Medicine Group . 2023 February 23;4(2):297-302. doi: 10.37871/jbres1674.
Life Habits, Frequency of Application and Long-Term Exposure to Cosmetic Products Containing Parabens Can Cause Higher Breast Cancer Risk among Women
Tea Gaberc1, Željka Roje2*, Zdenko Stanec3, Zlatko Vlajčić2, Krešimir Martić2, Domagoj Eljuga2, Božo Gorjanc2, Srećko Budi4, Franjo Rudman5, Ioannis Stasinopoulos6 and Rado Žic2
2Department for Plastic, Reconstructive and Aesthetic Surgery, University Hospital Dubrava, Zagreb, Croatia
3Edumed Polyclinic & Day Hospital, Zagreb, Croatia
4Special Hospital AGRAM - Polyclinic Zagreb, Zagreb, Croatia
5University Hospital for Tumors, University Hospital Center "Sestre milosrdnice", Zagreb, Croatia
6Ivi Med Clinic, Budapest, Hungary
- Breast cancer risk
- Deodorants
- Endocrine disruptors
- Life habits
- Parabens
Abstract
Background: Modern lifestyle involves everyday use of cosmetic products containing various sources of Endocrine Disruptors (EDs), parabens being the most common ones. Growing cancer burden globally, namely the increased breast cancer incidence, suggests a lifestyle cause. Could the continuous and prolonged exposure to parabens be accountable for the higher breast cancer risk on a global scale?
Methods: A Questionnaire was filled out on the subjects' anamnestic data, anthropometric measurements, sociodemographic characteristics, the presence of risk factors for the development of breast cancer, and the beginning and frequency of use of deodorants, body lotions and antiperspirants. Total of seventy-two (n = 72) patients were enrolled in the study. All obtained data was compared with risk factors for the development of breast cancer.
Results: Women with breast cancer have longer been exposed to cosmetic products usage (an average of 36 years in total) in comparison to healthy women (an average of 24 years in total). Healthy women tend to start using cosmetic products earlier, at the average age of 16, and tend to use them more often as part of their everyday routine. In contrast, women with breast cancer start later on, at the average age of 19.
Conclusion: Our study supports the fact that the most important risk factor for breast cancer is age, but also suggests that long term exposure to parabens could play a crucial role in breast cancer manifestation, even more so at an earlier age. Shift to healthier alternatives to parabens in cosmetic products would contribute to both disease prevention as well as greener environment.
Introduction
Healthy living requires a balance of responsible and smart health choices: eating right, daily physical activity, ensuring adequate sleep, avoiding harmful drugs, alcohol, tobacco, stress, and safeguarding a good work-life balance [1]. High incidence of NCDs (Chronic Noncommunicable Diseases) nowdays, specifically endocrine disorders, suggests that environmental factors may have a more significant impact on expression of endocrine disorders compared to genetics. Endocrine Disrupting Chemicals (EDCs) are defined by the World Health Organization (WHO) as “exogenous substances or mixtures that influence the endocrine system and cause adverse health effects in an intact organism, or its progeny, or (sub)populations” [1]. Studies have shown the adverse effects of EDs and have highlighted namely parabens among them, one of the most popular preservatives used in various cosmetics, pharmaceutical products and food, and their possible contribution to development of hormone-dependent cancers [2-7]. Furthermore, breast cancer has now overtaken lung cancer as the world’s mostly commonly-diagnosed cancer, according to statistics released by the International Agency for Research on Cancer (IARC) in December 2021. Breast cancer is the leading cause of cancer death among women. WHO and the cancer community have in June 2022 responded to the growing cancer burden globally that is straining individuals, communities and health systems via newly established Global Breast Cancer Initiative which aims to reduce deaths from breast cancer by promoting breast health, improving timely cancer detection and ensuring access to quality care [8,9]. The increased breast cancer incidence suggests a lifestyle cause since hereditary breast tumors account for less than 10% of all breast cancers respectively. Among the most common modifiable risk factors for breast cancer, which account for up to 90% of breast cancer occurrence respectively, exposure to EDCs has been the most concerning [2,4,5]. Considering modern lifestyle involves everyday use of cosmetic products containing various sources of EDs, parabens being the most common ones, the plausible question arises - could the continuous and prolonged exposure to parabens be accountable for the higher breast cancer risk and current incidence on a global scale [10-12]?
