complex structure than land plant pectins. It is important to note that the design of its molecule consists of several structural blocks, which include various sugars (arabinose, galactose, rhamnose, mannose, apiose, ribose) [3,4]. The main block of the carbohydrate chain consists of α (1→4) polygalacturonic acid interconnected (1→2) by rhamnose bonds (Figure 1) [4]. About a quarter of the zosteran molecule is apigalacturonan containing D-apiosa attached by (1→2) and (1→3) bonds to galacturonan. Other side chains contain D-xylose, D-galactose, L-arabinose and 2-O-methyl-D-xylose. Also, zosteran, in contrast to the pectins of land plants, has an extremely low degree of methoxylation [4].
The structural features of the zosteran molecule ensure its practical application [3,5]. In the course of experimental studies, the following pharmacological effects were revealed in zosteran pectin: hypocholesterolemic, enterosorption, antibacterial, antiviral, antitumor, antiulcer, immunomodulatory, antioxidant [6-11]. Currently, there is an active search for pectins for the production of new functional biomaterials based on pectin gels with sorption, anti-adhesive, superhydrophobic, and other valuable properties [12]. The results of scientific studies on the effect of pectins on bacterial adhesion are contradictory.
Some authors show that pectins and pectin oligosaccharides have antiadhesive properties against pathogenic microorganisms [13,14]. Other authors describe the rapid adhesion of bacteria when pectin is added to the habitat [15]. There are several theories describing adhesion from different positions (adsorption, mechanistic, chemical, electronic, electrical, diffusion). All theories are based on the intermolecular interaction of substances with each other. Any interaction (chemical or physical) is provided by the formation of complexes. The low degree of methoxylation and the unique structure of the zosteran molecule with a high content of functional groups (carboxylic, hydroxyl) ensure its high complexing activity [3,4,6-8,16,17]. It is important to note that scientific studies on the adhesive properties of zosteran have not been found to date. Therefore, in our study, we used the method of adhesion of microorganisms to the surface of erythrocytes under the influence of zosteran.
It is known that the pectins of some plants have cryoprotective properties [18] due to the content of active groups -OH and -COOH in the molecular structure. Active groups underlie the effective protective action of all known cryoprotectants [19]. Zosteran contains these carboxyl and hydroxyl functional groups. Therefore, this pectin was studied as a cryoprotectant using cryoscopic analysis and the method of cell cryopreservation. Thus, for the first time, we will identify and evaluate new properties of zosteran as a substance that affects the adhesive properties of microorganisms, and a substance that has a cryoprotective effect. It will also be given the opportunity to assess whether this pectin has the potential for further effective use in practice.
Materials and Methods
Characterization of the pectins
In this study, two pectins were used. Zosteran is a pectin from a sea flowering plant. Apple pectin "Classic AU-701" - pectin from a terrestrial flowering plant.
The biologically active additive "Zosterin - Ultra" (LLC "Aquamir", St. Petersburg) was used in the work, with the content of low molecular weight fraction (from 1 to 30 kD) not less than 30%. "Zosterin - Ultra" is made from sea grass of the Zosteraceae family using a special technology that does not include chemical components. It is a natural multifunctional pectin of a new generation, acting on the body simultaneously as an enterosorbent, hemosorbent and immunomodulator. Contains 60% D-galacturonic acid and 40% neutral monosaccharides. One of the main neutral monosaccharides is D-apiosis – 3-8%. It also contains D-xylose, L-arabinose, L-rhamnose. The degree of esterification of pectin is 5% [5].
Commercial apple pectin "Classic AU-701" (Herbstreith & Fox KG, Germany) was used for comparison. The structure of the apple pectin molecule is completely linear, since AU-701 pectin contains 91% of galacturonic acid residues. This area of homogalactorunan consists of 1, 4-linked α-D-galactopyranosyluronic acid residues, and these regions are joined by one or two L-rhamnopyranose residues. The rest of the molecule (9%) consists of neutral monosaccharides. The percentage of each type of monosaccharide in apple pectin is Gal-2.4%, Ara-0.3%, Rha-1.4%, Glc-1.6%, Xyl-2.9%. Pectin esterification degree is 38-40% [12,20].
Experimental procedure for adhesion of lactobacilli
As a culture of microorganisms, the drug Lactobacterin (FSUE NPO Microgen, Moscow) was used, which is a biomass of a live, antagonistic active strain of lactobacilli (LactobacilIus plantarum 8P-A3 or Lactobacillus fermentum 90T-C4), lyophilized with added sucrose in the culture medium -gelatinous milky drying habitat. A suspension of microorganisms was prepared in isotonic sodium chloride solution at a concentration of 109 CFU / mL. The adhesive properties were studied according to the method of V.I. Brilis, with used erythrocytes as models to assess the adhesiveness of microorganisms [21]. According to the literature, the results of this technique can be interpreted for intestinal cells [22,23]. In the work, we used a solution of pectins (zosteran and apple "Classic AU-701") - at concentrations of 1.2%, 0.6%, 0.3%, 0.15%. A pectin solution was prepared using distilled water as a solvent.
