Medicine Group . 2023 February 22;4(2):258-268. doi: 10.37871/jbres1670.
Campylobacter jejuni Infections: Epidemiology, Pathophysiology, Clinical Manifestations and Management
Rozan O Al-Khreshieh1*, O’la Al-Fawares1 and Abu-Taleb EM2
2Department of Medical Laboratory Sciences, AL-Salt Government Hospital Al-Salt, Jordan
Abstract
Campylobacter spp has become one of the most important foodborne pathogens. Moreover, Campylobacter can cause an economic burden on the human population since it can cause about 8.4% of diarrheal cases worldwide. In addition, Campylobacteriosis outbreaks have been reported sporadically in association with untreated drinking water. Water that does not take from a licensed water supplier is considered the main cause of water contamination. The most common risk factor for Campylobacteriosis transmission to humans is raw undercooked chicken. Contamination among poultry may be the result of the environmental conditions inside the farms or chicken factories that could be spread very fast among others. This infection is usually self-limited with no signs or symptoms but it can also present with severe symptoms, including diarrhea that can last for more than one week and can ultimately lead to dehydration, fever, and abdominal pain. However, the main recognized sequelae are Guillain-Barré Syndrome (GBS), Reactive Arthritis (REA), Irritable Bowel Syndrome (IBS), and rarely Bacteremia. Recently, many cases of Campylobacter spp show important resistance to various antibiotics such as tetracyclines and fluoroquinolones. Thus, the prevention and monitoring of this infection play an essential role. Campylobacteriosis is self-limiting, and most of the cases does not need to be treated. Some medical interventions such as electrolytes replacement, as well as hydration may be followed to treat immunocompromised patients, patients suffering from severe symptoms, pregnant women, and the elderly.
Introduction
Campylobacteraceae is the largest and the most diverse family composed of two genera, Campylobacter and arcobacter that are considered to be commensals on both humans and animals [1]. Campylobacter genus was described as small Gram-negative, bacteria of a spiral rod shape. When Campylobacter bacterial cells grouped together, they form an “S” or a “V” like shape so; it will appear as a gull-wing. The majority of Campylobacter species are motile via a single polar flagellum that is unsheathed, which occurs on either one or both ends of the bacterial cell, creating a corkscrew-like motion. There are some exceptions like Campylobacter gracilis that is non-motile, and Campylobacter showae, which has multiple flagella [2]. All Campylobacter species have an oxidase activity, except for Campylobacter gracilis. In addition, most Campylobacter species did not utilized carbohydrates; instead, they gain energy from amino acids, or tricarboxylic acid [3].
Theodore Escherich, who described a spiral-shaped bacterium that was non- cultivable [4], reported the first report, which gave the primary description concerning Campylobacter, in 1886. By the year 1913, two scientist McFadyean and Stockman from aborted bovine fetuses isolated these microorganisms. In 1927, Smith and Orcutt isolated a group of bacteria, from cattle with diarrhea, and named it Vibrio jejuni. After about Seventeen years later, in 1944, a different vibrio was isolated by Doyle from pigs with diarrhea and classified them as Vibrio coli [3].
In 1963 Campylobacter genus was first distinguished from the true Vibrio app, by Sebald and Véron, and that's due to the differences in Campylobacter characteristic in comparison to the true Vibrio species, such as their low DNA base composition, non- fermentative features and their requirement of a microaerophilic conditions to live [3]. As a source of animal diseases, Campylobacters have been known since 1909, but as a disease source for humans they have been recognized only since about 1980 (Table 1) [4].
| Table 1: Members of the family Campylobacteraceae [5]. | ||
| Family Member | Known Source(s) | Disease Associations |
| C. coli | Pigs, poultry, cattle, sheep, birds | Gastroenteritis, septicemia |
| C. concisus | Human | Periodontal disease, gastroenteritis |
| C. curvus | Human | Periodontal disease, gastroenteritis |
| C. fetus subsp. Fetus | Cattle, sheep | Abortion, gastroenteritis, meningitis, septicemia |
| C. fetus subsp. Venerealis | Cattle | Septicemia |
| C. gracilis | Human | Periodontal disease, empyema, abscesses |
| C. helviticus | Cats, dogs | None at present |
| C. hyointestinalis subsp. Hyointestinalis |
Pigs, cattle, hamsters, deer | Gastroenteritis |
| C. hyointestinalis Subsp. Lawsonii |
Pigs | None at present |
| C. hyoilei | Pigs | None at present |
| C. jejuni subsp. Doylei | Human | Gastroenteritis, gastritis, septicemia |
| C. jejuni subsp. Jejuni | Poultrty, pigs, cattle, sheep, dogs, cats, water, birds, mink, rabbits, insects |
Gastroenteritis, septicemia (GBS) meningitis, abortion, proctitis |
| C. lari | Birds, poultry, water, dogs, cats, monkeys, horses | Gastroenteritis, septicemia |
| C. mucosalis | Pigs | None at present |
| C. rectus | Human | Periodontal disease |
| C. showae | Human | Periodontal disease |
| C. sputorum bv. Sportum | Human, cattle, pigs | Abscesses, gastroenteritis |
| C. sputorum bv. Faecalis | Sheep, bulls | None at present |
| C. upsaliensis | Dogs, cats | Gastroenteritis, septicemia, abscesses |
| C. insulaenigrae | Seals, porpoises | None at present |
| C. lanienae | Cattle, pigs and humans | None at present |
| C. hominis | Humans | Gastroenteritis in immunocompromised |
| C. corcagiensis | captive lion-tailed macaques | None at present |
| C. bilis | chickens with spotty liver | None at present |
| C.blaseri sp | common seals | None at present |
| C. geochelonis | Hermann's tortoise | None at present |
Campylobacter is small (0.2 to 0.9 µm in width and 0.5 to 5 μm in length) and they move through a single polar flagellum at one or both of the two ends of the bacterial cell [6]. Campylobacter species are considered as thermophilic since they are able to grow between 37°C and 42°C, in which the optimum temperature is 41.5°C, whereas they cannot grow below 30°C. The optimum pH for Campylobacter survival is at about 6.5-7.5; for that, Campylobacter will not service below a pH of 4.9 and above pH 9.0. The bacteria are microaerophilic, growing in a low oxygen tension atmosphere (5% oxygen, 10% carbon dioxide, 85% nitrogen) [4].
