Biology Group . 2023 April 21;4(4):747-759. doi: 10.37871/jbres1733.
Biostatistical Analysis on Synergetic Therapy of the Three Plants
Bin Zhao1* and Xia Jiang2
2Hospital, Hubei University of Technology, Wuhan, Hubei, China
- Liver
- Hepatotoxins
- CCl4
- Hepatoprotective activities
- Plants
Abstract
The liver is an important metabolic organ consisting of four different sized lobes in our bodies. The important function of liver includes the elimination of toxic material, detoxication and regulation of the blood, blood clotting, and manufacture of vital hormones. It also plays a crucial role in other activities, such as pathogen protection, synthesis of bile salts, food supply, and blood collecting. There is currently no obvious mechanism that includes direct hepatotoxicity and unfavorable immune response for several study reports on hepatic damage mechanisms. Pharmaceutical bioactivation creates reactive chemical metabolites that interact with lipids, nucleic acid, and proteins in the cell, producing DNA damage, protein malfunction, lipid peroxidation, and oxidative stress. Many illnesses also affect the liver due to its strategic position. Hepatotoxins, due to the overdose of drugs, xenobiotics, alcohol and industrial toxins, can harm the liver. However, Natural and synthetic chemicals are used to treat liver diseases as hepatoprotective agents. This study was conducted to evaluate the hepatoprotective potential of Geranium wallichianum, Elaeagnus parvifolia and Taraxacum officinale against Carbon Tetrachloride (CCl4) induced hepatotoxicity in experimental rats. Extract of all three plants was induced (200 mg/kg) and combination of all three plants were given in (100 mg/kg). Liver enzymes including Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP) and total bilirubin as well as liver histopathology were evaluated to see the effect of herbal treatments. Results of this study suggest that all three plants have hepatoprotective effect and highest effect was noted in synergetic therapy.
Introduction
The liver is one of the important organ that play an important role in digestion, glycogen synthesis, plasma protein synthesis and the direction of homeostasis in the body [1,2]. Likewise, it plays an important role in performing various functions including protection against microorganisms, supplements, salt production, and blood collection. Liver disease is a critical problem for healthcare professionals all around the world and traditional medicine has been used in the treatment of liver disorders and still may not be safe [3].
The liver is less susceptible to damage from drugs and substances due to its function of using substances closely related to intestines [4]. The intestinal tract delivers 75% of whole blood to the liver through venous gates that deliver drugs and xenobiotics to the system. Various studies are in progress to diagnose liver damage, it is important to have specialized tools that can cover hepatotoxicity and adverse effects. Liver disease is usually caused by taking drugs to produce metabolites that interact with cellular macromolecules i.e. nucleic acids, lipids and proteins that stimulate DNA damage, lipid peroxidation, protein breakdown and oxidative stress [5]. Not caused by problems with hepatocytes and bile duct cells, the amount of corrosive bile increases in the liver, which can lead to further liver damage. There is elimination of the active cells which form a perfect environment in the cells that go through and cause liver failure [6]. Damage and stress on the liver triggers the onset of symptoms that activate various cellular functions.
The neonatal immune system consists of Neurons (NK), Kupffer Cells (KC), and Neurons (TKT) that trigger liver function by supporting inflammatory bowel disease and the release of chemokines. It is formed by a combination of inflammatory cytokines (interferon (IFN)-γ, interleukin (IL-1β) and tumor degradation factor (TNF)-α) which occur during injury and influence persistent tissue damage [7,8].
Hepatitis is one of the most devastating illnesses that suffered many families. Complications of hepatitis such as Hepatocellular Carcinoma (HCC) have become the fifth most common cause of death worldwide [9-11]. Hepatitis C Virus (HCV) and Hepatitis B Virus (HBV) are sources and cause chronic and persistent liver disease and hepatocellular carcinoma [12,13]. The best-known goal for the development of HCC is associated with the risk of Hepatitis B as well as the addition of Hepatitis C infection (HBV/HCV) [14,15].
The population (almost 80%) of developing countries is not capable to approach modern therapeutic strategies according to WHO [16] and they continue to rely on traditional herbal drugs.
Plant materials are traded in various countries worldwide to meet the rising demand for herbal therapeutic medicines. Since ancient times, herbal medicines have been used to improve human health and to treat various illnesses [17]. Plants have numerous active phytochemicals that maintain body biological balance without noticeable side effects. The presence of various bioactive compounds results in fewer or uncommon side effects than natural or synthetic medicines [18].
Plant related medicines were used as key medical mediators in developing counties. The majority of medicines used have recently been derived either directly or indirectly from different medicinal plants sources. The conventional treatment approach of herbal medicines has been reported to be commonly used by many people [19]. Various benefits are achieved with herbal derived drug usage such as improved tolerability, rejuvenation function, less side effects, natural in structure, maintaining balance, reduced allergy, smoother effects, effectiveness and harmony in body physiology [20]. Plants are simple treatment options because there are present half million therapeutic plants around the world and some therapeutic properties of herbal plants have no yet discovered and their medicinal/ therapeutic characteristics might have potential for various diseases management.
Medicinal plants exhibit many characteristics when they are used as follows:
- Synergic medicine: All plant constituents work together to supplement or harm other ingredients and to avoid adverse factors.
- Official medication help: The best-proven plant agents are used to treat dangerous diseases such as cancer.
- Preventive medicine: Plant parts are also characterized by the ability to resist such as infections. It helps to reduce the use of drug treatments and pharmaceutical side effects [21].
Most of the Asian countries are among the world's richest countries in the field of medicinal plants where the medicinal plants sector is still a long-standing practice today. Pakistan has been blessed by nature from wonderful resources especially with various therapeutic agents [22]. Medicinal plants have a prominent position in the traditional medicine’s framework for the management of wide range of diseases [23].
The synergistic cooperation that occurs between compounds of natural order or botanicals is very important. Synergies often explain the adequacy of conception, especially when small doses are needed. The bioactivity, or compatibility of the compounds in local combinations will steadily decrease when included from the mixture. This works well with a single plant arrangement like phytomedicine with multiple plants [24]. Hepatotoxicity can be induced in exploratory organisms by treatment with Carbon Tetrachloride (CCl4), an unstable, odorless and unpleasant compound [25].