Materials and Methods
Patients
The study was conducted from April 2017 to November 2019 and a total of seventy- two (n = 72) patients were enrolled in the study. The inclusion criteria were otherwise healthy patients (ASA (American Society of Anesthesiology) category 2 (52.8%) or ASA category I (38.9%)), 18-80 years of age, who gave their consent to the proposed study. The patients who received adjuvant oncological therapy, either chemo-, radio - or hormone therapy, and patients with other oncological diagnosis and systemic infections were excluded.
The first group of patients (“the unhealthy”) included 49 (68.1%) female patients with malignant breast tumor. The second group (“the healthy”) included 23 (31.9%) patients, namely 13 (18.1%) patients with benign breast tumors (pathohistological finding of fibroadenoma) and 10 (13.9%) patients with benign hypertrophy of glandular tissue (pathohistological finding of benign fibrocystic mastopathy) (Table 1).
| Table 1: Study groups. | ||||
| Group | N | Label | Age (years) | Inclusion Criteria [PHD] |
| Healthy | 49 | B-biopsy, R-reduction | 18-80 | Fibroadenoma, Benign Fibrocystic Mastopathy |
| Unhealthy | 23 | Ca-Carcionoma | Malignant Breast Tumor = Carcinoma | |
Outcomes of the study
The primary outcome of the study was to determine how frequency of application and long-term exposure to cosmetic products containing parabens can cause endocrine disruption and whether it leads to higher breast cancer incidence among women.
Secondary outcomes were the duration of exposure to paraben containig products, the correlation between lifestyle habits and breast cancer incidence, and the effect of physical activity on breast cancer risk.
Study design
Upon admission to the Clinic, the ward doctor filled out a Questionnaire (Study Protocol) on the subjects' anamnestic data, anthropometric measurements, sociodemographic characteristics, the presence of risk factors for the development of breast cancer, and the beginning and frequency of use of deodorants, body lotions and antiperspirants. All obtained data was compared with risk factors for the development of breast cancer. The data obtained in this study via the Questionnaire is available upon request of the respective author. The novel FPSE-based (Fabric-Phase Sorptive Extraction) pretreatment method for the selective extraction of parabens from the breast tissue samples as well as the novel HPLC-DAD methodology were successfully implemented to real cancerous and non- cancerous tissue samples derived from different sub regions of women’s breast obtained from diverse individuals [13-15].
Compliance with ethical standards
Prior to being included in the study, the patients were informed in detail about the procedure and possible risks and complications. The study protocol was approved by the Ethics Review Board of the University Hospital Dubrava, Zagreb, Croatia.
Statistical analysis
For statistical analysis we used the GraphPad Prism 6.0 software (San Diego, CA, USA) to run one-way ANOVA followed by Dunnett’s multiple comparison test to compare mean values of individual treatments with control means. Significance threshold was set at p < 0.05. In the case where no significant deviation from normality was observed, the mean and standard deviation was used to describe a distribution of a quantitative variable. Median and range were used for asymmetrically distributed quantitative or ordinal variables. Absolute and relative frequencies were used to describe the distribution of categorical variables. The significance of differences in quantitative variables between the study groups was assessed with the nonparametric independent-samples Kruskal–Wallis test. The Chi-square test was used to assess differences in the distribution of categorical data. In cases when the frequency of events was low, Fisher exact test was used instead.