In 2000 Campylobacter jejuni genome sequence (NCTC 11168) was published and described to have a circular chromosome of 1,641,481 base pairs in length. The Campylobacter genome is unfamiliar in which there are no insertion or phage- associated sequences and only a few repeated sequences [7]. One of the most important findings concerning the Campylobacter genome is the presence of hypervariable sequences. This high rate of variation may be involved in the survival strategy of C. jejuni [8].
For the isolation of Campylobacter species different selective agars can be used such as, Butzler, Preston, and Charcoal Cefoperazone Deoxycholate (CCDA) agars. Campylobacter detection methods are not commonly used in the routine laboratory practices, because it is fastidious and difficult to be cultivated [9]. To quickly find and validate the presence of Campylobacter species, a number of techniques have been developed, including filtering, latex agglutination, and Fluorescence In Situ Hybridization (FISH). However, most effective confirmation methods are based on Polymerase Chain Reaction (PCR) and most of the epidemiological studies got benefited from the use of different molecular techniques such as, PCR and Pulse-Field Gel Electrophoresis (PFGE) [4].
C. jejuni lacks some classical mechanisms and virulence factors that enable the organism to adapt the different growth challenges. Such as type III secretion system and RNA-polymerase sigma factor-mediated global stress response [10]. Instead, particular mechanism investigated recently in C. jujeni shows that poly-P molecular metabolism has a potential role on the survival of this bacterium as well as, its adaptation and control [11]. Poly-P act as a source of energy (ATP) for about 500 reaction, including the activation of the precursors of fatty acids, phospholipids, nucleic acids, and polypeptides. This poly-P is a linear polymer made up of orthophosphate residues is associated with the prebiotic evolution of the bacteria. Poly-P is also important for the fidelity of DNA replication and its entry through the membrane channels, biofilm formation, quorum sensing, antibiotic resistance, invasion, and host colonization [11].
Virulence and Survival Factors of Campylobacter jejuni
Campylobacter jujeni has the ability to perform N-linked glycosylation of more than 30 proteins, that are responsible for various stages of Campylobacter pathogenesis, including colonization, adherence, and invasion. Twenty-four proteins produced by C. jujeni as heat shock response, but there is no data received from C. jujeni genome sequencing shows the ability of the genus to produce cold shock proteins at a temperature as low as 4°C. Therefore, C. jujeni cannot grow or multiply, but it still able to generate ATP and perform respiration in these cold conditions [12]. However, virulence factors produced by C. jejuni are associated with Campylobacter pathogenicity are summarized at the table 2.
| Table 2: Virulence factors associated with Campylobacter pathogenicity. | |||
| Virulence Factors | Functions | Remarks | References |
| hipO gene | Cleaves hippuric acid into benzoic acid and glycine. Reporter of gene expression |
It is expressed only by C. jujeni | [13] |
| asp gene | catalyzes the phosphorylation of the amino acid aspartate |
It is specific for C. coli | [14] |
| CadF | binding and colonization of human intestinal cell |
It is a genus specific gene for all Campylobacter spp |
[14] |
| CiaB gene | CiaB protein has a role in both colonization and invasion of the intestinal cells | If a mutation occurs in this gene, this will result in a decrease in the number of bacterial cells, comparing to the wild type isolate |
[15] |
| PldA gene | It stands for phospholipase A, which is responsible for the synthesis of the bacterial cell outer membrane phospholipase | PldA gene is also related to host cell invasion | [14] |
| dnaJ gene | This virulence gene is considered as a chaperon protein | Enable Campylobacter bacteria to cope up with various changes and physiological stress | [14] |
| Cdt genes | Cdt stands for the Cytolethal distending toxin, which is considered one of the main Campylobacter virulence genes that are encoded by three adjacent genes (CdtA, CdtB, and CdtC). | All the three genes are required for a complete toxic activity, in which CdtA and CdtC genes are responsible for binding and delivery of CdtB into the interior of the host cell. CdtB is correlated with the active toxic effect that blocks the cell cycle and breaks the double-strand DNA. It exhibits DNase-I like enzyme | [16] |
| Flagella Composed of: FlaA (Major flagellin) FlaB(Minor flagellin). | Main function: Motility Complex function: Non- flagellar proteins secretion which modulate virulence. | Motility is rapid and darting. | [17] |
| Chemotaxis | This mechanism is used by motile bacteria, which sense, and move towards more suitable conditions. | [18] | |
| proteins: Che A, B, R, W, Y and Z. methyl- accepting chemotaxis proteins (MCPs) called (Tlps) |
|||
| PEB1 CadF Type VI secretion system |
Binding, adhesion and colonization. | Is a major cell adherence molecule of Campylobacter An outer membrane protein Depends on the contact with competing bacteria or host cells to release toxins affecting them to facilitate the colonization. |
[14] |
| Iam Serine protease HtrA |
Invasion | Invasion associated marker. Cell attachment and invasion |
[18] |
| Resistance factor Campylobacter polysaccharide capsule (CPS) |
Resistance to complement killing, invasion | It was noted that reduced invasion correlated with reduced capsule expression. | [19] |
Epidemiology of Campylobacter Infection
According to the WHO, Campylobacter was considered as the most common cause of bacterial gastroenteritis worldwide. The reservoir of Campylobacter is the farm animals, mainly the poultry. The major infection caused by Campylobacter is acute diarrhea, which can be triggered by an ingested dose of a few as 500-800 CFU from the bacterial cells [20]. However, the ingestion of 100 bacterial cells or less could also correlated with human infection [20]. The most common risk factor for Campylobacteriosis transmission to humans is the raw undercooked chicken. Contamination among poultry may be the result of the environmental conditions inside the farms or chicken factories that could be spread very fast among others. Campylobacter may also spread vertically from poultry to their offspring’s, or horizontally from contaminated water, contact with fecal materials, rodents and farm personnel. Campylobacter does not spread to poultry from their feed, as it is too dry for the survival of the bacteria [20]. Campylobacter can cause an economic burden on the human population since it can cause about 8.4% of the diarrheal cases worldwide. In addition, the emerging of Campylobacter foodborne outbreaks might influence the economic situation of the community, in which these outbreaks are most commonly triggered by the consumption of the poultry meat. Considering the age of the infected people, Campylobacteriosis is common in children below 4 years old and aged people above 75 years old [21].
