Biology Group . 2023 March 16;4(3):440-445. doi: 10.37871/jbres1693.
Studying free Proline and Soluble Sugars Accumulation in the Fungus Aspergillus creber Exposed to Salt Stress
John Pouris*, Konstantina Kapnopoulou, Sofia Papadopoulou and Ioanna Pyrri
- Aspergillus creber
- Fungi
- Osmoprotective
- Salt tolerance
- Carbohydrates
- Proline
Abstract
Salinity is one of the most important factors influencing plant growth by participating in abiotic stress. Plants have developed a number of physiological mechanisms responding to abiotic stress. A well-studied response is the concentration of free proline and soluble sugars, which through various mechanisms enhances plant resistance to abiotic stress. What is less studied is that the mechanism of proline and sugars is present in other categories of organisms such as fungi. There are even less bibliographic data on a relationship of the mechanism of proline and soluble sugars accumulation in fungi. The purpose of this study is to investigate proline and soluble sugars accumulation in the fungus Aspergillus creber under salt stress tolerance.
Introduction
Aspergillus series Versicolores members, can be found in a wide range of environments such as indoor environments, food, clinical materials, soil, caves, marine or hypersaline ecosystems [1]. Aspergillus creber belonging to the revised group Aspergillus section Versicolores [2]. This newly characterized fungus is airborne and according to recent literature data, produces the substance Sterigmatocystin (ST), that is a carcinogenic precursor to aflatoxin B1 [3]. Very little bibliographic data have been gathered so far regarding the physiology of Aspergillus creber. It is of even greater interest to study the physiology of this fungus under stress conditions and more specifically under salinity stress.
Two of the well-studied mechanisms of plants response to abiotic stress is the accumulation of free proline [4-6] and soluble sugars [7-8]. here is a lack of data on the variation of these substances in fungi under abiotic stress.
The study of free proline and soluble sugars accumulation in the fungus Aspergillus creber aims to understand how this organism responds to stress in a more comprehensive way. Both free proline and soluble sugars have been shown to accumulate in response to abiotic stress, and their accumulation may play a role in protecting the fungus against the adverse effects of stress. By investigating the mechanisms underlying the accumulation of both these compounds in Aspergillus creber, hope to gain insight into the fungal stress response and develop strategies for improving the organism's resilience in agricultural and industrial settings. In addition, this research may have wider implications for understanding how other fungi and even other organisms respond to environmental stress. Ultimately, a better understanding of how Aspergillus creber responds to stress could have significant practical and theoretical implications for the field of microbiology.
Overall, measuring soluble sugars and free proline in Aspergillus creber provides valuable insights into the metabolic and stress response processes of this fungus. This information can be useful in understanding the biology of Aspergillus creber and can help researchers identify potential targets for intervention or manipulation to improve its growth and productivity.
The Role of Proline
Proline is produced in plant cells either in the cytoplasm or the chloroplasts from glutamate, which is reduced to Glutamine-Hemialdehyde (GSA) by D-1-Pyrroline-5-Carboxylate Synthetase (P5CS). GSA can be converted to Pyrroline 5-Carboxylate (P5C), which is then further reduced from P5C Reductase (P5CR) to proline. Proline is degraded in the mitochondria by Proline Dehydrogenase (ProDH) and P5C Dehydrogenase (P5CDH) to glutamate [9-11].
Intracellular proline levels are determined by biosynthesis, catabolism, intercellular transport, and different cell compartments. Predictions by computational models and experimental data suggest the detection of biosynthetic enzymes (P5CS1, P5CS2 and P5CR) in the cytosol, while a mitochondrial detection is predicted for enzymes involved in proline catabolism, such as PDATH, PDHH/OD5, PDHH/ERD. The partitioning of proline metabolism implies that extensive intracellular and apoplastic transfer of proline must take place between the cytoplasm, chloroplasts and mitochondria [12].