As a biological object, we used venous blood cells from volunteer donors with their informed consent in accordance with the “Law of the Russian Federation on the donation of blood and its components”. Blood was taken in an amount of 6 ml into special test tubes. Venous blood and one of the pectin solutions (in the above concentrations) were mixed in equal volumes (50 μL each). The resulting mixture in a volume of 100 μL was mixed with 100 μL of microorganisms and incubated at 37°С for 30 min, shaking regularly (every 3 min). After that, a smear was prepared, dried, and stained according to the May-Grunwald and Romanovsky method. The study of adhesion was carried out using a light microscope with a count of 100 erythrocytes. The concentration of erythrocytes was within the normal range of 4.4-4.7 × 1012/L. The total number of studies with each pectin in the indicated concentrations was 240. The results of the experiment were assessed by the Microorganism Adhesiveness Index (IAM), which characterizes the average number of microbial cells on one erythrocyte participating in the adhesion process and by the erythrocyte participation Coefficient (CUE)-the percentage of erythrocytes having a adhered microbes to its surface.
Cryoosmotic analysis
The osmometer-cryoscope OSKR-1 device (NPP Burevestnik, St. Petersburg) was used to determine osmolar concentrations (absolute error in the measurement range from 0 to 500 mOsm/L was 2.0) and freezing temperatures (absolute error in the range from -0.930 to -3.720°C was ± 0.010) aqueous solutions of cryoprotectants: dimethylsulfoxide (DMSO) - 5%, dimethylacetamide (DMAC) - 5%; aqueous solutions of each cryoprotectant with zosteran in the concentration range 0.1%-1% (wt%). All solutions were taken at the final concentration. Ten tests were performed with each solution. The investigated solution with a volume of 0.3 mL was placed in a plastic cuvette; the measuring element was immersed in it, installed in the thermostatted chamber of the device and measured. The operation of this device is based on the cryoscopic method, which makes it possible to measure the decrease in the freezing point (crystallization) of a solution in comparison with the freezing point of a pure solvent (water).
Cryopreservation of cells
As a biological object, we used venous blood cells from volunteer donors with their informed consent in accordance with the “Law of the Russian Federation on the donation of blood and its components”. Venous blood was frozen in a cryoprotectant medium DMSO (control group) and in a mixture of DMSO and zosteran pectin (experimental group). The viability of leukocytes as the most sensitive to cryopreservation of cells containing a nucleus and a multicomponent granular complex was assessed.
Before freezing, the blood was mixed (1:1) with a cryopreservative containing zosteran (0.4%) and DMSO (10%). The final concentration of substances in the solution was 0.2% and 5%, respectively. The choice of the indicated concentrations of substances was based on previously obtained data on the use of pectins in cryoprotective mixed [24]. The mixture was cooled according to slow nonlinear programs using electric freezers. After 15 min of exposure of blood with a cryopreservative at room temperature in cryovials, the mixture was placed in an alcohol bath (96% ethanol), chilled at -20°C (electric freezer "Derby", Denmark) for another 15 min. Then the cryovials were transferred for further freezing and storage in the chamber air of an electric freezer at -80°C "Vestfrost" (Denmark). The average cooling rate from 20°C to -20°C was 2.6°C/min, then down to -80°C at 3.5°C/min. After 1 day of storage, the samples were warmed up a temperature of +38°C in a water bath with vigorous shaking of the cryovial for 20-25 sec. A total of 15 procedures were performed for freezing and warming blood cells in mixed with a combined preservative.
The following parameters were investigated using light microscopy (Nikon H550S, Japan) before cooling and after warming: the degree of cryoresistance of various cell populations in smears stained according to May-Grunwald and Romanovsky, the integrity of the cell membrane of leukocytes in samples with 1.0% solution supravital dye eosin, the percentage of phagocytic neutrophils using inert latex particles with a diameter of 0.08 μm (Sigma-Aldrich, Germany) [25]. For the analysis of each parameter, 100 cells were evaluated. The parameter value before freezing was taken as 100%.
Statistical analysis
Statistical data processing consisted in calculating the arithmetic value and standard deviation. The Wilcoxon signed rank test was used to determine the statistical significance of the differences between the values using the «BIOSTAT, 2007» software. Values were considered significant at p ≤ 0.05 [26].