Moreover, some studies identified domestic animals like cats and dogs as a host for Campylobacter infections. Therefore, these pets have the ability to transmit the bacteria to other animals or to humans when its fecal materials contaminate human foods. Indeed, Baker, et al. reported that, 55% of the cats and 49% of the dogs were found positive for Campylobacter species [22]. In developing countries, a lower number of research and national surveillance programs were conducted compared to the developed countries. Recently, researches and control programs in developing countries are growing; because of the continuous increase in the cases of Campylobacteriosis that exceeded those of Shigella and Salmonella infections. In addition, a potential increase in mortality rate was reported in Human Immunodeficiency Virus (HIV) patients associated with Campylobacter infection [23]. In Jordan, a four-year epidemiological study from 1997 to 2000 on Jordanian population suffering from diarrhea and gastroenteritis has been performed [24]. Pathogenic bacteria were identified in 343 cases out of 1400 patients, in which most of them were children. The most frequent bacterial cause accounting for these cases was Salmonella (10.7%), followed by Entero Pathogenic Escherichia coli (EPEC) in 3.9%, Campylobacter spp. in 0.9%, and Shigella spp. in 0.8% of the study cases [24]. Further, In the United States an annual incidence of 14.3 per 100,000 population for Campylobacteriosis has been reported, through The U.S. Food-Borne Diseases Active Surveillance Network (FBDAS) [25]. In 2012, the incidence of Campylobacter infection increased 14% compared to the 2006-2008 period. However, one of the important things to be considered is that the incidence of Cryptosporidium, Listeria, Salmonella, Shiga-Toxigenic Escherichia coli (STEC) O157:H1, Shigella and Yersinia infections, decreased over the same period. The FBDAS Network, also considered Campylobacter as the leading cause of travel-associated gastroenteritis; as it accounts for 41.7% of the cases, followed by Salmonella 36.7%, then Shigella 13.0% [25]. In Mexico C. jujeni was reported as the most common cause of acute gastroenteritis [19]. In Europe most of the epidemiological studies reveal that the incidence of Campylobacteriosis in 27 European Union (EU) states in 2009 were ranged from 13.5% to 29.9% However, the highest incidence was detected in Bulgaria, whereas the lowest was in Finland and Sweden [26]. This incidence equates to 9.2 million cases compared to Salmonellosis cases that were 6.2 million over the same period [27].
Recently, the Czech Republic reported the highest incidence of Campylobacter infection worldwide (215 per 100,000 in 2019) [28], followed by Australia (146.8 per 100,000 in 2016) [28]. and New Zealand (126.1 per 100,000 in 2019), Japan had a varied number of cases from (1,893 to 3,272 ) each year [29], Korea which has also a varied number of cases each year, for instance, Campylobacteriosis cases were the lowest in 2017 (103 cases) and highest in 2016 (902 cases). However, reported low incidence of Campylobacteriosis, ranging from 4.7 per 100,000 in 2014 to 2.0 in 2020 [28]. In France, a continuous increase in Campylobacteriosis cases was observed from 2014 to 2020, with the reported incidence increasing from 45.2 per 100,000 in 2014 to 58.8 in 2020 [28].
Campylobacteriosis outbreaks have been reported sporadically in association with untreated drinking water. Water that does not take from a licensed water supplier considered the main cause of water contamination [22]. Furthermore, an annual incidence of 35.2 cases per 100.000 people was reported in Quebec and Canada between the periods of 1996-2006, in which there were 28,521 cases of Campylobacterosis. A higher incidence in Ontario, Canada of 49.69 cases per 100.000 persons has been reported in the period from 1990 to 2004. The consumption of poultry, raw milk, and untreated water was considered the main rout of Campylobacteriosis [25]. For example, in 2011, an outbreak of Guillain-Barre’ Syndrome (GBS) associated with C. jujeni infection in both Sonora in Mexico, and Arizona, USA was recorded [31]. In October 2012, Campylobacteriosis outbreak associated with a long- Distance Obstacle Adventure Race in Nevada has been reported by Nellis Public Health [32]. Also, from 2013 through 2014, Campylobacteriosis outbreak associated with the consumption of undercooked chicken liver pate has been reported in Ohio and Oregon [33]. And last but not least, in 2014, Campylobactereosis outbreak associated with the consumption of raw milk has been reported in Utah, by the Utah Public Health Laboratory (UPHL) [34].
Pathogenicity of Campylobacter jejuni Infection
Campylobacter pathogenesis must begin with the intestinal colonization of the host that depends mainly on the bacterial motility and chemotaxis Colonization process, requires the bacteria to move on into the intestinal cells mucus layer and penetrate its barriers using both its polar flagella that create the "crock-screw" motility of Campylobacter, and chemotaxis that mediate the subsequent binding of bacterial subpopulation [35].
After binding and entry of the bacterial cells into the host cells, subsequent invasion will take place leading to mucosal damage and inflammation. In addition to invasion, a lot of attention should be focused on the cytopathic effects and toxic activity of Campylobacter, which mostly carried out by the cytolethal distending toxins that block the G2 phase in cell cycle, and thus induce the progresses into cell death [35]. However, other factors contribute to Campylobacter pathogenesis such as Iron acquisition. Campylobacter acquire iron using system consist of an outer-membrane receptors that internalize the essential nutrient (iron) into the bacterial cells [36]. In addition, Surface polysaccharide structures. The different polysaccharide structures that found on the bacterial cell surface considered to be as a very important virulence factors, which helps in adhesion, endotoxicity, and serum resistance [36]. Campylobacter must deal with the toxic Oxygen byproducts that produced normally through the different infection stages. Defense against this can be achieved via two oxidative stress defense systems; peroxide defense system, and superoxide defense system [36]. Moreover, Campylobacter species must cope up with the continuous changing in the temperature, as they can found in different hosts with different body temperature, for that Campylobacter should express type of proteins called heat shock proteins HSPs [20].