Results and Discussion
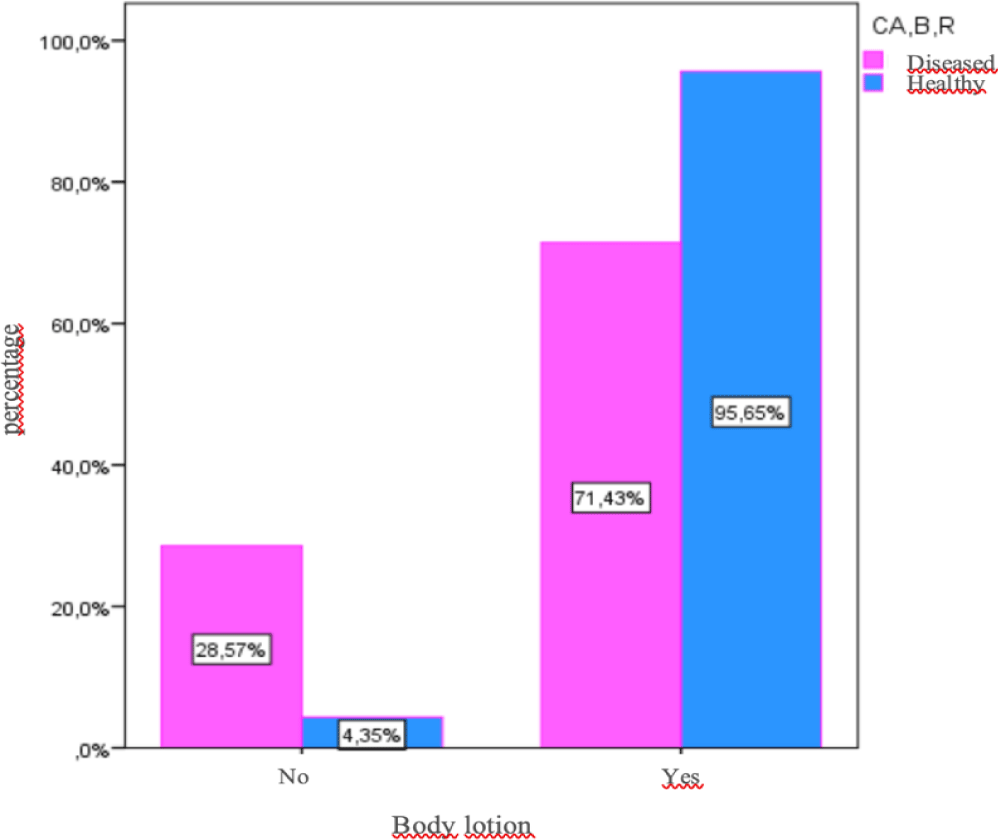
According to the Questionnaire, the most commonly used brand is Nivea. If we take into account the number of exposure years in total, there is a significant difference (p < 0,05) between the two study groups, which indicated that women with breast cancer have longer been using deodorants and body lotions (Table 2). The results have shown that women with breast cancer have been exposed to average of 32, 97 years in total to body lotions, and to average of 36, 88 years in total to deodorants. On the contrary, healthy women have been exposed to average of 22, 14 years in total to body lotions, and to average of 24, 22 years in total to deodorants (Table 3). The average beginning age of body lotion usage is 24, 29 for women with breast cancer compared to 17, 86 for healthy women (Figure 1). Although the diseased women started to use cosmetic products at an older age, they have been using deodorants and body lotions longer, thus suggesting positive correlation between the length of exposure to EDs and the risk of developing breast cancer.
| Table 2: The length of exposure in years in total. | ||||
| N | x̄ | SD | ||
| Age - Beginning age of body lotion usage |
Unhealthy | 35 | 32.97 | 11,094 |
| Healthy | 22 | 22.14 | 9,677 | |
| Age - Beginning age of deodorant usage |
Unhealthy | 42 | 36.88 | 9,554 |
| Healthy | 23 | 24.22 | 9,798 | |
| Table 3: The beginning age of body lotion/deodorant usage. | ||||||||||||
| N | x̄ | SD | p* | |||||||||
| Beginning age of deodorant and antiperspirant usage | Unhealthy | 42 | 19.98 | 4,635 | 0.02 | |||||||
| Healthy | 23 | 16.83 | 5,828 | |||||||||
| *Fisher’s exact test. | ||||||||||||
| Unhealthy = Breast Carcinoma | Healthy = Reductions [Benign Mastopathy] | p* | ||||||||||
| Percentile 25 | Median | Percentile 75 | Percentile 25 | Median | Percentile 75 | |||||||
| Age - Beginning age of body lotion usage | 26 | 32 | 41 | 15 | 22 | 29 | <0.001 | |||||
| Age - Beginning age of deodorant usage | 31 | 37 | 43 | 17 | 24 | 31 | <0.001 | |||||
The association between BMI (Body Mass Index) and breast cancer risk has received much attention, since adipose tissue is no longer considered to be an inert tissue that stores fat, but is in fact an endocrine organ [16,17]. The average BMI among women with breast cancer is 26.24 which falls into the overweight range (average height being 164.41 cm and weight 71.59 kg respectively). The average BMI among healthy women is 24.97 which falls within the healthy range (average height being 167.39 cm and weight 69.74 kg) (Table 4). All of the participants rated their everyday physical activity on a scale of 1 to 10 (1 = sedentary activity, 3 = everyday house chores, 10 = high intensity sports). Using Fisher's exact test which resulted with level of 0,150, we did not demonstrate a significant difference among healthy and diseased women (Table 5). But, the results showed that 28, 6% women with breast cancer are not physically active (mean scale score 3 out of 10) in comparison to healthy women (mean scale score 6 out of 10). Moreover, the diseased women tend to have higher BMI, suggesting that the excess fat tissue could serve as a bioaccumulation reservoir of paraben and therefore are at higher risk of developing larger and more aggressive tumors with higher levels of Ki-67 proliferation index [18]. And vice versa, studies have shown that circulating plasma concentrations of parabens induce adipocyte differentiation that can lead to higher BMI [16-19].