Results
Influence of pectins on the adhesive properties of microorganisms
It was found that in the presence of the pectins used in the cell habitat, the adhesiveness index of lactobacilli does not change. However, if pectin zosteran is present in the habitat at a concentration of 0.3%, the percentage of erythrocytes with attached microbes on their surface is statistically significantly increased compared to those without pectin added (Table 1).
| Table 1: Influence of pectins at different concentrations on the adhesion indices of Lactobacteria microorganisms to human erythrocytes (M ± σ, n = 10). | ||||
| Series | Pectin Concentration, % | IAM | CUE% | |
| Control | 0 | 1.57 ± 0.25 | 38 ± 7.57 | |
| Apple pectin "Classic AU-701" | 1.2 | 1.65 ± 0.32 | 34 ± 5.96 | |
| 0.6 | 1.61 ± 0.25 | 37 ± 6.96 | ||
| 0.3 | 1.75 ± 0.13 | 39 ± 6.48 | ||
| 0.15 | 1.78 ± 0.24 | 40 ± 10.91 | ||
| Zosteran | 1.2 | 1.60 ± 0.16 | 38 ± 5.64 | |
| 0.6 | 1.66 ± 0.42 | 43 ± 12.38 | ||
| 0.3 | 1.75 ± 0.23 | 47 ± 9.64*#γ | ||
| 0.15 | 1.75 ± 0.22 | 43 ± 9.30γ | ||
| IAM: Microorganism Adhesion Index (average number of microbial cells on one red blood cell). CUE%: Erythrocyte participation coefficient (the percentage of red blood cells with adherent microbes on their surface). *: Significance of difference from control series at p < 0.05. #: Significance of difference from series with the value of the zosteran pectin concentration indicator 1.2% at p < 0.05. γ: Significance of difference from the series with the value of the indicator of the concentration of apple pectin 1.2% at p < 0.05. |