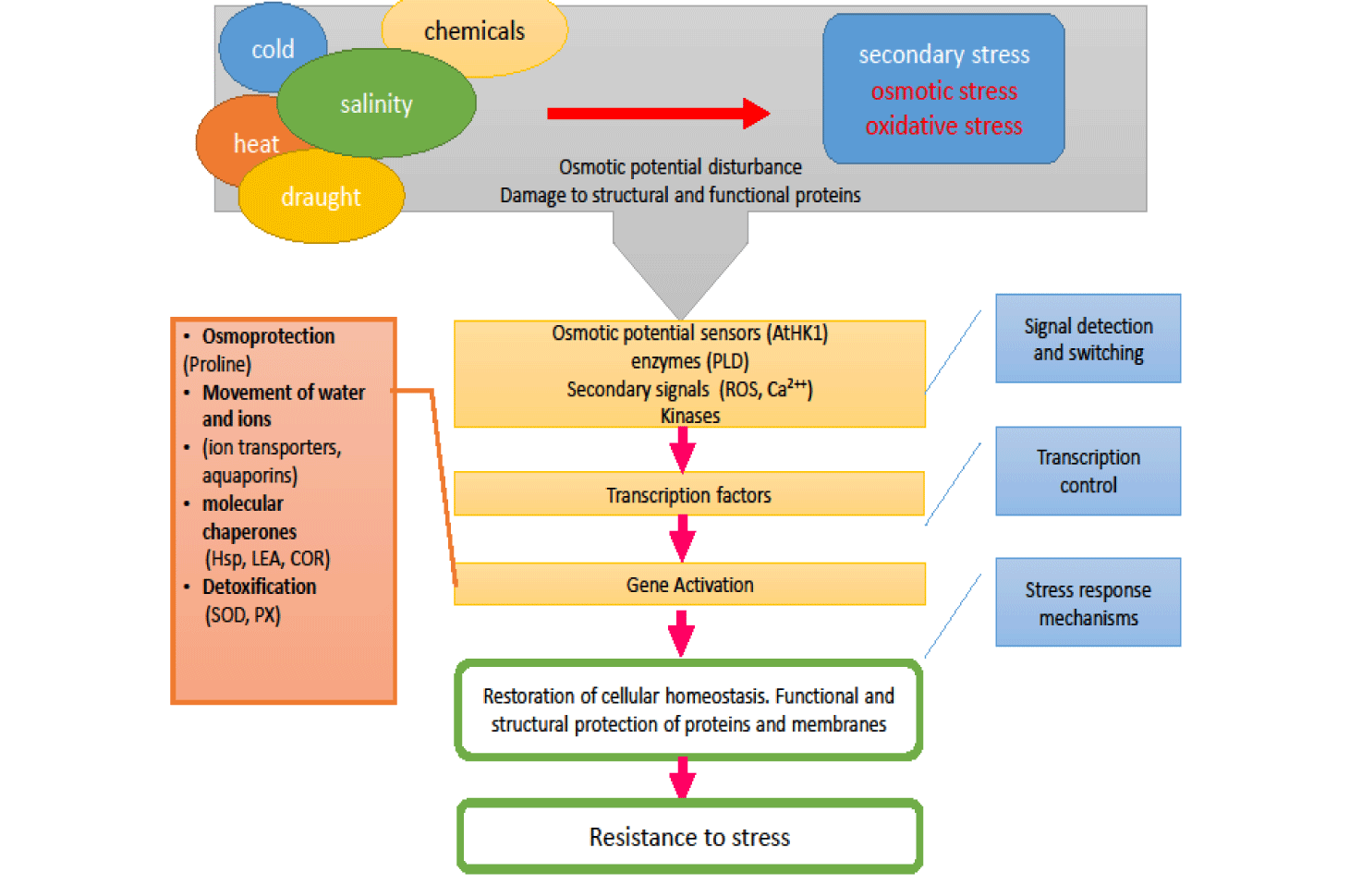
It is well known that the ability of plant tissues to accumulate proline has been associated with resistance to abiotic stress. Proline is thought to act as an osmolyte associated with the degradation of ROS, as well as a molecular companion by stabilizing the structure of proteins [13], thus protecting cells from damage caused by stress (Figure 1). Accumulation of proline in drought conditions, salinity, high light and ultraviolet radiation, heavy metals and in response to abiotic stress has been reported by numerous colleagues [14-18].