Biofilm Formation
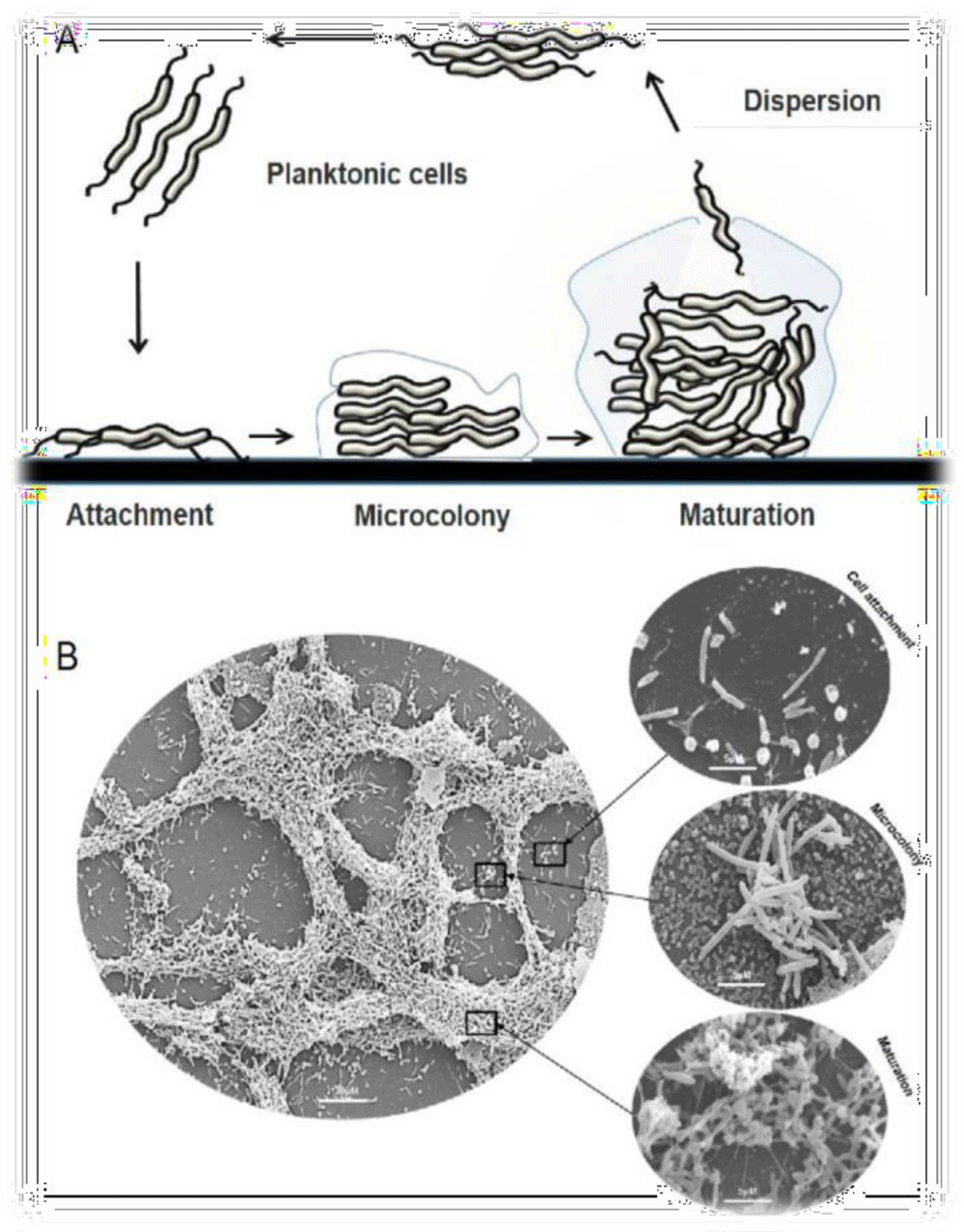
In order to protect itself from different environmental stresses such as; UV radiation, desiccation, and many other physiological stresses, Campylobacter tends to form encased matrix around its surface known as (Biofilm formation) [37]. Campylobacter that formed biofilm exhibit a1000 folds resistant to disinfectants and antimicrobial activity [38]. Campylobacter biofilm composition shown to be similar to that produced by other bacterial species, one of its defined components is a fiber like matrix that appears as a net like matrix other crucial component is the extra-cellular DNA that maintain and support the Campylobacter biofilm [39].
Well, Campylobacter species uses its flagella, outer membrane proteins, and other adhesions proteins to initiate the first stage of biofilm formation, by which the planktonic cells will attach either by cell-surface, or cell-cell interactions. In the second stage the attached cells produces substances extracellularly such as; Proteins, polysaccharides, and extracellular DNA. The third stage involve the formation of (3D) structure of biofilm, which will ultimately protect the cells from antibiotics, and other disinfectants (Figure 1) [40].
One of the most important components of the biofilm is the Extracellular Polymeric Substance (EPS) supports the three-dimensional (3D) structure of the biofilm, acts as an adhesive material between the bacterial cells, and aids in the maturation of the biofilm. This crucial component protects the bacterial cells from toxic compounds such as antibiotics. Biofilm detaches when bacterial cells undergo autolysis, thus the cells will be released in a process called dispersion into the environmental niche, this process is crucial for the bacterial cell survival propagation and self-renewal of bacterial communities [41].
Clinical Manifestations of Campylobacteriosis and Related Complications
Campylobacteriosis typically occurs as a result of undercooked poultry and poultry product consumption. This infection is usually self-limited with no signs or symptoms but it can also present with severe symptoms, including diarrhea that can last for more than one week and can ultimately lead to dehydration, fever, and abdominal pain. Campylobacteriosis is usually resolved without dissemination to other parts of the body [42]. However, in individuals with immunodeficiency disorders, it sometimes extends to extra-intestinal sites causing a wide spectrum of conditions including hepatitis, bacteremia, and other focal infections. On the fifth day of infection, antibodies against Campylobacter start to appear in the blood to reach their peak in 2-4 weeks and then decline [43]. The main recognized complications are Reactive Arthritis (REA), Guillain-Barré Syndrome (GBS), Miller-Fisher Syndrome, irritable bowel syndrome, and Bacteremia [1].Campylobacteriosis typically occurs as a result of undercooked poultry and poultry product consumption. This infection is usually self-limited with no signs or symptoms but it can also present with severe symptoms, including diarrhea that can last for more than one week and can ultimately lead to dehydration, fever, and abdominal pain. Campylobacteriosis is usually resolved without dissemination to other parts of the body [42]. However, in individuals with immunodeficiency disorders, it sometimes extends to extra-intestinal sites causing a wide spectrum of conditions including hepatitis, bacteremia, and other focal infections. On the fifth day of infection, antibodies against Campylobacter start to appear in the blood to reach their peak in 2-4 weeks and then decline [43]. The main recognized complications are Reactive Arthritis (REA), Guillain-Barré Syndrome (GBS), Miller-Fisher Syndrome, irritable bowel syndrome, and Bacteremia [1].