| Table 4: Anthropometric measurements among study groups. | |||||||
| Unhealthy | Healthy | p* | |||||
| Percentile 25 | Median | Percentile 75 | Percentile 25 | Median | Percentile 75 | ||
| Birth year | 1954.00 | 1960.00 | 1967.00 | 1970.00 | 1978.00 | 1988.00 | 0.001 |
| Age (2019-X) | 52 | 59 | 65 | 31 | 41 | 49 | 0.001 |
| Height | 162.00 | 165.00 | 168.00 | 164.00 | 168.00 | 170.00 | 0.09 |
| Weight | 65 | 71 | 78 | 63 | 69 | 78 | 0.531 |
| BMI | 23.8 | 26.7 | 28.8 | 22.6 | 24.3 | 27.6 | 0.185 |
| *t-test. | |||||||
| Table 5: Everyday physical activity rated on a scale of 1 to 10 (1 = sedentary activity, 3 = everyday house chores, 10 = high intensity sports). | ||||||
| Unhealthy | Healthy | Total | p* | |||
| Physical activity | 2 | N | 7 | 1 | 8 | 0.150 |
| % | 14.3 | 4.3 | 11.1 | |||
| 3 | N | 14 | 2 | 16 | ||
| % | 28.6 | 8.7 | 22.2 | |||
| 4 | N | 5 | 3 | 8 | ||
| % | 10.2 | 13.0 | 11.1 | |||
| 5 | N | 5 | 4 | 9 | ||
| % | 10.2 | 17.4 | 12.5 | |||
| 6 | N | 9 | 4 | 13 | ||
| % | 18.4 | 17.4 | 18.1 | |||
| 7 | N | 5 | 3 | 8 | ||
| % | 10.2 | 13.0 | 11.1 | |||
| 8 | N | 3 | 4 | 7 | ||
| % | 6.1 | 17.4 | 9.7 | |||
| 9 | N | 0 | 2 | 2 | ||
| % | 0.0 | 8.7 | 2.8 | |||
| 10 | N | 1 | 0 | 1 | ||
| % | 2.0 | 0.0 | 1.4 | |||
| Total | N | 49 | 23 | 72 | ||
| % | 100.0 | 100.0 | 100.0 | |||
There was no significant difference between study group’s smoking cigarettes habits or hard liquor consumption (p > 0.05), as well as for well known breast cancer risk factors - having the first pregnancy after age 30, not breastfeeding and birth control pill intake [20]. Most of the participants stated that they drink alcohol occasionally (59.7%). Most of the participants declared themselves as non-smokers (70.8%). On the contrary, 62.5% of all participants have a positive family history of cancer (Table 6). The most common among them is breast cancer (32%) followed by lung, prostate, colon, endometrial, and thyroid cancer, which further supports the plausible correlation between the higher incidence of hormone dependent cancer nowdays with constant exposure to endocrine disruptors. Using Fisher's exact test which resulted with level of 0.789, we did not demonstrate a significant difference between the healthy (34.8%) and diseased group (30.6%) as far as family cancer history is concerned. These results suggest higher awareness among women with family cancer burden and better prevention measures, early detection and better treatment options. As far as dietary habits are concerned, healthy women eat more fruit and meat, but less vegetables compared to women with breast cancer. There was no significant difference between group’s carbohydrates intake (Table 7).