The Role of Soluble Sugars
Non-structural carbohydrates, such as solube sugars, are the main reserves in fungi, necessary to support the phenological events that occur in them and to from abiotic stress. Soluble sugars (sucrose, glucose and fructose) play an important role in maintaining the overall structure and growth of fungi acting as both nutrients and regulators of metabolism, growth and response to stress throughout the life of the fungi [19]. In addition, sugars play an active role in regulating growth, carbon distribution, carbohydrate and lipid metabolism, osmotic homeostasis, protein synthesis and gene expression, as well as stabilizing membranes during various abiotic strains. Studies have shown that fungi, under conditions of osmotic stress or drought, activate various mechanisms that result in the accumulation of osmotically active substances or the accumulation of substances that act as osmoprotectants [20,21]. Osmotic stress is caused by a decrease in osmotic potential. The osmotic potential is reduced (at more negative values) by the accumulation of water-soluble molecules such as sugars, to reduce the intake of e.g. water in drought conditions Therefore, the soluble sugar content can be used as a physiological indicator for assessing resistance to osmotic stress.
Fungi and Proline
It is suggested via the literature that the presence of increased proline in fungal media is associated with resistance to abiotic stress [22,23]. Fungi belong to heterotrophic organisms. They do not synthesize proline and are therefore bound to the uptake of proline by their substrate through specific membrane transporters [24].
Materials and Methods
The fungus was used for the experiments was Aspergillus creber ATHUM 9676. This strain has been isolated from the air and comes from ATHUM Culture Collection of Fungi of the National and Kapodistrian University of Athens Mycetotheca.
Four different Sabouraud Dextrose Agar (SDA) nutrients were prepared with increased concentration of NaCl: SDA (control), SDA + 2% NaCl, SDA + 4% NaCl, SDA + 8% NaCl. The cultivation of the fungus was carried out at 25°C until they were fully developed [25]. The fungus were carefully removed from the nutrient medium. The nutrient material SDA contains 2.84% proline [26]. The fungus were then dried in an oven at 45°C for 5 days and were ground. Proline content was determined spectrophotometrically [27]. The powder was homogenized with sulphosalicylic acid (20 mL, 3% w/v), and then filtered through Whatman # 2 filter paper. Two ml of the filtrate reacted with acid-ninhydrin solution (2 mL) and glacial acetic acid (2 mL) in triplicate test tubes. The tubes were heated at 100°C for 1 h in a water bath and the reaction terminated in cool water. After cooling, the reaction mixture was extracted with 5 mL toluene, and homogenized in a vortex. The toluene that contains the chromophore used to measure the absorbance at λ = 520 nm and toluene was used as blank sample. The results of proline concentration are given in µmol g-1 d.w.; L-proline (Serva, Germany) solutions were used for the standard curve.
Soluble sugar content was estimated spectrophotometrically [28]. Soluble sugars were extracted from dried leaf tissue (dwt) with 80% ethanol (v/v) and tissue residues were used to determine starch content. The ground sample was placed in 10 ml of 80% ethanol (v/v) in a shaker and the extract was filtered using Whatman No. 2 filter paper. Soluble sugar concentrations were determined using a modified phenol-sulfuric acid method [29].
Statistical Analysis
ANOVAs indicated no significant treatment between experiments, and thus, means were averaged over experiments. No data transformation took place judging from the normality and homogeneity of variance tests. Statistical analysis was performed using SPSS 20 (IBM, Armonk, NY, USA). Both ANOVA and Tukey’s tests were set at p ≤ 0.05.
Results
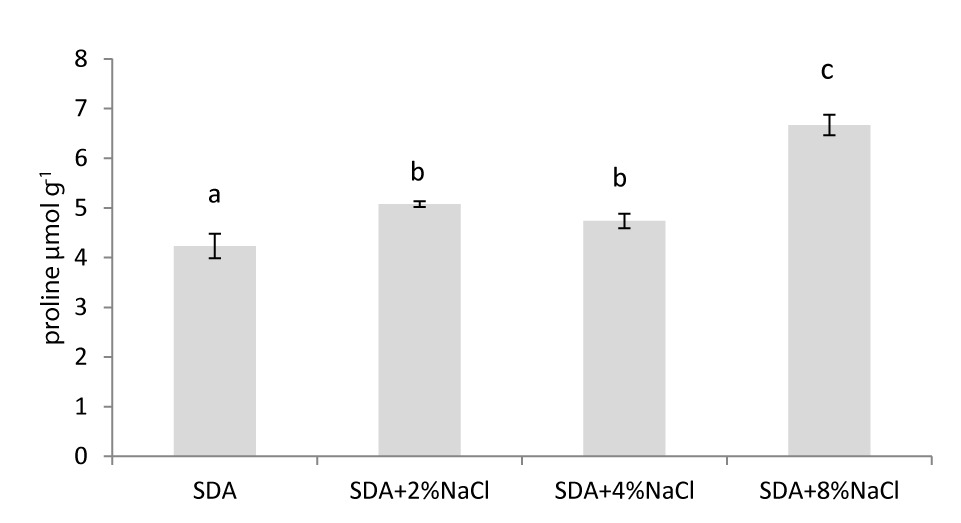
There is an increase in free proline accumulation in Aspergillus creber which grows in an environment of increased salinity. There are statistically significant differences (p ≤ 0.05) between fungi that grew in nutrient medium with NaCl compared to those that grew in nutrient medium without NaCl (control). Compared to control, fungi grown in 2% NaCl showed a 17.14% increase in free proline concentration, in 4% NaCl proline increased by 11.90% while in 8% NaCl 57.57%.