Campylobacter Species and Guillain-Barré Syndrome
Guillain-Barré Syndrome (GBS) is a self-limited immune-mediated disease that affects the Peripheral Nervous System (PNS). GBS now becomes the most common cause of acute flaccid paralysis after the eradication of polio cases worldwide [44]. Patients with GBS show heterogeneous presentation of the disorder, but they usually present with symmetrical weakness of the limbs and the respiratory tract [45]. GBS is thought to be caused by an aberrant molecular mimicry and a cross-reactive immune response to infections that result in damage to peripheral nerves. C. jujeni considered as the firmly established causative agent of GBS among various microbial infections. Antibodies produced in the response to this infection will cross- react with the host PNS gangliosides [45]. GBS occurs among all age groups with an incidence rate of 6 to 4 cases per 100,000 population every year [38]. Females are less frequently affected than males (1.25:1) and the incidence appears to increase with age [45].
GBS that associated with C. jujeni infection was noted to be more severe related to extensive axonal injury, respiratory inadequacy, the need for mechanical ventilation, and an increase in the risk of permanent neurological damage. There is a relationship between the development of GBS and C. jujeni serotyping, in which the risk of its development is increased with certain C. jujeni serotypes. In South Africa, panner type 0:41 is the most commonly serotype associated with GBS, while in the United States, panner type of 0:19 is frequently reported with GBS [44]. Although Campylobacteriosis is a common infection in the general population, GBS development remains quite low (0.001%), suggesting that there is a role of host genetic factors in the pathogenesis and the development of this disorder [45].
Campylobacter and Reactive Arthritis
Reactive Arthritis (ReA) develops after the occurrence of an infection in another part of the body. It is mostly triggered by urogenital infection, and enteric Infections associated with Salmonella, Shigella, Yersinia, and Campylobacter. At present, the exact pathogenesis by which Campylobacter increase the risk of ReA still unknown, but there are some suggestions that an interaction of certain bacteria with Human Leukocyte Antigen (HLA) B27 has a crucial role in the development of ReA. People that are (HLA) B27 positive tend to develop ReA more than others that don't have this antigen [46]. Patients were suffered from joint inflammation, pain and swelling [47].
Campylobacter Bacteremia
Campylobacter bacteremia is a very rare consequence that occurs after Campylobacter reaching to the bloodstream, this disease occurs mainly in patients with serious conditions, for instance, hypogammaglobulinemia, liver diseases, Human Immunodeficiency Virus (HIV) Infection. In addition, the rate of bacteremia relapsing increased in those who suffer from serious conditions [48]. One of the methods used to determine the place of acquisition of the Bloodstream Infection (BSI) is the conventional criteria, by which BSI was considered as community-acquired if the first positive blood culture specimen was drawn within the first 48 hours of admission. After this period the infection was considered hospital-acquired [48].
The initial treatment for Campylobacter bacteremia is decided after in vitro susceptibility testing to various antibiotics. Nowadays, and because of the increase in antibiotic resistance some cases show no susceptibility test result, at this stage Campylobacter fetus isolates were assumed to be susceptible to cefotaxime and ceftriaxone, whereas C jejuni and C coli isolates were assumed to be resistant to all third-generation cephalosporins [49].
Miller Fisher Syndrome and Campylobacter Infection
The Miller Fisher Syndrome (MFS) is a neuropathy that occurs because of immune response against host gangliosides, which has molecular mimicry between C. jejuni lipopolysaccharides (LPS) and gangliosides. MFS may be preceded by other infectious illnesses, but the most commonly identified microorganism in association with MFS is the enteric pathogen Campylobacter jejuni [50].
Irritable Bowel Syndrome and Campylobacter Infection
Irritable Bowel Syndrome (IBS) is a gastrointestinal disease characterized by abdominal discomfort, bloating and diarrhea. This disorder may occur following an exposure to Acute Gastroenteritis (GE) which may be caused by different entero- pathogens like Shigella and Campylobacter Species [51].
Methods of Isolation and Identification of Campylobacter Species
Campylobacter species are thermophilic bacteria that cannot grow below about 31°C, instead of that it can grow at 42°C-43°C. The media that designed to isolate Campylobacter are selective media containing mixture of antibiotics to neutralize the toxic effect of light and oxygen formed substance. It is it necessary to incubate culture plates under microaerophilic conditions containing (5-7% oxygen, 10% carbon dioxide and 80% nitrogen and/or hydrogen). These conditions can achieve by using gas- generating envelope and anaerobic jar, Candle jar, and burning ethanol [52]. The most commonly used isolation media for the thermophilic Campylobacters are Modified Skirrow, Campy BAP, Preston agar, charcoal cefoperazone deoxycholate (mCCD) agar, Modified Charcoal Cefoperazone Deoxycholate (mCCD) agar, Karmali agar, Abeyta- Hunt-Bark agar [52].
Identification of Campylobacter is initially carried out by gram staining and microscopic examination of the bacterial shape. A unique corkscrew like motility observed by wet mount preparation is an important tool used for identification. A group of biochemical tests, as well as different molecular methods, which offer fast, sensitive, and specific diagnosis and can be used to confirm the genus of the isolate, will follow this. Several methods can be used for Campylobacter typing, including Phage typing, serotyping, 16S rRNA sequencing. Multilocus Sequence Typing (MLST), and Pulsed- Field Gel Electrophoresis (PFGE) are frequently used to source track foodborne pathogens [20].
Antimicrobial Susceptibility of Campylobacter Species
The levels of resistance of a large number of Campylobacter isolates to different types of antibiotics have changed, especially their resistance to fluoroquinolone. Before 1992 it was rare for a Campylobacter strain to be resistant to this antibiotic, however, afterward a marked increase in the resistance to fluoroquinolone was noticed. The resistance rate of Campylobacter in many countries in Asia and Africa reached 80% for this antibiotic. However, the rate of resistance of Campylobacter isolates to different types of antibiotics differ between countries [53]. The mechanisms by which Campylobacter resist fluoroquinolones is by a point mutation in the DNA gyrase, specifically in the quinolone resistance-determining region. Unlike other intestinal bacteria such as Salmonella and Escherichia coli, Campylobacter needs a single point mutation to become resistant to fluoroquinolones [53].