The hepatotoxic effects of Carbon Tetrachloride (CCl4) are well established. In labs, CCl4 is used to cause acute toxic liver damage in animals [26,27]. Due to the importance of medicinal plants as a source of hepatoprotective drugs and the rising number of cases of liver damage, a study was conducted to test the hepatoprotective effects of ethanolic extracts of some medicinal plants collected from various locations in the Poonch division of Azad Kashmir, Pakistan, against CCl4-induced toxicity in mice.
Materials and Methods
Basic collection
Plant samples (Geranium wallichianum, Elaeagnus parvifolia and Taraxacum officinale) were collected from the Poonch district in Rawalakot, Azad Kashmir. The Department of Botany, University of Poonch Rawalakot, taxonomically recognized and validated the plant samples. Then each sample is ground to a powder.
Plant extract preparation
The plant material is dried in the shade until all water was removed and the plant is well dried for grinding. After cleaning, the contents of the plant were ground into a fine powder fitted with a stirrer and stored in an airtight container with a suitable sealant for future use.
Hot water extraction
Mixed 5 g of finely powdered herbs in a beaker with 200 ml warm water. The mixture was cooked for 20 minutes in a roasting oven at 30-40°C, then the extract was filtered by filter paper before being used for phytochemical tests. The extracts were stored in the refrigerator untile use.
Solvent extraction
The Solvent extraction system is used for crude plants extract. About 20 g of powder was collected individually in a thimble and extracted in 250 ml of 70% alcohol solvent. The extraction was carried out for another 24 hours, or until the solvent in the syphon tube had turned colorless. The resulting mixture is placed in a beaker and boiled in an oven preheated to 30-40°C until all solvents have evaporated. The dried extract was kept in the refrigerator at 4°C.
Animal model oral toxicity test
With minimal variation, the toxicity of all plants, Plant 1, 2, 3 (Geranium wallichianum, Elaeagnus parvifolia, Taraxacum officinale) is investigated according to the "OECD-425 Organization for “Economic Co-operation and Development" criteria [28]. For animals administered orally, limit the test dose to 2000 mg/kg. Twenty BALB/C female mice of the same weight (30 g) were recruited for this investigation and divided into four groups (from G-I to G-IV).
Hydroalcoholic extraction doses (2000 mg/kg) from individually selected plants were administered to the G-II to G-IV groups, whereas the G-I was the control group (P1-P3). The one-on-one mice (G-II to G-IV) that fasted at night had potable water. These three animals (one fasting in each group) gave 2000 mg/kg of specimens per plant one at a time. Animals are seen for 24 hours for death. All treated animals were live (G - II-GIV) and four other animals in each treatment group (groups II to IV) were tested using a plant sample (P1-P3) individually so that 5 animals tested. It might potentially be one of them (for a total of 15 animals in all three groups). For the first 30 minutes following treatment, all animals were attentively observed, and then at regular intervals for the next 24 hours. Hyperactivity, ataxia, tremors, convulsions, rescue, diarrhoea, coma, weariness, and dizziness were all noted as behavioral alterations. For fourteen days all animals were viewed on a daily basis [28].
Hepatoprotective activity on animal model preliminary trial/evaluation of hepatotoxin objective
Many chemicals with various concentrations have been reported by the researcher [29-32] to induce hepatotoxicity. Following chemicals were reevaluated through animal trial to select the most appropriate hepatotoxin.
- Carbon tetrachloride at 30% for two weeks, 3 ml/kg body weight twice a week.
- A single dosage of 100 percent carbon tetrachloride (0.7 ml/kg body weight).
- 70% carbon tetrachloride 3 ml/kg body weight as a single dose.
- 100% carbon tetrachloride 3 ml/kg body weight as a single dose.
Induction of hepatotoxicity by CCl4
70% carbon tetrachloride 3 ml/kg body weight was given to induced hepatotoxicity as a single dose.
Experimental animals
The Government Collage University Faisalabad housed the experimental animals in an animal room. It might potentially be one of them (for a total of 15 animals in all three groups). For the first 30 minutes following treatment, all animals were attentively observed, and then at regular intervals for the next 24 hours. The experiment began ten days after acclimatisation at 26 degrees Celsius (2 degrees Fahrenheit), with 12 hour light/dark cycles, food, and drink. All procedures were carried out in compliance with the Institutional Animal Care and Use Committee (IACUC) recommendations of the Government Collage University Faisalabad.
Calculation of dose
Each solution was dissolved in normal saline separately (0.9%). The total dosage was determined using the OECD-423 directive, which is outlined below.
Normal saline
For 30 g rats, the daily saline requirement was 0.6 ml, 3.6 ml of saline is required for six dosages each minute. The overall dosage was 100.8 ml over the course of 28 days. One 30 g rat (1) required 0.6 ml per day, while six rats required 6 × 0.6 = 3.6 ml per day. Six mice required 3.6 x 28 = 100.8 ml of water throughout the course of 28 days.
Plants extract
For 30 g mice, the recommended dose is 6 mg (200 mg/kg) per day. For up to six doses, the recommended dose is 36 mg and for 28 days the recommended dose for each plant is 1008 mg. 108 mg of each plant was measured and transported separately in 150 ml Falcon tubes. Then, using a syringe, 100.8 ml of saline was injected into each Falcon tube. The extract was dissolved using a sonicator, and the treatment dosage (200 mg/kg) was given to six rats.
Standard drug
Standard hepatoprotective drug (Silymarin) was calculated for 6 animals with the same standard (GIII) containing 100 mg/kg body weight. The recommended dose is for 3 mg/day, as well as for 6, the recommended dose is 18 mg/day and for 28 days is 504 mg. A total of 504 mg of the standard drug was weighted and transferred to a 150 ml Falcon tube. With a syringe, 100.8 ml of saline is introduced to the falcon tube, and the medication is dissolved using a sonicator equipment.