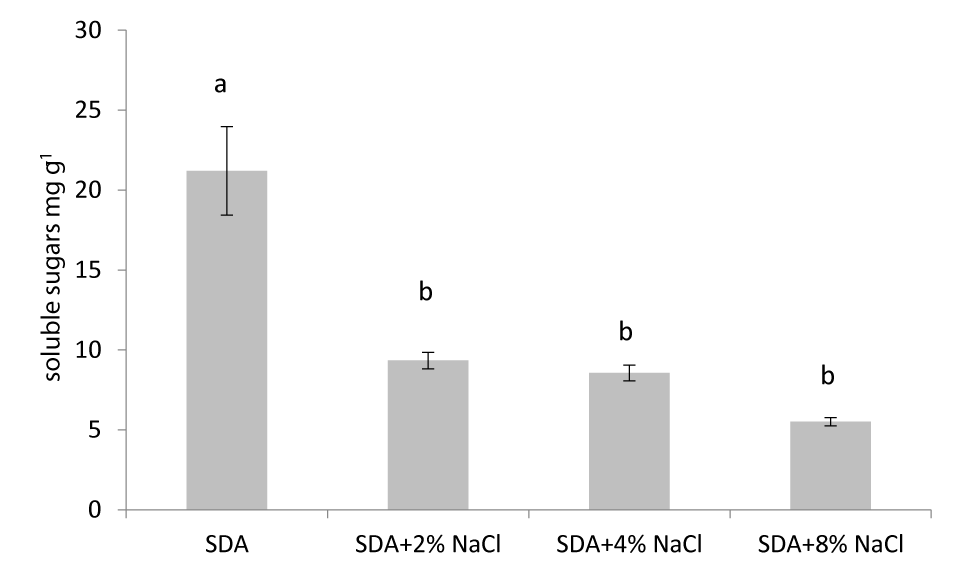
There is a decrease in soluble sugars accumulation in Aspergillus creber which grows in an environment of increased salinity. There are statistically significant differences (p ≤ 0.05) between fungi that grew in nutrient medium free of NaCl (control) compared to those that grew in nutrient medium with NaCl. Compared to control, fungi grown in 2% NaCl showed a 55.95% decrease in soluble sugars concentration, in 4% NaCl soluble sugars decreased by 59,65% while in 8% NaCl 73,99%.
Discussion
Free proline appears to be accumulated in the fungus Aspergillus creber under salinity conditions. The well-studied mechanism of free proline accumulation in plants might be involved in fungus life cycle. However, the macroscopic picture of the fungal colonies did not differ between fungus exposed to salinity 8% NaCl) and control fungal specimens. The fungus has the ability to absorbs and accumulate proline from its substrate contributing to endurance to abiotic stress.
Total soluble sugars serve as a source of energy for cells and are involved in various cellular processes, such as osmotic regulation and signaling. Our results showed that the levels of total soluble sugars were significantly higher in control compared to the treatments, suggesting that this fungus has a high demand for energy under salinity. It seems that the increase in salinity leads to a decrease in their concentration in the fungus. There is evidence that the reduced concentration of sugars in fungi may be due to increased metabolic rates in order to cope with the increased salinity [30].