Furthermore, Campylobacter resists macrolide either by point mutation or via enzyme- mediated methylation that performs modification of the ribosomal target. Resistance to tetracycline is achieved by means of a ribosomal protection protein produced from the tet (O) gene [20]. This gene is found widely in Campylobacter strains, and it is believed that the expressed protein identifies a specific site on the bacterial ribosome and attaches to it, inducing conformational changes and causing tetracycline to separate from its site and leading to bacterial resistance. Another possible mechanism for resistance is by the active efflux that is mediated by multidrug-efflux transporters, as well as the low permeability of the Campylobacter membrane. Among these drugs are streptogramin B, novobiocin, vancomycin, trimethoprim, and bacitracin [54].
Campylobacter Treatment
Campylobacteriosis is self-limiting, and in most of cases, it does not need to be treated. Some medical interventions such as electrolytes replacement, as well as hydration. We may turn to treatment with antibiotics in immunocompromised patients, patients suffering from severe symptoms, pregnant women, and the elderly [20].
The treatment of choice for Campylobacter infection became macrolides after the increase in the resistance of Campylobacters to fluoroquinolones. In pregnant women and children, Erythromycin is considered safe to some extent. The recommended dose of erythromycin in adults is 500 mg/day for five days. For children, the recommended dose is 40 mg/kg/day for five days, but divided into two doses. For Azithromycin the recommended dose for adult is 500 mg/day for 3 days. For children the recommended dose is 10 mg/kg for 3 days [54].
References
- Facciolà A, Riso R, Avventuroso E, Visalli G, Delia SA, Laganà P. Campylobacter: from microbiology to prevention. J Prev Med Hyg. 2017 Jun;58(2):E79-E92. PMID: 28900347; PMCID: PMC5584092.
- Vandamme P. Taxonomy of the family Campylobacteraceae in Campylobacter. American Society for Microbiology. 2000;3-27.
- On SL. Taxonomy of Campylobacter, Arcobacter, Helicobacter and related bacteria: current status, future prospects and immediate concerns. Symp Ser Soc Appl Microbiol. 2001;(30):1S-15S. doi: 10.1046/j.1365-2672.2001.01349.x. PMID: 11422556.
- Silva J, Leite D, Fernandes M, Mena C, Gibbs PA, Teixeira P. Campylobacter spp. as a Foodborne Pathogen: A Review. Front Microbiol. 2011 Sep 27;2:200. doi: 10.3389/fmicb.2011.00200. PMID: 21991264; PMCID: PMC3180643.
- Silva MF, Pereira G, Carneiro C, Hemphill A, Mateus L, Lopes-da-Costa L, Silva E. Campylobacter portucalensis sp. nov., a new species of Campylobacter isolated from the preputial mucosa of bulls. PLoS One. 2020 Jan 10;15(1):e0227500. doi: 10.1371/journal.pone.0227500. PMID: 31923228; PMCID: PMC6953823.
- Bronowski C, James CE, Winstanley C. Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol Lett. 2014 Jul;356(1):8-19. doi: 10.1111/1574-6968.12488. Epub 2014 Jun 19. PMID: 24888326.
- Golz JC, Epping L, Knüver MT, Borowiak M, Hartkopf F, Deneke C, Malorny B, Semmler T, Stingl K. Whole genome sequencing reveals extended natural transformation in Campylobacter impacting diagnostics and the pathogens adaptive potential. Sci Rep. 2020 Feb 28;10(1):3686. doi: 10.1038/s41598-020-60320-y. PMID: 32111893; PMCID: PMC7048796.
- Kelley BR, Ellis JC, Large A, Schneider LG, Jacobson D, Johnson JG. Whole-Genome Sequencing and Bioinformatic Analysis of Environmental, Agricultural, and Human Campylobacter jejuni Isolates From East Tennessee. Front Microbiol. 2020 Nov 5;11:571064. doi: 10.3389/fmicb.2020.571064. PMID: 33224113; PMCID: PMC7674308.
- Nachamkin I, Nguyen P. Isolation of Campylobacter Species from Stool Samples by Use of a Filtration Method: Assessment from a United States-Based Population. J Clin Microbiol. 2017 Jul;55(7):2204-2207. doi: 10.1128/JCM.00332-17. Epub 2017 May 3. PMID: 28468859; PMCID: PMC5483923.
- Kreling V, Falcone FH, Kehrenberg C, Hensel A. Campylobacter sp.: Pathogenicity factors and prevention methods-new molecular targets for innovative antivirulence drugs? Appl Microbiol Biotechnol. 2020 Dec;104(24):10409-10436. doi: 10.1007/s00253-020-10974-5. Epub 2020 Nov 13. PMID: 33185702; PMCID: PMC7662028.
- Kassem II, Rajashekara G. An ancient molecule in a recalcitrant pathogen: the contributions of poly-P to the pathogenesis and stress responses of Campylobacter jejuni. Future Microbiol. 2011 Oct;6(10):1117-20. doi: 10.2217/fmb.11.94. PMID: 22004028.
- Dasti JI, Tareen AM, Lugert R, Zautner AE, Gross U. Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol. 2010 Apr;300(4):205-11. doi: 10.1016/j.ijmm.2009.07.002. Epub 2009 Aug 8. PMID: 19665925.
- Caner V, Cokal Y, Cetin C, Sen A, Karagenc N. The detection of hipO gene by real-time PCR in thermophilic Campylobacter spp. with very weak and negative reaction of hippurate hydrolysis. Antonie Van Leeuwenhoek. 2008 Nov;94(4):527-32. doi: 10.1007/s10482-008-9269-4. Epub 2008 Jul 30. PMID: 18665452.
- Reddy S, Zishiri OT. Genetic characterisation of virulence genes associated with adherence, invasion and cytotoxicity in Campylobacter spp. isolated from commercial chickens and human clinical cases. Onderstepoort J Vet Res. 2018 Feb 15;85(1):e1-e9. doi: 10.4102/ojvr.v85i1.1507. PMID: 29781670; PMCID: PMC6238761.
- Konkel ME, Klena JD, Rivera-Amill V, Monteville MR, Biswas D, Raphael B, Mickelson J. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J Bacteriol. 2004 Jun;186(11):3296-303. doi: 10.1128/JB.186.11.3296-3303.2004. PMID: 15150214; PMCID: PMC415756.