||||
It was found that when apple pectin was added to the habitat, there were no statistically significant differences with the indicator without pectin added. It was found that the CUE% index with the addition of zosteran at a concentration of 0.15 and 0.3% is statistically significantly higher than with the addition of apple pectin.
Change in cryosmotic properties of cryoprotectants under the action of pectins
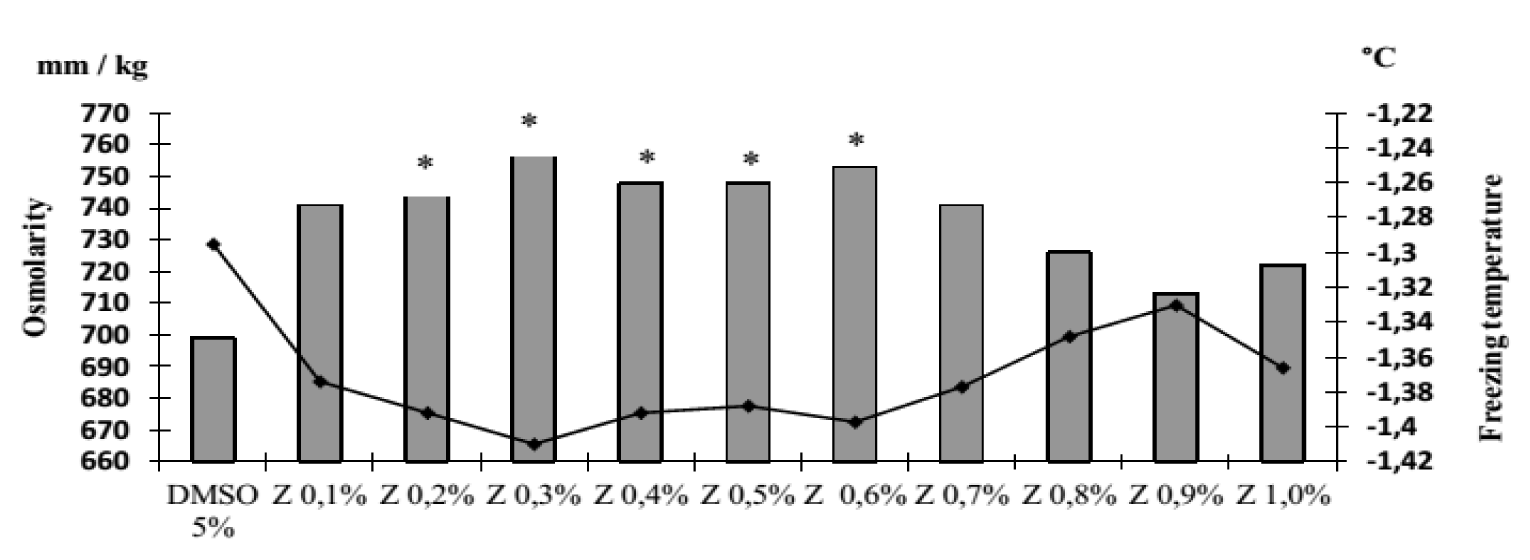
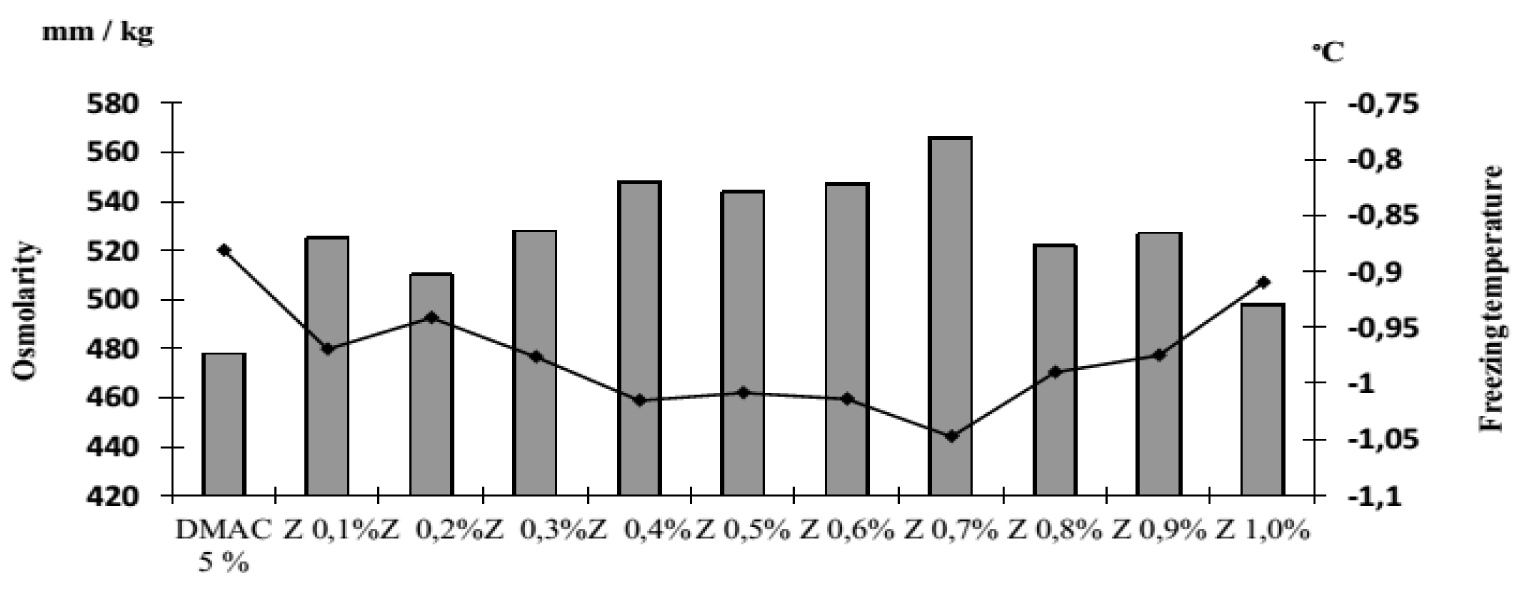
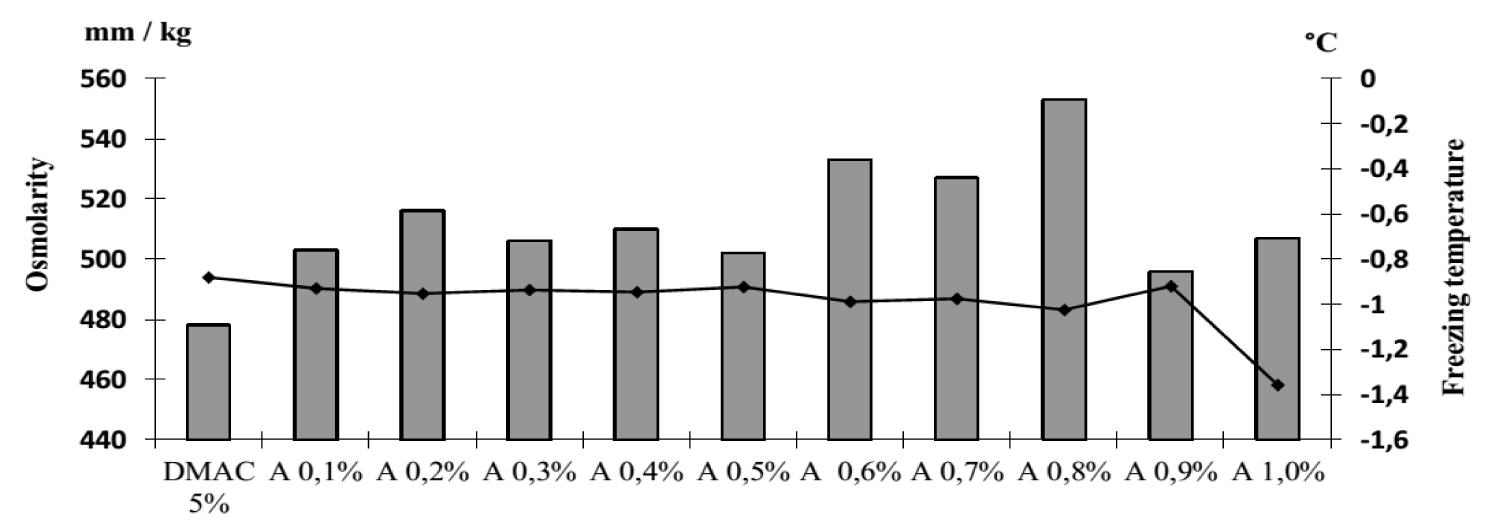
It was revealed that the osmolarity and freezing point of the cryoprotectants used in the work differ. So, the osmolarity of DMAC is 478 mOsm/L, and its freezing occurs at -0.881°C, respectively. DMSO has a higher osmolarity of 699 mOsm/L and a lower freezing point of -1.295°C. The osmolarity and freezing point of a 1% solution of zosteran are 40 mOsm/L and -0.073°C respectively. We found that in the presence of zosteran at concentrations from 0.2% to 0.6%, the osmolarity and freezing point of DMSO (5%) increased statistically significantly (Figure 2). However, when this pectin was added at any indicated concentration to DMAC (5%), the studied parameters did not change (Figure 3).