Our results showed a significant increase in the accumulation of free proline in Aspergillus creber compared to the control, indicating that this fungus is likely experiencing osmotic stress and responding to it by accumulating free proline. One of the main roles of proline in osmotic regulation is to act as an osmoprotectant. Osmoprotectants are small molecules that help cells maintain their shape and function under conditions of high osmotic stress. Proline, in particular, can stabilize proteins and cell membranes, preventing damage caused by changes in water potential. Additionally, proline can act as a signaling molecule, helping to regulate various cellular processes under stress conditions. Accumulation of proline and sugars in fungi as a result of response to abiotic stress is an understudied field. There is not enough literature data on whether these mechanisms exist in a large number of fungi. In plant organisms it has been established that these mechanisms are present in the majority of them, which has led to practical applications. More studies should be conducted in this field including more fungal species.
References
- Sklenář F, Glässnerová K, Jurjević Ž, Houbraken J, Samson RA, Visagie CM, Yilmaz N, Gené J, Cano J, Chen AJ, Nováková A, Yaguchi T, Kolařík M, Hubka V. Taxonomy of Aspergillus series Versicolores: species reduction and lessons learned about intraspecific variability. Stud Mycol. 2022 Dec;102:53-93. doi: 10.3114/sim.2022.102.02. Epub 2022 Nov 16. PMID: 36760461; PMCID: PMC9903908.
- Jurjevic Z, Peterson SW, Horn BW. Aspergillus section Versicolores: nine new species and multilocus DNA sequence based phylogeny. IMA Fungus. 2012 Jun;3(1):59-79. doi: 10.5598/imafungus.2012.03.01.07. Epub 2012 Jun 21. PMID: 23155501; PMCID: PMC3399103.
- Jurjević Z, Peterson SW, Solfrizzo M, Peraica M. Sterigmatocystin production by nine newly described Aspergillus species in section Versicolores grown on two different media. Mycotoxin Res. 2013 Aug;29(3):141-5. doi: 10.1007/s12550-013-0160-4. Epub 2013 Feb 17. PMID: 23417508.
- Rhizopoulou S, Diamantoglou ST, Passiakou l. Free proline accumulation in leaves, stems and roots of four mediterranean native phrygana species. Acta Oecologica. 1990;11(4):585-593.
- Maurizio T, Roberto M, Paolo C. Multiple roles of proline in plant stress tolerance and development. Rendiconti Lincei. 2008;19:325-346. doi: 10.1007/s12210-008-0022-8.
- John P, Meletiou-Christou M, Chimona C, Rhizopoulou S. Seasonal functional partitioning of carbohydrates and proline among plant parts of the sand daffodil. Agronomy. 2020;10(4):539. doi: 10.3390/agronomy10040539.
- Chysanthi C, Sophia R. Water economy through matching plant root elongation to Mediterranean landscapes. World Journal of Research and Review. 2017;5:22-24.
- Rosa M, Prado C, Podazza G, Interdonato R, González JA, Hilal M, Prado FE. Soluble sugars-metabolism, sensing and abiotic stress: a complex network in the life of plants. Plant Signal Behav. 2009 May;4(5):388-93. doi: 10.4161/psb.4.5.8294. Epub 2009 May 26. PMID: 19816104; PMCID: PMC2676748.
- Hare PD, Cress WA. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regulation. 1997;21:79-102. doi: 10.1023/A:1005703923347.
- Roberto M, Paolo C.
- Maurizio T, Costantino P, Mattioli R. Proline accumulation in plants: not only stress. Plant Signaling & Behavior. 2009;4(11):1016-1018. doi: 10.4161/psb.4.11.9797.
- Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot. 2012 Feb;63(4):1593-608. doi: 10.1093/jxb/err460. Epub 2012 Jan 30. PMID: 22291134; PMCID: PMC4359903.
- Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010 Feb;15(2):89-97. doi: 10.1016/j.tplants.2009.11.009. Epub 2009 Dec 23. PMID: 20036181.
- Rhizopoulou S, Mitrakos K. Water relations of evergreen sclerophylls. I. Seasonal changes in the water relations of eleven species from the same environment. Annals of Botany. 1990;65(2):171-178. doi: 10.1093/oxfordjournals.aob.a087921.
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002 Feb;25(2):239-250. doi: 10.1046/j.0016-8025.2001.00808.x. PMID: 11841667.