Experimental design for antioxidants and hepatoprotective
Group I (n = 6): normal mice treated with carboxymethyl cellulose (1 ml/kg 1 percent w/v p/o); Group II (n = 6): intoxicated mice (CCl4 1 m/kg, I/P); Group III (n = 6): standard drugs (silymarin 100 mg/kg, P/O); Group IV (n = 6): P1 extract (200 mg/kg, P/O); Group V (n = 6): P2 extract (200 mg/kg, P / O); Group VI(n = 6): P3 extract (200 mg/kg, P/O) and Group VII (n = 6): Combine extract (100 mg/kg, P / O). A 28- day treatment regimen was devised based on the findings of prior trials [17]. Standard dose of silymarin with daily oral administrated for 4 weeks in respective groups [33].
Biochemical and histopathological studies
Food was stop, 12 hours before rat sacrificing. On 29 days, mice were sacrificed for biochemical markers and liver histopathology studies for each group.
Collection of blood: Blood was drawn into a glass tube with a syringe and allowed to chill for 40-45 minutes after heart puncture. Centrifugation (3000 rpm for 15 minutes) was used to separate blood serum and 4 for further analysis.
Biochemical analysis: Biochemical markers in the serum of mice, such as Serum Glutamate Pyruvate Transaminase (SGPT), Glutamic Oxaloacetic Transaminase (SGOT), Alkaline Phosphate (ALP), and serum bilirubin, were evaluated using commercially available kits on a chemistry analyzer.
Tissue samples: Liver fragments were also separated by dissection and washed with water; Buffer formalin (10%) was added for 24 hours until histopathological examination.
Histopathology: Liver dehydration was treated with several dilutions of ethyl alcohol (70%, 80% and 100%) and cleared with xylene. 4-5 μm thick portion of liver was prepared using a microtome by dipping the tissue into paraffin-containing bees wax and placing it in a preheated oven at 56°C for 24 hours. After tissue preparation, sections were mounted on slides and Hematoxylin-Eosin (H&E) staining was done for historical microscopic analysis to detect changes in hepatocytes [34]. The camera was used to takes picture.
Statistical analysis: The data was interpreted as means ± standard deviations for all variables. The mathematical review would require a one-way ANOVA. The meaning of the 0.05 "p- value" was calculated.
Results and Discussion
Liver disease is a major cause of mortality globally, and it has a negative impact on many families. Current treatments are not only costly, but the cost of treatment is also very low, with many side effects. This study focused on finding alternative therapies using Natural remedies for liver disease having minimum or no negative effects. Three medicinal herbs (Geranium wallichianum, Elaeagnus parvifol and Taraxacum officinale) were chosen and their hepatoprotective effects were tested in various groups in this study.
Oral toxicity test
At the time of the current study, a dose of 2000 mg/kg per selected plant (Taraxacum officinale, Geranium wallichianum, Elaeagnus parvifolia) given orally to rats showed no adverse effects on plants. Selected non-toxic and at LD50 more than 2000 mg/kg from any plant. Daily monitoring of crude extract of selected plants at a dose (2000 mg/kg) in a dose for 14 days did not cause any toxicity. All of the plants used in our study were suitable for further investigation of their hepatoprotective effect on hepatotoxicity caused by CCl4. (2000 mg/kg) for plant extraction's hepatoprotective effects in rats are also seen by [28,35,36].