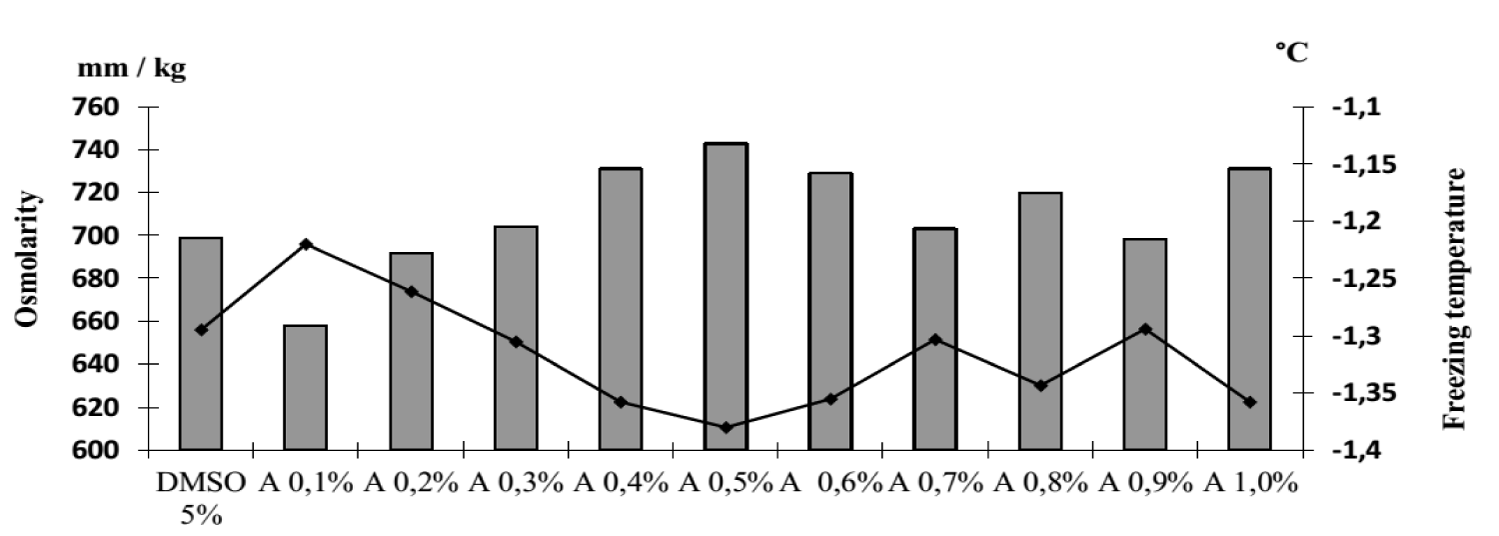
Osmolarity and freezing point of 1% apple pectin solution are 15 mOsm/L and -0.028°C. It was found that in the presence of apple pectin in the range of indicated concentrations, the osmolarity and freezing temperatures of the studied cryoprotectants did not change (Figures 4,5).
Positive effect of zosteran in cryopreservation of leukocytes
When assessing the viability of leukocytes, it was found that after 1 day of storage at -80°C in DMSO and zosteran, the cell indices were statistically significantly higher than when they were frozen and stored only in DMSO (Table 2). So the number of leukocytes with an intact membrane increased by 18%, the total number of granulocytes - by 16%, and the level of phagocytic activity of neutrophils, as the main indicator of viability, increased by 20%, in comparison with the level of cell preservation in DMSO.