- de Carvalho AF, da Silva DM, Azevedo SS, Piatti RM, Genovez ME, Scarcelli E. Detection of CDT toxin genes in Campylobacter spp. strains isolated from broiler carcasses and vegetables in São Paulo, Brazil. Braz J Microbiol. 2014 Jan 15;44(3):693-9. doi: 10.1590/s1517-83822013000300005. PMID: 24516435; PMCID: PMC3910176.
- Guerry P. Campylobacter flagella: not just for motility. Trends Microbiol. 2007 Oct;15(10):456-61. doi: 10.1016/j.tim.2007.09.006. Epub 2007 Oct 24. PMID: 17920274.
- Bolton DJ. Campylobacter virulence and survival factors. Food Microbiol. 2015 Jun;48:99-108. doi: 10.1016/j.fm.2014.11.017. Epub 2014 Dec 25. PMID: 25790997.
- Guerry P, Poly F, Riddle M, Maue AC, Chen YH, Monteiro MA. Campylobacter polysaccharide capsules: virulence and vaccines. Front Cell Infect Microbiol. 2012 Feb 15;2:7. doi: 10.3389/fcimb.2012.00007. PMID: 22919599; PMCID: PMC3417588.
- Al-Khresieh RO, Al-Daghistani HI, Abu-Romman SM, Abu-Niaaj LF. Genetic Signature and Serocompatibility Evidence for Drug Resistant Campylobacter jejuni. Antibiotics (Basel). 2022 Oct 17;11(10):1421. doi: 10.3390/antibiotics11101421. PMID: 36290079; PMCID: PMC9598221.
- Igwaran A, Okoh AI. Human campylobacteriosis: A public health concern of global importance. Heliyon. 2019 Nov 14;5(11):e02814. doi: 10.1016/j.heliyon.2019.e02814. PMID: 31763476; PMCID: PMC6861584.
- Whiley H, van den Akker B, Giglio S, Bentham R. The role of environmental reservoirs in human campylobacteriosis. Int J Environ Res Public Health. 2013 Nov 8;10(11):5886-907. doi: 10.3390/ijerph10115886. PMID: 24217177; PMCID: PMC3863877.
- Forson AO, Adjei DN, Olu-Taiwo M, Quarchie MN, Asmah HR. Characterization of Campylobacter associated gastric enteritis among patients with Human Immunodeficiency Virus (HIV) in a hospital in Accra, Ghana. PLoS One. 2020 Oct 15;15(10):e0240242. doi: 10.1371/journal.pone.0240242. PMID: 33057408; PMCID: PMC7561167.
- Battikhi MN. Epidemiological study on Jordanian patients suffering from diarrhoea. New Microbiol. 2002 Oct;25(4):405-12. PMID: 12437219.
- Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global Epidemiology of Campylobacter Infection. Clin Microbiol Rev. 2015 Jul;28(3):687-720. doi: 10.1128/CMR.00006-15. PMID: 26062576; PMCID: PMC4462680.
- Larrosa-Haro A, Macias-Rosales R, Sánchez-Ramírez CA, Cortés-López MC, Aguilar-Benavides S. Seasonal variation of enteropathogens in infants and preschoolers with acute diarrhea in western Mexico. J Pediatr Gastroenterol Nutr. 2010 Oct;51(4):534-6. doi: 10.1097/MPG.0b013e3181df5b66. PMID: 20706147.
- Havelaar AH, Ivarsson S, Löfdahl M, Nauta MJ. Estimating the true incidence of campylobacteriosis and salmonellosis in the European Union, 2009. Epidemiol Infect. 2013 Feb;141(2):293-302. doi: 10.1017/S0950268812000568. Epub 2012 Apr 13. PMID: 22717051; PMCID: PMC9152072.
- Liu F, Lee SA, Xue J, Riordan SM, Zhang L. Global epidemiology of campylobacteriosis and the impact of COVID-19. Front Cell Infect Microbiol. 2022 Nov 28;12:979055. doi: 10.3389/fcimb.2022.979055. PMID: 36519137; PMCID: PMC9742372.
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021 Dec 13;19(12):e06971. doi: 10.2903/j.efsa.2021.6971. PMID: 36329690; PMCID: PMC9624447.
- Yoshikura H. Declining Vibrio parahaemolyticus and Salmonella, Increasing Campylobacter and Persisting Norovirus Food Poisonings: Inference Derived from Food Poisoning Statistics of Japan. Jpn J Infect Dis. 2020 Mar 24;73(2):102-110. doi: 10.7883/yoken.JJID.2019.247. Epub 2019 Oct 31. PMID: 31666496.
- Jackson BR, Zegarra JA, López-Gatell H, Sejvar J, Arzate F, Waterman S, Núñez AS, López B, Weiss J, Cruz RQ, Murrieta DY, Luna-Gierke R, Heiman K, Vieira AR, Fitzgerald C, Kwan P, Zárate-Bermúdez M, Talkington D, Hill VR, Mahon B; GBS Outbreak Investigation Team. Binational outbreak of Guillain-Barré syndrome associated with Campylobacter jejuni infection, Mexico and USA, 2011. Epidemiol Infect. 2014 May;142(5):1089-99. doi: 10.1017/S0950268813001908. Epub 2013 Aug 7. PMID: 23924442; PMCID: PMC6527315.
- Zeigler M, Claar C, Rice D, Davis J, Frazier T, Turner A, Kelley C, Capps J, Kent A, Hubbard V, Ritenour C, Tuscano C, Qiu-Shultz Z, Leaumont CF; Centers for Disease Control and Prevention (CDC). Outbreak of campylobacteriosis associated with a long-distance obstacle adventure race--Nevada, October 2012. MMWR Morb Mortal Wkly Rep. 2014 May 2;63(17):375-8. PMID: 24785983; PMCID: PMC4584888.
- Scott MK, Geissler A, Poissant T, DeBess E, Melius B, Eckmann K, Salehi E, Cieslak PR; Centers for Disease Control and Prevention (CDC). Notes from the field: campylobacteriosis outbreak associated with consuming undercooked chicken liver pâté - Ohio and Oregon, December 2013-January 2014. MMWR Morb Mortal Wkly Rep. 2015 Apr 17;64(14):399. PMID: 25879900; PMCID: PMC5779543.