Preliminary evaluation of toxin
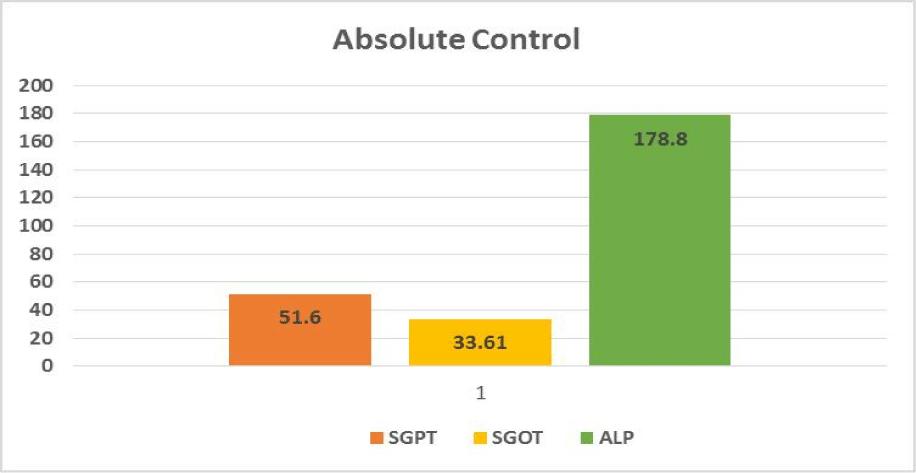
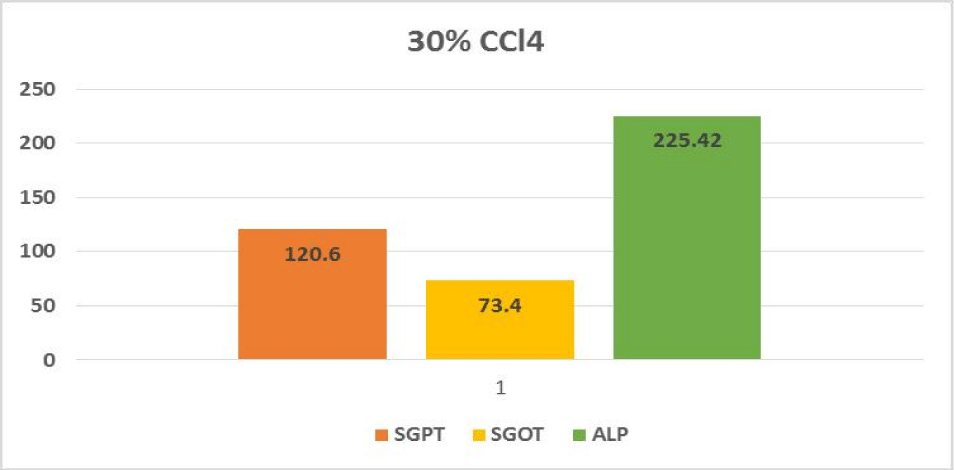
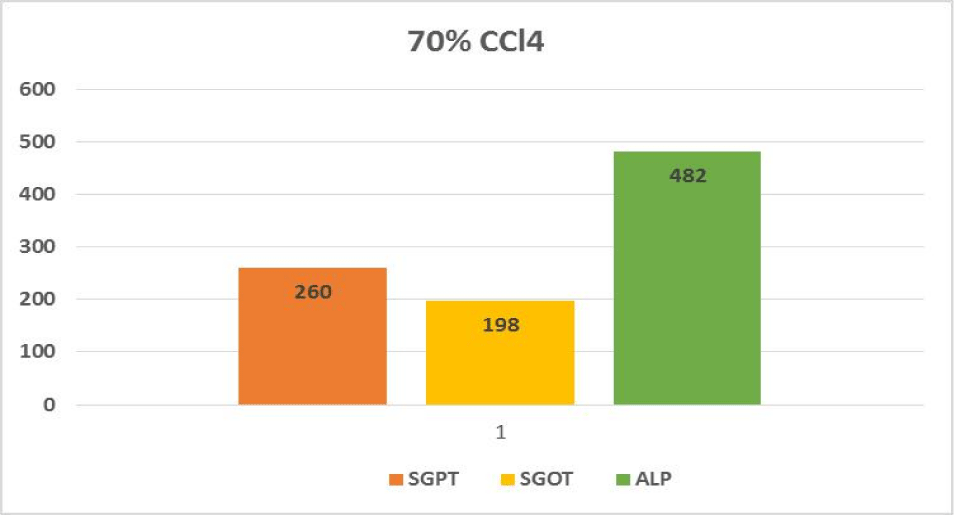
Hepatotoxic fluids were collected in CCl4-exposed rats at various dosages, was utilised to determine the optimal hepatotoxin. Figures 1-3 shows the outcomes of the SGPT, SGOT, and ALP level in different groups respectively. Because of the gradual development of all biochemical biomarkers (SGPT, SGOT, and ALP) suggest 70% CCl4 in this treatment appears to be the most hepatotoxic in this therapy.
Conclusion preliminary evaluation of toxin
Based on the biochemical analysis of different groups during the first experiment, 70% CCl4 (3 ml/kg body weight per dose) was the most effective chemical for inducing hepatotoxicity. Therefore, it was decided that CCl4 as capdoxin would be used for clinical trials prior to this study.
Animal trial
Toxin: Initial experiments showed that CCl4 gradually fails the liver and has nothing to do with it, so it is used to improve hepatotoxicity in pre-treatment tests (animal models). Experiments with animals at the Animal house of the Government College University Faisalabad. To stimulate hepatotoxicity, CCl4 (3 ml/kg body weight per dose) is used.
Herbal medicine: Treatment was started with medicinal plants (Geranium wallichianum, Elaeagnus parvifolia, Taraxacum officinale) after poisoning in experimental animals to test their hepatoprotective potential.
Blood tests: Blood samples were obtained from these mice by jugular vein. A thin needle syringe is used to extract approximately 3 ml of blood from each mice, which is then kept in a Guangzhou Improvement Medical Instruments Co. Ltd. infusion tube. The utilized serum is separated from blood samples, and numerous biochemical processes are evaluated.
Biochemical analysis: Liver cytolysis caused by CCl4 was detected in the effect of plant extraction as well as regulating the levels of serum enzymes SGPT, SGOT, ALP and total bilirubin. All of these biochemical markers have been identified. The measurement of liver enzymes in the blood is a useful quantitative indicator of the severity and type of hepatocyte injury.
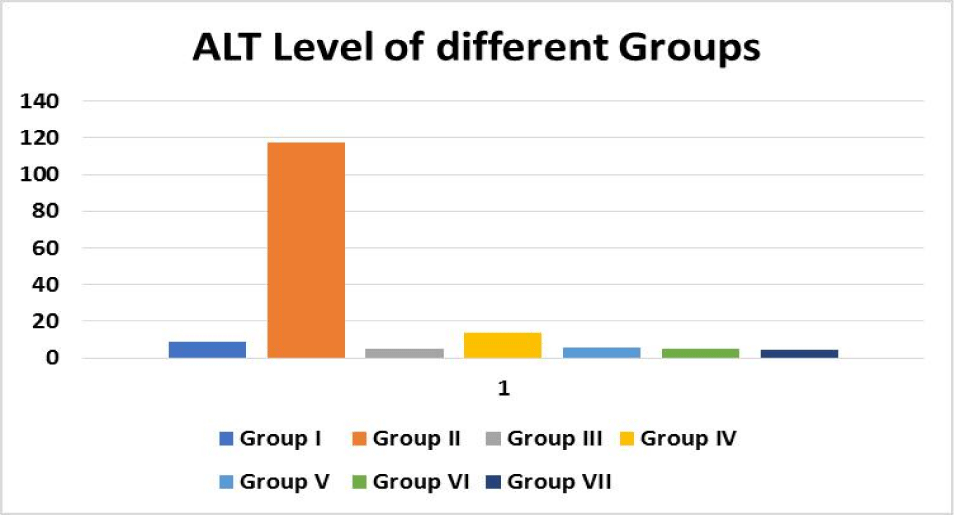
Serum glutamic pyruvic transaminase
SGPT (Serum Glutamic Pyruvic Transaminase) is an enzyme found in the liver cells. When the liver is injured, SGPT is released into the bloodstream. As a result, when the liver is damaged, the SGPT levels in the blood are high (for example, from viral hepatitis). SGPT levels can also be raised by several medicines. Alanine aminotransferase is another name for Alanine Aminotransferase (ALT). Professionals utilise SGPT to diagnose liver illness. ALT catalyses the transfer of an amino group from L-alanine to - ketoglutarate, yielding pyruvate and L-glutamate as products of this reversible transamination process.