When freezing and storing leukocytes under the same conditions in a mixture of DMSO and apple pectin, the level of functional activity of the cells remained the same as when using only DMSO (Table 2).
| Table 2: Leukocyte preservation indices (М ± σ, n = 15) after storage for 1 day at -80°С in various solutions. | |||
| Substance | Indicators of Preservation, % | ||
| Eosin Resistance | Number of Granulocytes | Phagocytic Activity of Neutrophils | |
| DMSO (5%) | 74 ± 3.02 | 70 ± 9.21 | 45 ± 10.54 |
| DMSO (5%) + Zosteran (0.2%) | 92 ± 3.89* | 86 ± 8.60* | 65 ± 8.73* |
| DMSO (5%) + Apple pectin (0.2%) | 72 ± 3.36 | 71 ± 7.12 | 43 ± 8.45 |
| Note: Data are presented as a percentage in relation to the level before freezing, taken as 100%. *: Significance of difference from series with the value of the DMSO indicator at p < 0.05. |
|||
Discussion
According to the results of recent studies, it is known that the ability of pectins to have adhesive or anti-adhesive properties depends on its molecular structure. It turned out that fast cell adhesion is associated with a lower degree of esterification of pectin, a higher molecular weight of the substance and the specific viscosity of pectin [15,27]. Based on the data we received on the effect of pectins on the adhesive properties of microorganisms, it can be concluded that zosteran pectin can be used as a substance that will create the best conditions for the life of lactobacilli and increase the ability of cells to perceive microorganisms on their surface in the zone of their natural existence. Probably, the unique structure of the zosteran molecule, a very low degree of its esterification (5%), and a high molecular weight [3] provide the formation of a layer covering the erythrocyte surface and increasing the ability of cells to perceive lactobacilli. It is known that low esterified pectins form gels due to intermolecular bridges using calcium and magnesium ions. Intermolecular bridges have a network structure that probably provides new space or new free sites for bacteria to attach. It should be noted that apple pectin has its own characteristics in comparison with zosteran: a linear molecular chain structure and a higher degree of esterification (38-40%). This is probably why apple pectin did not affect the adhesion of lactobacilli. In our study, zosteran at a concentration of 0.3% increased the number of red blood cells capable of attaching bacteria to their surface. Therefore, such a concentration of pectin is the most effective for the formation of optimal conditions for the attachment of microorganisms. In this regard, it is impractical to increase or decrease the concentration of zosteran. Thus, zosteran as a complex carbohydrate polymer providing a change in the conditions for the adhesive activity of bacteria can be used to enhance the perception of microorganisms by intestinal cells for representatives of normal microflora or to reduce it for pathogens of infectious diseases. This expands the list of previously described pharmacological effects of zosteran [4,8-11].
When evaluating the interaction of zosteran in various concentrations with cryoprotectants, it was found that its addition entails a change in the osmolarity and freezing point of only a mixture of pectin with DMSO. Probably, this result is associated with the structure of the molecules of substances. Zosteran has a more complex structure than pectins of terrestrial plants [4,10,25]. It has a straight and ramified area. The branched spatial structure is similar to a bundle of tangled threads, consisting of cells of different sizes between the main linear chains and their lateral branches. The main chain in the zosteran molecule is 60% and is represented by residues of galacturonic acid, the rest of the molecule consists of neutral monosaccharides (apiosis, xylose, arabinose, rhamnose). It is known that unmethoxylated residues of galacturonic acid in pectins form intermolecular bridges using calcium and magnesium ions [24]. These bridges are formed by ionic and hydrogen bonds between carboxyl and hydroxyl groups, which leads to the formation of a network structure. This complex molecular structure provides the high complexing activity of zosteran that was previously described [4,6-10,16].
Note that according to Shrago MI, et al. [19] all cryoprotectants used in cryobiology have a number of specific functional groups, for example, DMSO (= S = O-CH3), DMAC (-CH3). From the discussion of the structure of the cryopreservative components, it follows that the complex was probably formed upon contact of the functional groups of zosteran (-OH, -CH3) and DMSO (= S = O-CH3), but not DMAC (-CON (CH3) 2). This complex is a network for capturing more water molecules. As a result, the osmolarity of the solution increases, and the temperature of its crystallization decreases. Note that a change in the cryoosmotic parameters of an aqueous solution of DMSO occurred with the addition of zosteran in the concentration range of 0.2-0.6%. Probably, such a number of zosteran molecules (in particular, free active groups -OH and -COOH) is sufficient to interact with free functional groups of DMSO and membrane components.
Above, we found that in the presence of zosteran, the freezing point of the DMSO solution decreases. In cryobiology, there is an opinion that lowering the freezing point of a cryoprotectant can help reduce the risk of cell damage during freezing [28]. To test the effectiveness of the interaction between zosteran and DMSO, an additional series of studies on cell cryopreservation of highly functional nucleated cells was carried out.