- Health, disease control and prevention, epidemiology. Communicable disease rule; R386-702. Salt Lake City, UT: Utah Department of Health. 2016.
- Haddad N, Marce C, Magras C, Cappelier JM. An overview of methods used to clarify pathogenesis mechanisms of Campylobacter jejuni. J Food Prot. 2010 Apr;73(4):786-802. doi: 10.4315/0362-028x-73.4.786. PMID: 20377972.
- Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007 Sep;5(9):665-79. doi: 10.1038/nrmicro1718. PMID: 17703225.
- Chang WS, van de Mortel M, Nielsen L, Nino de Guzman G, Li X, Halverson LJ. Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water-limiting conditions. J Bacteriol. 2007 Nov;189(22):8290-9. doi: 10.1128/JB.00727-07. Epub 2007 Jun 29. PMID: 17601783; PMCID: PMC2168710.
- Elgamoudi BA, Korolik V. Campylobacter Biofilms: Potential of Natural Compounds to Disrupt Campylobacter jejuni Transmission. Int J Mol Sci. 2021 Nov 10;22(22):12159. doi: 10.3390/ijms222212159. PMID: 34830039; PMCID: PMC8617744.
- Brown HL, Reuter M, Hanman K, Betts RP, van Vliet AH. Prevention of biofilm formation and removal of existing biofilms by extracellular DNases of Campylobacter jejuni. PLoS One. 2015 Mar 24;10(3):e0121680. doi: 10.1371/journal.pone.0121680. PMID: 25803828; PMCID: PMC4372405.
- Rendueles O, Ghigo JM. Multi-species biofilms: how to avoid unfriendly neighbors. FEMS Microbiol Rev. 2012 Sep;36(5):972-89. doi: 10.1111/j.1574-6976.2012.00328.x. Epub 2012 Mar 8. PMID: 22273363.
- Tram G, Day CJ, Korolik V. Bridging the Gap: A Role for Campylobacterjejuni Biofilms. Microorganisms. 2020 Mar 23;8(3):452. doi: 10.3390/microorganisms8030452. PMID: 32210099; PMCID: PMC7143964.
- Otsuka Y, Hagiya H, Takahashi M, Fukushima S, Maeda R, Sunada N, Yamada H, Kishida M, Fujita K, Otsuka F. Clinical characteristics of Campylobacter bacteremia: a multicenter retrospective study. Sci Rep. 2023 Jan 12;13(1):647. doi: 10.1038/s41598-022-27330-4. PMID: 36635328; PMCID: PMC9837072.
- Wakerley BR, Yuki N. Risk of guillain–barré syndrome from fresh chicken in the United Kingdom. Journal of Acute Medicine. 2016;6(4):105-106. doi: 10.1016/j.jacme.2016.09.002.
- Nyati KK, Nyati R. Role of Campylobacter jejuni infection in the pathogenesis of Guillain-Barré syndrome: an update. Biomed Res Int. 2013;2013:852195. doi: 10.1155/2013/852195. Epub 2013 Aug 13. PMID: 24000328; PMCID: PMC3755430.
- Vucic S, Kiernan MC, Cornblath DR. Guillain-Barré syndrome: an update. J Clin Neurosci. 2009 Jun;16(6):733doi: 10.1016/j.jocn.2008.08.033. Epub 2009 Apr 7. PMID: 19356935.
- Fischer GH, Paterek E. Campylobacter. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
- Fernández-Cruz A, Muñoz P, Mohedano R, Valerio M, Marín M, Alcalá L, Rodriguez-Créixems M, Cercenado E, Bouza E. Campylobacter bacteremia: clinical characteristics, incidence, and outcome over 23 years. Medicine (Baltimore). 2010 Sep;89(5):319-330. doi: 10.1097/MD.0b013e3181f2638d. PMID: 20827109.
- Pacanowski J, Lalande V, Lacombe K, Boudraa C, Lesprit P, Legrand P, Trystram D, Kassis N, Arlet G, Mainardi JL, Doucet-Populaire F, Girard PM, Meynard JL; CAMPYL Study Group. Campylobacter bacteremia: clinical features and factors associated with fatal outcome. Clin Infect Dis. 2008 Sep 15;47(6):790-6. doi: 10.1086/591530. PMID: 18699745.
- Spyromitrou-Xioufi P, Ntoulios G, Ladomenou F, Niotakis G, Tritou I, Vlachaki G. Miller Fisher Syndrome Triggered by Infections: A Review of the Literature and a Case Report. J Child Neurol. 2021 Aug;36(9):785-794. doi: 10.1177/0883073820988428. PMID: 34448412.
- Berumen A, Lennon R, Breen-Lyles M, Griffith J, Patel R, Boxrud D, Decuir M, Farrugia G, Smith K, Grover M. Characteristics and Risk Factors of Post-Infection Irritable Bowel Syndrome After Campylobacter Enteritis. Clin Gastroenterol Hepatol. 2021 Sep;19(9):1855-1863.e1. doi: 10.1016/j.cgh.2020.07.033. Epub 2020 Jul 23. PMID: 32711045; PMCID: PMC8994162.
- Jribi H, Sellami H, Mariam S, Smaoui S, Ghorbel A, Hachicha S, Benejat L, Messadi-Akrout F, Mégraud F, Gdoura R. Isolation and Identification of Campylobacter spp. from Poultry and Poultry By-Products in Tunisia by Conventional Culture Method and Multiplex Real-Time PCR. J Food Prot. 2017 Oct;80(10):1623-1627. doi: 10.4315/0362-028X.JFP-16-321. PMID: 28853632.
- Elhadidy M, Ali MM, El-Shibiny A, Miller WG, Elkhatib WF, Botteldoorn N, Dierick K. Antimicrobial resistance patterns and molecular resistance markers of Campylobacter jejuni isolates from human diarrheal cases. PLoS One. 2020 Jan 17;15(1):e0227833. doi: 10.1371/journal.pone.0227833. PMID: 31951631; PMCID: PMC6968864.
- Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM, Zhang Q. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 2009 Mar;4(2):189-200. doi: 10.2217/17460913.4.2.189. PMID: 19257846; PMCID: PMC2691575.
- Wieczorek K, Osek J. Antimicrobial resistance mechanisms among Campylobacter. Biomed Res Int. 2013;2013:340605. doi: 10.1155/2013/340605. Epub 2013 Jun 24. PMID: 23865047; PMCID: PMC3707206.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.