Decreased SGPT levels were found in all groups treated with different medicinal plants compared to the same control group. Similarly, a lower proportion of therapeutic agents compared with control agents indicated that the input of the selected drug plant began to resume liver function. All herbal drugs were treated differently than control agents (silymarin treated) but higher SGPT levels in the same control group showed better response in combination therapy (Figure 4).
Serum glutamic oxaloacetic transaminase
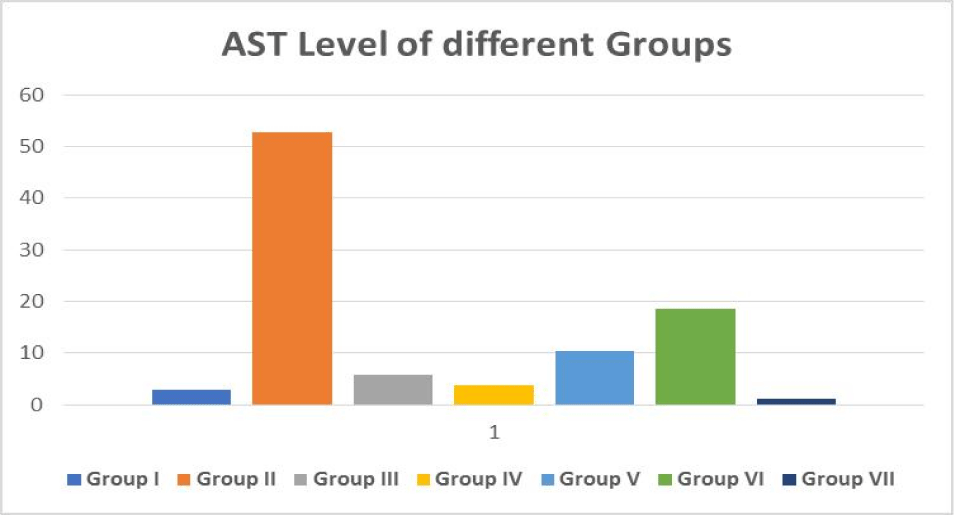
SGOT (Serum Glutamic Oxaloacetic Transaminase) is an enzyme found in the liver cells. When the liver is injured, SGOT is released into the bloodstream. As a result, when the liver is damaged, the blood SGOT levels rise (for example, from viral hepatitis). SGOT levels can also be raised by several medicines. Aspartate Aminotransferase is another name for SGOT (AST) (Figure 5).
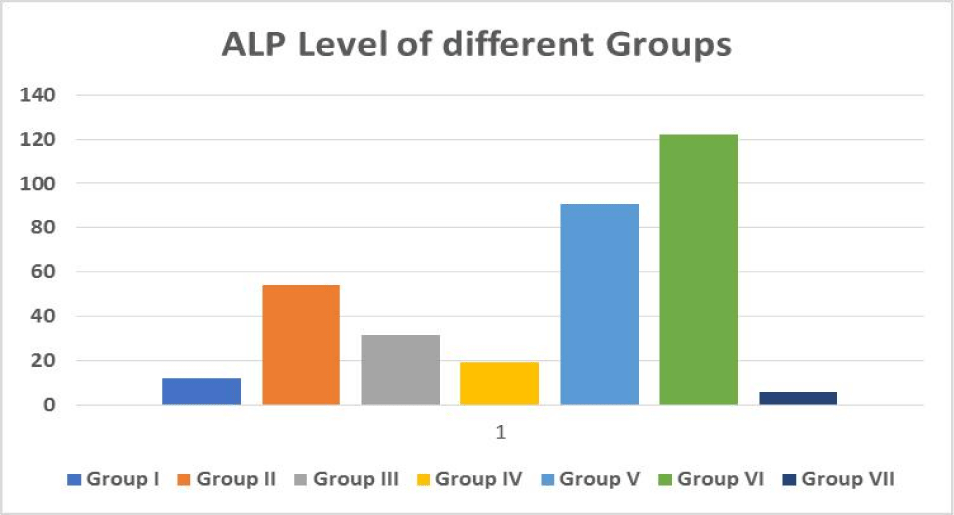
ALP is an enzyme that may be discovered in your blood. It aids in the breakdown of proteins in the body and comes in a variety of forms depending on where it comes from.
ALP is produced mostly in the liver, although it is also produced in the bones, intestines, pancreas, and kidneys. The placenta produces ALP in pregnant women.
Many tissues, including the intestines, bones, liver, and placenta, contain Alkaline Phosphatase (ALP). The use of serum ALP in the identification of two significant problems, for example, is critical. Increased bone function and hepatobiliary illness. ALP levels that are abnormally high in blood usually signal a problem with your liver, gallbladder, or bones (Figure 6).
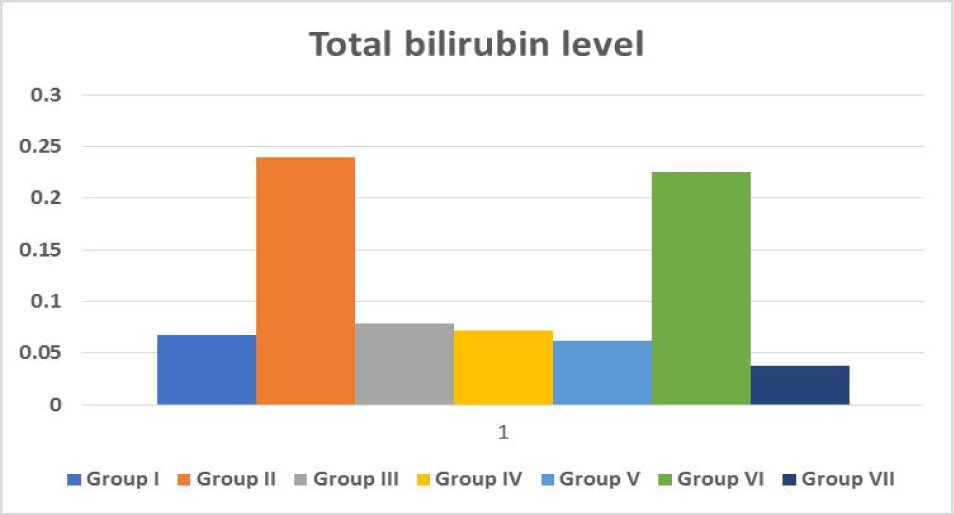
Total bilirubin level
The quantity of bilirubin in your blood is measured by a bilirubin test. It's used to figure out what's causing symptoms including jaundice, anaemia, and liver illness. Bilirubin is an orange-yellow pigment produced by the breakdown of a portion of red blood cells (Figure 7). The bilirubin in blood is taken by your liver, which alters its chemical make-up such that the majority of it is passed through your stool as bile. If bilirubin levels are greater than normal, it means either red blood cells are breaking down at an extraordinary pace or your liver isn't adequately breaking down waste and removing bilirubin from blood (Figure 7).