The data obtained on the preservation of leukocytes confirm that a decrease in the freezing point of a DMSO solution in the presence of zosteran helps to reduce the risk of cell damage during freezing. Let us assume that the displacement of the crystallization temperature of water in the cells to a lower temperature range at the initial stages of cooling contributes to the formation of a fine-meshed, low-traumatic ice structure. Also, when DMSO and zosteran were combined, their functional groups interacted not only with each other, but also with biological structures, which probably ensured the safety of biological objects during freezing and thawing, and also reduced the toxicity of the cryopreservative. Thus, the combination of zosteran with DMSO in 5% concentration promotes the preservation of nuclear cells at a temperature (-80°C) for 1 day.
Conclusion
In this study, new properties of zosteran pectin from the marine plant Zostera marina (L.) were discovered - adhesive and cryoprotective. Realizing the first property, namely, increasing the adhesiveness of lactobacilli to the surface of cells, zosteran can contribute to a more rapid and effective restoration of the normal intestinal microflora after an infectious disease suffered by the body. Realizing the second property, zosteran in combination with DMSO cryoprotectant can be used as an effective combined cryopreservative for the conservation of various biological objects at low temperatures. The results obtained are especially important because not all pectins have similar properties, because for this they need to have a number of physical and chemical features. So, on the example of apple pectin with a linear molecular structure and a higher degree of esterification than that of zosteran, the absence of an effective action was shown in all analyzes. Thus, despite the wide range of already discovered properties, our results will expand the possibilities of using zosteran pectin not only in medicine, but also in other industries that require long-term low-temperature storage of biological objects.
Conflict of Interest
The authors declare no conflict of interest.
Funding
The research was carried out within the framework of the research topic of the IF FRC Komi SC UB RAS FUUU-2022-0065 (№ 1021051201894-0).
Informed Consent Statement
The donors' venous blood was used as a biological object, which was kindly provided by them with their written informed consent during the voluntary blood donation procedure. This is in line with the principles of the Declaration of Helsinki (Helsinki, 1964), with all subsequent amendments, as well as the "Law of the Russian Federation on the donation of blood and its components." The supervisory board of Institute of Physiology of Komi Science Centre of the Ural Branch of the Russian Institute approved all tests.
References
- Enerstvedt KH, Lundberg A, Sjøtun IK, Fadnes P, Jordheim M. Characterization and seasonal variation of individual flavonoids in and Zostera noltii from Norwegian coastal waters. Biochemical Systematics and Ecology. 2017;4:42-50. doi: 10.1016/j.bse.2017.08.003.
- Komeichev ON, Ovodova RG, Ovodov YS. Isolation and investigation of influence of zosteran, pectic polysaccharide from the White Sea phanerogams on the endocrine and immune systems. Syktyvkar, Russia: Dal’nauka Publishers; 1997.
- Turkina Mya, Pecherina TV. Zosterin - A new sorbent for efferent therapy. Efferent Therapy. 2007;13:39-44.
- Loenko JN, Artjukov AA, Kozlovskaja JP, Miroshnichenko VA, Eljakov GB. Zosterin. Vladivostok, Russia: Dal’nauka Publishers; 2013.
- Certificate of state registration. № RU.77.99.11.003.E.005751.05.14 dated May 18, for the product biologically active food supplement "ZOSTERIN-ULTRA 30". 2014.
- Khasina EI, Kolenchenko EA, Sgrebneva MN, Kovalev VV, Khotimchenko YuS. Antioxidant activities of a low etherified pectin from the sea grass Zostera marina. Russian Journal of Marine Biology. 2003;29:259-261. doi: 10.1023/A:1025493128327.
- Kolenchenko EA, Sonina LN, Khotimchenko YuS. Comparative in vitro assessment of antioxidant activities of low-etherified pectin from the eelgrass Zostera marina and antioxidative medicines. Russian Journal of Marine Biology. 2005;31:331-334. doi: 10.1007/s11179-005-0098-2.
- Sonina LN, Khotimchenko MY. The effectiveness of pectin isolated from the sea grass Zostera marina in case of liver damage by lead. Biology of the Sea. 2007;33:240-241.
- Gloaguen V, Brudieux V, Closs B, Barbat A, Krausz P, Sainte-Catherine O, Kraemer M, Maes E, Guerardel Y. Structural characterization and cytotoxic properties of an apiose-rich pectic polysaccharide obtained from the cell wall of the marine phanerogam Zostera marina. J Nat Prod. 2010 Jun 25;73(6):1087-92. doi: 10.1021/np100092c. PMID: 20465284.
- Markov PA, Popov SV, Nikitina IR, Ovodova RG, Ovodov YS. Anti-inflammatory activity of pectins and their galacturonan core. Khimija Rastitel’nogo Syr’ja. 2010;1:21-26.
- Greco M, Sáez CA, Contreras RA, Rodríguez-Rojas F, Bitonti MB, Brown MT. Cadmium and/or copper excess induce interdependent metal accumulation, DNA methylation, induction of metal chelators and antioxidant defences in the seagrass Zostera marina. Chemosphere. 2019 Jun;224:111-119. doi: 10.1016/j.chemosphere.2019.02.123. Epub 2019 Feb 19. PMID: 30818189.