| Table 6: Family Cancer’s history. | ||||||
| Unhealthy | Healthy | Total | p* | |||
| Family’s Cancer History | No | N | 34 | 15 | 49 | 0.789 |
| % | 69.4% | 65.2% | 68.1% | |||
| Yes | N | 15 | 8 | 23 | ||
| % | 30.6% | 34.8% | 31.9% | |||
| Total | N | 49 | 23 | 72 | ||
| % | 100.0% | 100.0% | 100.0% | |||
| *Fisher's exact test. | ||||||
| Table 7: Dietary habits. | ||||||||
| Unhealthy | Healthy | Total | p* | |||||
| N | % | N | % | N | % | |||
| Vegetables | 1 | 28 | 57.1% | 7 | 30.4% | 35 | 48.6% | 0.001 |
| 2 | 16 | 32.7% | 9 | 39.1% | 25 | 34.7% | ||
| 3 | 5 | 10.2% | 1 | 4.3% | 6 | 8.3% | ||
| 4 | 0 | 0.0% | 6 | 26.1% | 6 | 8.3% | ||
| Total | 49 | 100.0% | 23 | 100.0% | 72 | 100.0% | ||
| Fruit | 1 | 3 | 6.1% | 0 | 0.0% | 3 | 4.2% | 0.004 |
| 2 | 11 | 22.4% | 1 | 4.3% | 12 | 16.7% | ||
| 3 | 13 | 26.5% | 16 | 69.6% | 29 | 40.3% | ||
| 4 | 22 | 44.9% | 6 | 26.1% | 28 | 38.9% | ||
| Total | 49 | 100.0% | 23 | 100.0% | 72 | 100.0% | ||
| Meat | 1 | 11 | 22.4% | 10 | 43.5% | 21 | 29.2% | 0.036 |
| 2 | 15 | 30.6% | 5 | 21.7% | 20 | 27.8% | ||
| 3 | 10 | 20.4% | 0 | 0.0% | 10 | 13.9% | ||
| 4 | 13 | 26.5% | 8 | 34.8% | 21 | 29.2% | ||
| Total | 49 | 100.0% | 23 | 100.0% | 72 | 100.0% | ||
| Carbohydrates | 1 | 7 | 14.3% | 6 | 26.1% | 13 | 18.1% | 0.066 |
| 2 | 7 | 14.3% | 8 | 34.8% | 15 | 20.8% | ||
| 3 | 20 | 40.8% | 6 | 26.1% | 26 | 36.1% | ||
| 4 | 15 | 30.6% | 3 | 13.0% | 18 | 25.0% | ||
| Total | 49 | 100.0% | 23 | 100.0% | 72 | 100.0% | ||
| *Fisher's test. | ||||||||
Conclusion
Women with breast cancer have longer been exposed to cosmetic products usage (an average of 36 years in total) in comparison to healthy women (an average of 24 years in total). Healthy women tend to start using cosmetic products earlier, at the average age of 16, and tend to use them more often as part of their everyday routine. In contrast, women with breast cancer start later on, at the average age of 19. The obtained data supports the fact that the most important risk factor for breast cancer is age, but also suggests that long term exposure to EDs, parabens namely, plays a big role nowdays. Considering modern lifestyle involves everyday use of cosmetic products with confirmed negative or at least debatable effects on health as a whole, the obtained data should be of use in terms of creating better preventive measures which aim to reduce breast cancer risk via promoting everyday physical activity. Awareness raising campaigns should inform and educate consumers on the topic of cosmetic products quality. The positive shift to healthier alternatives to parabens would contribute to both individual health and disease prevention as well as greener and cleaner environment.
Acknowledgments
Author contributions
All authors have read and agreed to this version of the manuscript.
Funding
No funding occurred for this study.
Informed consent statement
Patients were informed in detail, and informed consent was obtained to use the data for this study.
Data availability statement
The data presented in this study is available upon request of the respective author. Due to the protection of personal data, the data is not publicly available.
Conflicts of interest
The authors declare no conflict of interest.
References
- Kickbusch I. The contribution of the World Health Organization to a new public health and health promotion. Am J Public Health. 2003 Mar;93(3):383-8. doi: 10.2105/ajph.93.3.383. PMID: 12604477; PMCID: PMC1447748.
- Bergman Å, Heindel JJ, Jobling S, Kidd K, Zoeller TR, Organization WHO. State of the science of endocrine disrupting chemicals 2012. World Health Organization. 2013.
- Mandic T. The effect of EDs on women's reproductive health. Zagreb, University in Zagreb, School of Medicine. 2016.
- Nowak K, Ratajczak-Wrona W, Górska M, Jabłońska E. Parabens and their effects on the endocrine system. Mol Cell Endocrinol. 2018 Oct 15;474:238-251. doi: 10.1016/j.mce.2018.03.014. Epub 2018 Mar 27. PMID: 29596967.
- Rodgers KM, Udesky JO, Rudel RA, Brody JG. Environmental chemicals and breast cancer: An updated review of epidemiological literature informed by biological mechanisms. Environ Res. 2018 Jan;160:152-182. doi: 10.1016/j.envres.2017.08.045. Epub 2017 Oct 6. PMID: 28987728.
- DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019 Nov;69(6):438-451. doi: 10.3322/caac.21583. Epub 2019 Oct 2. PMID: 31577379.
- Roje Ž, Ilić K, Galić E, Pavičić I, Turčić P, Stanec Z, Vrček IV. Synergistic effects of parabens and plastic nanoparticles on proliferation of human breast cancer cells. Arh Hig Rada Toksikol. 2019;70(4):310-314. doi: 10.2478/aiht-2019-70-3372. PMID: 32623858.
- World Health Organisation. New global breast cancer intiative highlights renewed commitment to improve survival. 2022.
- Stanec Z, Žic R, Budi S, Stanec S, Milanović R, Vlajčić Z, Roje Z, Rudman F, Martić K, Held R, Božo G. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg. 2014;73(5):485-91. doi: 10.1097/SAP.0b013e31827a30e6. PMID: 24378808.
- Darbre PD, Harvey PW. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol. 2008 Jul;28(5):561-78. doi: 10.1002/jat.1358. PMID: 18484575.
- Boberg J, Taxvig C, Christiansen S, Hass U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol. 2010 Sep;30(2):301-12. doi: 10.1016/j.reprotox.2010.03.011. Epub 2010 Apr 8. PMID: 20381602.
- Liao C, Chen L, Kannan K. Occurrence of parabens in foodstuffs from China and its implications for human dietary exposure. Environ Int. 2013 Jul;57-58:68-74. doi: 10.1016/j.envint.2013.04.001. Epub 2013 May 14. PMID: 23685225.
- Alampanos V, Kabir A, Furton KG, Roje Ž, Vrček IV, Samanidou V. Fabric phase sorptive extraction combined with high-performance-liquid chromatography-photodiode array analysis for the determination of seven parabens in human breast tissues: Application to cancerous and non-cancerous samples. J Chromatogr A. 2020;1630:461530. doi: 10.1016/j.chroma.2020.461530. Epub ahead of print. PMID: 32950814.
- Rigkos G, Alampanos V, Kabir A, Furton KG, Roje Ž, Vrček IV, Panderi I, Samanidou V. An improved fabric-phase sorptive extraction protocol for the determination of seven parabens in human urine by HPLC-DAD. Biomed Chromatogr. 2021;35(2):e4974. doi: 10.1002/bmc.4974. PMID: 32893361.
- Parla A, Zormpa E, Paloumpis N, Kabir A, Furton KG, Roje Ž, Samanidou V, Vinković Vrček I, Panderi I. Determination of Intact Parabens in the Human Plasma of Cancer and Non-Cancer Patients Using a Validated Fabric Phase Sorptive Extraction Reversed-Phase Liquid Chromatography Method with UV Detection. Molecules. 2021; 26(6):1526. doi: 10.3390/molecules26061526. PMID: 33799523; PMCID: PMC8002076.
- Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013 Apr 20;9(2):191-200. doi: 10.5114/aoms.2013.33181. Epub 2013 Feb 10. PMID: 23671428; PMCID: PMC3648822.
- Liu K, Zhang W, Dai Z, Wang M, Tian T, Liu X, Kang H, Guan H, Zhang S, Dai Z. Association between body mass index and breast cancer risk: evidence based on a dose-response meta-analysis. Cancer Manag Res. 2018 Jan 18;10:143-151. doi: 10.2147/CMAR.S144619. PMID: 29403312; PMCID: PMC5783020.
- Hu P, Chen X, Whitener RJ, Boder ET, Jones JO, Porollo A, Chen J, Zhao L. Effects of parabens on adipocyte differentiation. Toxicol Sci. 2013 Jan;131(1):56-70. doi: 10.1093/toxsci/kfs262. Epub 2012 Sep 5. PMID: 22956630; PMCID: PMC3621350.
- Regnier SM, Sargis RM. Adipocytes under assault: environmental disruption of adipose physiology. Biochim Biophys Acta. 2014 Mar;1842(3):520-33. doi: 10.1016/j.bbadis.2013.05.028. Epub 2013 Jun 2. PMID: 23735214; PMCID: PMC3823640.
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012 Nov;13(11):1141-51. doi: 10.1016/S1470-2045(12)70425-4. Epub 2012 Oct 17. PMID: 23084519; PMCID: PMC3488186.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.