Silymarin provides protection against this toxin by blocking the site of toxins as well as increasing the number of liver cells. Silibinin from the milk thistle inhibits the liver when applied against hepatotoxicity that causes liver damage [37,38]. Silymarin protects the liver from carbon tetrachloride, paracetamol, valedin, halothane, and thioacetamide toxins. It also defends the liver against ionising radiation, iron insufficiency, and ischemia injury [39,40].
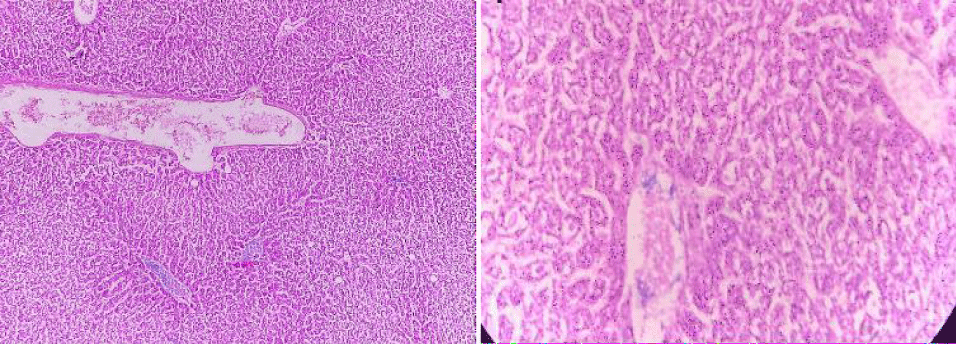
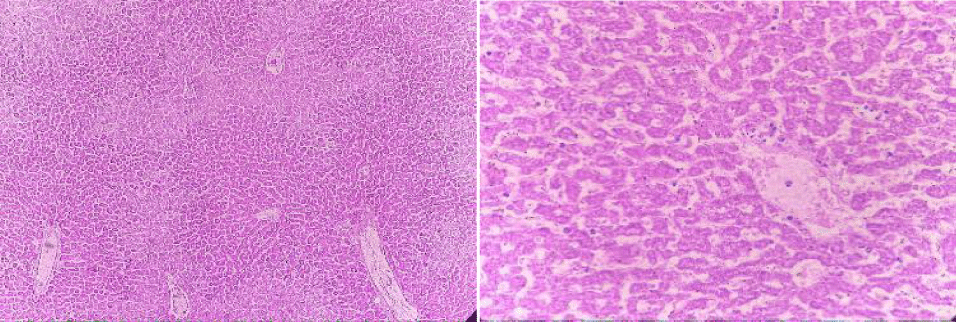
Histopathology
Selected animals are slaughtered at the end of the procedure and macroscopic and histopathological examinations performed on the liver tissue were performed.
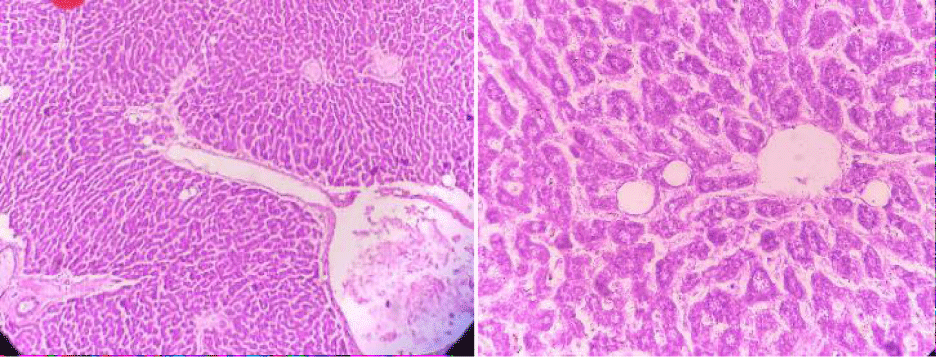
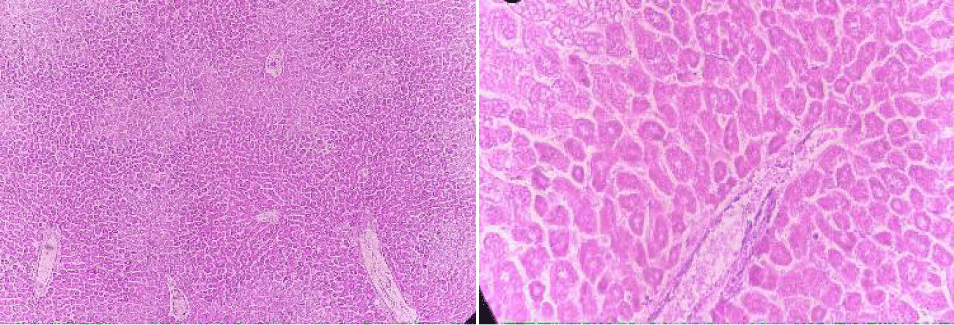
Absolute control
In this group, the tested animals were normal control showing normal histopathology. Hepatic parenchyma is normal. Hepatocyte nuclei are also common in the form of holes containing prominent nucleoli and chromatin material (Figure 8).