- Ben Rejeb K, Abdelly C, Savouré A. La proline, un acide aminé multifonctionnel impliqué dans l'adaptation des plantes aux contraintes environnementales [Proline, a multifunctional amino-acid involved in plant adaptation to environmental constraints]. Biol Aujourdhui. 2012;206(4):291-9. French. doi: 10.1051/jbio/2012030. Epub 2013 Feb 19. PMID: 23419256.
- Liang X, Zhang L, Natarajan SK, Becker DF. Proline mechanisms of stress survival. Antioxid Redox Signal. 2013 Sep 20;19(9):998-1011. doi: 10.1089/ars.2012.5074. Epub 2013 May 23. PMID: 23581681; PMCID: PMC3763223.
- Miransari M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol (Stuttg). 2010 Jul 1;12(4):563-9. doi: 10.1111/j.1438-8677.2009.00308.x. PMID: 20636898.
- Nehls U, Göhringer F, Wittulsky S, Dietz S. Fungal carbohydrate support in the ectomycorrhizal symbiosis: a review. Plant Biol (Stuttg). 2010 Mar;12(2):292-301. doi: 10.1111/j.1438-8677.2009.00312.x. PMID: 20398236.
- Willetts HJ. The survival of fungal sclerotia under adverse environmental conditions. Biological Reviews. 1971;46(3):387-407. doi: 10.1111/j.1469-185X.1971.tb01050.x.
- Chen C, Dickman MB. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc Natl Acad Sci U S A. 2005 Mar 1;102(9):3459-64. doi: 10.1073/pnas.0407960102. Epub 2005 Feb 7. PMID: 15699356; PMCID: PMC552905.
- Rodaki A, Bohovych IM, Enjalbert B, Young T, Odds FC, Gow NA, Brown AJ. Glucose promotes stress resistance in the fungal pathogen Candida albicans. Mol Biol Cell. 2009 Nov;20(22):4845-55. doi: 10.1091/mbc.e09-01-0002. Epub 2009 Sep 16. PMID: 19759180; PMCID: PMC2777113.
- Alwhibi MS, HashemA, Abd_AllahEF, Alqarawi AA , Soliman DWK , Wirth S, Egamberdieva D. Increased resistance of drought by trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. Journal of integrative agriculture. 2017;16(8):1751-1757. doi: 10.1016/S2095-3119(17)61695-2.
- Gournas C, Evangelidis T, Athanasopoulos A, Mikros E, Sophianopoulou V. The Aspergillus nidulans proline permease as a model for understanding the factors determining substrate binding and specificity of fungal amino acid transporters. J Biol Chem. 2015 Mar 6;290(10):6141-55. doi: 10.1074/jbc.M114.612069. Epub 2015 Jan 8. PMID: 25572393; PMCID: PMC4358254.
- Fomicheva GM, Vasilenko OV, Marfenina OE. [Comparative morphological, ecological, and molecular studies of Aspergillus versicolor (Vuill.) Tiraboschi strains isolated from different ecotopes]. Mikrobiologiia. 2006 Mar-Apr;75(2):228-34. Russian. PMID: 16758871.
- Polo J, Mata P. Evaluation of a Biostimulant (Pepton) Based in Enzymatic Hydrolyzed Animal Protein in Comparison to Seaweed Extracts on Root Development, Vegetative Growth, Flowering, and Yield of Gold Cherry Tomatoes Grown under Low Stress Ambient Field Conditions. Front Plant Sci. 2018 Jan 19;8:2261. doi: 10.3389/fpls.2017.02261. PMID: 29403513; PMCID: PMC5780448.
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973. doi: 10.1007/BF00018060.
- DuBois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Analytical chemistry. 1956;28(3):350-356. doi: 10.1021/ac60111a017.
- Buysse J, Merckx R. An improved colorimetric method to quantify sugar content of plant tissue. Journal of Experimental Botany, 1993;44(10):1627-1629. doi: 10.1093/jxb/44.10.1627.
- Li X, Han S, Wang G, Liu X, Amombo E, Xie Y, Fu J. The Fungus Aspergillus aculeatus Enhances Salt-Stress Tolerance, Metabolite Accumulation, and Improves Forage Quality in Perennial Ryegrass. Front Microbiol. 2017 Sep 4;8:1664. doi: 10.3389/fmicb.2017.01664. PMID: 28936200; PMCID: PMC5595160.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.