- Günter EA, Khramova DS, Markov PA, Popeyko OV, Melekhin AK, Beloserov VS, Martinson EA, Litvinets SG, Popov SV. Swelling behavior and satiating effect of the gel microparticles obtained from callus cultures pectins. Int J Biol Macromol. 2019 Feb 15;123:300-307. doi: 10.1016/j.ijbiomac.2018.11.081. Epub 2018 Nov 13. PMID: 30445072.
- Rhoades J, Manderson K, Wells A, Hotchkiss AT Jr, Gibson GR, Formentin K, Beer M, Rastall RA. Oligosaccharide-mediated inhibition of the adhesion of pathogenic Escherichia coli strains to human gut epithelial cells in vitro. J Food Prot. 2008 Nov;71(11):2272-7. doi: 10.4315/0362-028x-71.11.2272. PMID: 19044272.
- Lee W, Ahn CH, Hong S, Kim S, Lee S, Baek Y, Yoon J. Evaluation of surface properties of reverse osmosis membranes on the initial biofouling stages under no filtration condition. Journal of Membrane Science. 2010;351:112-122. doi: 10.1016/j.memsci.2010.01.035.
- Günter EA, Melekhin AK, Belozerov VS, Ananchenko BA, Martinson EA, Litvinets SG. Adhesive properties of calcium pectinate gels prepared from callus cultures pectins. International Journal of Biological Macromolecules. 2018;112:900-908. doi: 10.1016/j.ijbiomac.2018.02.053.
- Popov SV. Interaction of mammalian phagocytes with plant polysaccharides. Syktyvkar, Russia: Publishing House of Komi Scientific Center, Ural Branch of RAS; 2002.
- Zaporozhets TS. Mekhanizmy aktivatsii neĭtrofilov biopolimerami morskikh gidrobiontov [Neutrophil activation by sea hydrobiont biopolymers]. Antibiot Khimioter. 2003;48(9):3-7. Russian. PMID: 15002173.
- Serqushkina MI , Khudyakov AN , Polezhaeva TV , Bezmeltseva OM . The Ability of Pectins to Modulate the Action of Glycerol In the Freezing of Nucleated Cells. Cryo Letters. 2017 Nov/Dec;38(6):477-481. PMID: 29734444.
- Schrago MI, Guchok MM, Khanina LA, Kalugin YuV. About some of the ways to create cryoprotectans. Gematology and Transfuziology. 1981;16:3-8.
- Patova OA, Golovchenko VV, Ovodov YuS. Pectic polysaccharides: structure and properties. Russian Chemical Bulletin. 2014.63:1901-1924. doi: 10.1007/s11172-014-0681-9.
- Brilis VI, Brilene TA, Lentsner KhP, Lentsner AA. Metodika izucheniia adgezivnogo protsessa mikroorganizmov [Method of studying the adhesive process of microorganisms]. Lab Delo. 1986;(4):210-2. Russian. PMID: 2423751.
- Khrenov PA, Atlas EE. The adhesive properties of microorganisms isolated from the respiratory tract are the effect of modern antibacterial drugs. Bulletin of New Medical Technologies. 2016;23:51-54. doi: 10.12737/21748.
- Zandanova TN, Gogoleva PA. Selection of medium for obtaining bacterial concentrate of microbial consortium. Technical Sciences. 2018;5:227-232.
- Solomina ON, Svedentsov EP, Zaitseva OO, Polezhaeva TV, Ovodova RG, Golovchenko VV, Laptev DS, Khudyakov AN, Stepanova ES, Ovodov YS. Cryoprotective properties of some pectins. Dokl Biol Sci. 2010 Jan-Feb;430:20-2. doi: 10.1134/s0012496610010072. PMID: 20380171.
- Polezhaeva ТV, Zaitseva ОО, Khudyakov АN, Laptev DS, Golovchenko VV. Use of pectin polysaccharides for cryopreservation of biological objects. Archives of Biological Sciences. 2014;66:1025-1033. doi: 10.2298/ABS1403025P.
- Glanz S. Primer of Biostatistics. 4th ed. New York, USA: McGraw-Hill; 1997.
- Günter EA, Melekhin AK. Adhesion of Bacillus subtilis on the surface of pectin-calcium gel. Applied Biochemistry and Microbiology. 2015;151:70-74. doi: 10.7868/s0555109915010055.
- Gurruchaga H, Saenz del Burgo L, Hernandez RM, Orive G, Selden C, Fuller B, Ciriza J, Pedraz JL. Advances in the slow freezing cryopreservation of microencapsulated cells. Journal of Controlled Release. 2018;281:119-138. doi: 10.1016/j.jconrel.2018.05.016.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.