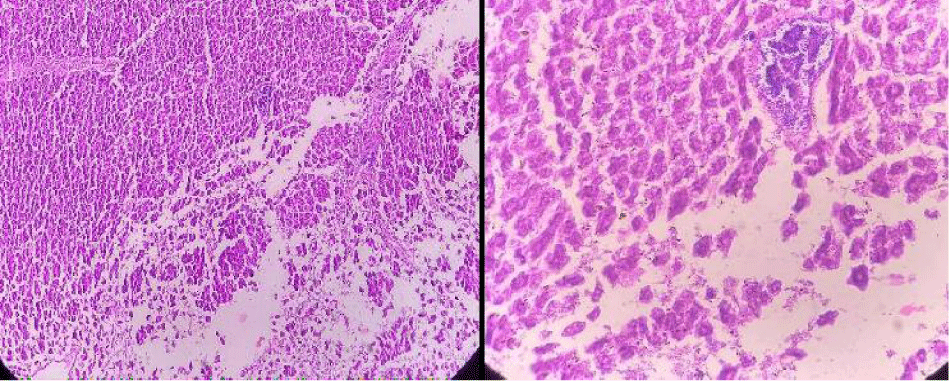
Positive control
In positive control, the animal is treated with toxins to stimulate liver damage. Examination of the history of this group showed a high degree of vacuum damage to the cytoplasm of hepatocytes. In some places, there is an excessive amount of single necrosis cell, as evidenced by hepatocyte nuclei. Perivascular tuberculosis, fibrosis and biliary hypertrophy were also present (Figure 9).
Group 1: Group 1 experimental animals were given liver-damaging toxins before being given standard drug (silymarin, 200 mg/kg/body weight) available in the local market. In liver tissue cells, significant swelling at small sinusoidal openings is shown. Bone vacuoles are found throughout the cytoplasm. A single necrotic cell is also present; No potency effect was shown (Figure 10).
Group 2: The animals in the second group were given poisons that induced liver damage before being given medicinal plant extract (Geranium wallichanum 200 mg/kg/body weight). Low levels of steric degeneration and hepatocyte cytoplasm were observed. Cell necrosis also occurs in some areas. Partial treatment is shown with a mixture of these medicinal plants (Figure 11).
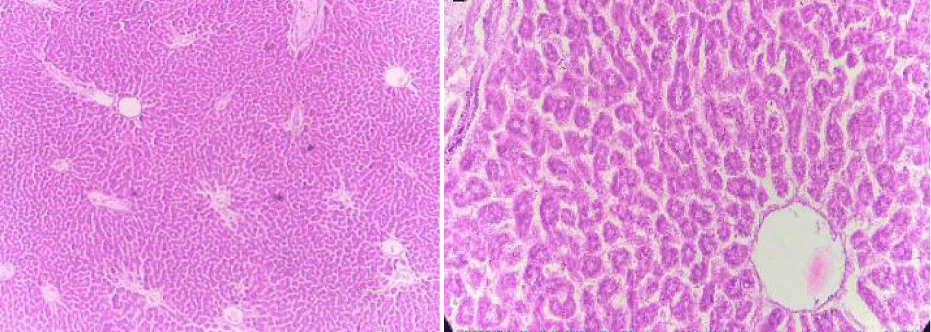
Group 3: Experimental animals included in this group were treated with liver-damaging toxins and then treated with plant extracts (Elaeagnus parvifolia 200 mg/kg/body weight). The liver parenchyma is normal. Hepatocytes form in the soft tissues of the liver and in some places it is a mild disease.
Unicellular necrosis is also present. Pocket space is normal, there is a small degree of hunger pangs in places and it feels like a casino. The anatomical effect is observed by histopathology (Figure 12).
Group 4: In this group, the animal is given toxins that cause liver damage before being given a medicinal plant extract that contains (Taraxacum officinale 200 mg/kg/body weight). Liver parenchyma shows moderate levels of vacuolar degeneration and hepatocyte cytoplasm. A small crowd also came. There is also a small cell entry level in the entry area. It showed a side effect in this group (Figure 13).
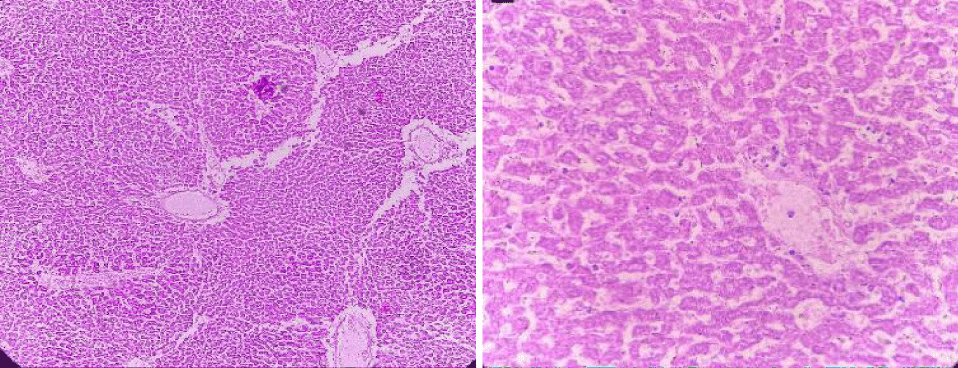
Group 5: In this group, the tested animals were treated with toxins that cause liver damage and treated with medicinal plants combination (Geranium wallichanum, Elaeagnus parvifolia and Taraxacum officinale) (100 mg/kg/weight). Hepatitis in the parenchyma is almost normal. Hepatocyte nuclei are also common in the form of holes containing prominent nucleoli and chromatin material. A full sensory effect is shown (Figure 14).
| Table 1: Effect of medicinal plants Extract and standard drug on biochemical serum parameter in mice with CCl4 induced toxicity. | |||||
| Group | ALT (U/l) Mean ± SE |
AST (U/l) Mean ± SE |
ALP (U/l) Mean ± SE |
TB Mean ± SE |
|
| Group I | Control | 8.5 ± 4.17 | 2.94 ± 1.72 | 12.11 ± 6.21 | 0.067 ± 0.03 |
| Group II | CCl4 (1 mL/kg) | 117.5 ± 54.97 | 52.88 ± 27.76 | 54 ± 29.11 | 0.24 ± 0.13 |
| Group III | Silymarin + CCl4 | 5.16 ± 2.37 | 5.66 ± 2.49 | 31.38 ± 17.63 | 0.078 ± 0.03 |
| Group IV | Plant 1 (200 mg/kg) + CCl4 |
13.66 ± 6.15 | 3.83 ± 1.73 | 18.83 ± 8.11 | 0.072 ± 0.03 |
| Group V | Plant 2 (200 mg/kg) + CCl4 |
5.72 ± 3.20 | 10.44 ± 5.81 | 90.55 ± 45.83 | 0.062 ± 0.03 |
| Group VI | Plant 3 (200 mg/kg) + CCl4 |
5.33 ± 2.65 | 18.66 ± 8.24 | 122.22 ± 55.81 | 0.225 ± 0.12 |
| Group VII | Combination of 3 (100 mg/kg) + CCl4 plants |
4.5 ± 1.89 | 1.166 ± 0.54 | 5.83 ± 3.33 | 0.038 ± 0.02 |
| Results are expressed as mean ± SD (n = 6). p < 0.05; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; TB: Total Bilirubin; ALP: Alkaline Phosphatase; Plant 1 (Geranium wallichanum), plant 2 (Elaeagnus parvifolia) and Plant 3 (Taraxacum officinale). | |||||
The results of liver function tests were linked to histopathological alterations in this research. Other studies testing the hepatoprotective effects of fluid regimens containing Artemisia capillaris, Lonicera japonica and Silybum marianum found similar alterations in liver function [41]. The control liver itself is in good shape and has a flat surface and liver cells without breaking down fat fibrosis. The liver is one of the most effective agents, it is yellow and hard to the touch, while destroying the hepatocytes and surrounding fibrous tissue, promoting the formation of a continuous fibrous septum. Different levels of lipid clearance are distributed throughout the liver tissue, indicating that CCl4 has successfully caused liver damage. With appropriate administration of silymarin, secretion was observed but remained unclear compared to CCl4-negative controls. In the subsequent AEF-treated group, disruption was significantly reduced and normal cell count increased with mild fibrosis and incomplete septal defects. Damaged liver histopathology shows fatty changes in hepatocytes and fibrosis [42]. Histopathology of liver tissue has shown that removal of wormwood Artemisia reduces necrosis of hepatocytes and leads to reduced adhesion of inflammatory cells [43]. In another study to determine the liver capacity of Dodonaea viscosa. Liver tissue histopathology showed normal central veins, hepatic intestine, and paranasal sinuses with normal untreated striations. Yellow necrotic areas after treatment with CCl4 with a single control (CCl4); Safe Control Protection 200 mg/kg silymarin (CCl4 + silymarin); Protection against methanol extraction from Dodonaea viscosa (CCl4 + MeOH). Methanol derivatives have been shown to be more effective than silymarin in terms of protection [44].
Kanbur M, et al. [45] found that Royal Jelly (RJ) protects rats and histologists from paracetamol induced liver impairment. No significant liver injury was found in the control group. Another treatment was found with the inclusion of RJ lymphocytes and liver parenchyma. Treatment with CCl4 showed hyperemia, severe bleeding and a nevus. Changes in degenerative, necrotic and hepatocytes, a slight increase in fibrous tissue and the introduction of lymphocytes into the portal area for the accumulation of aggregated erythrocytes in large hemorrhagic areas and in some sinuses, alterations of damage in those areas were observed. Cardiac parenchyma. This change is always one of our controlled approaches. Similar results have been described in other studies showing remodeling and depletion of hepatocytes with severe necrosis of the central lobe in the paracetamol group. The liver section showed normal cellular architecture with hepatocytes present in the sinusoidal lumen in a control. Although rats treated with alcohol-induced silymarin, Liv52 and acetaminophen showed minimal damage to the hepatocytes, they displayed significant immune function [29].
Discussion
The liver regulates environmental internal chemicals by producing exogenous and endogenous metabolites that have been reported to be harmful to the liver due to oxidative stress [46]. ROS produces liver diseases such as cirrhosis. Liver damage due to various hepatotoxins has serious consequences for the body. Despite significant developments in the treatment of liver disease with combination medication, acute chronic liver disease typically does not improve because it causes adverse effects [47]. Plant extracts have been proven to be effective in the treatment of liver disease in several investigations because they contain powerful antioxidants in liver protective properties [48-50]. Medicinal plants play an important role in pharmaceutical research and primary health care for 80% of the plant –based population in developing countries. Despite breakthroughs in synthetic medications, 25% of prescription and non-prescription drugs in developed countries are derived from plants [51].
Only a few numbers of hepatoprotective plants have been researched for safety and effectiveness [47]. To assist in the search for safe hepatoprotective agents, the current study focuses on the analysis of the hepatoprotective effects of selected medicinal plants collected from various sites at the Poonch Azad Jammu Department in Kashmir, Pakistan.
Carbon Tetrachloride (CCl4) is used as a hepatotoxic agent to induce hepatotoxicity in experimental animals and experiments in the pharmaceutical application of products derived from chemical derivative products [52,53]. Plant extracts improve liver function and reduce serum enzyme disturbances in the circulation. Silymarin (Flavonolignan from Silybum marianum) is an important antioxidant that contains powerful radicals and hepatoprotective agents. It is known for its liver protection and for commonly used restorative procedures such as anti-inflammatory, immunomodulatory, and liver therapy. Treatment with silymarin showed mild inflammation, mild fibrosis, and necrosis at low cost [54]. It is useful for hepatoprotective and bile duct infections, viral hepatitis, acute liver disease, and hepatotoxicity through drugs or poisoning. It plays a significant part in the restoration of damaged livers, is helpful for both acute and chronic hepatitis, and is safe, therapeutic, and patient- friendly, as well as cost-efficient [55]. In the present study, it was used as an indicator of SD, as a positive control with a hepatoprotective effect on the hepatotoxicity of CCl4.
In this study, the hepatoprotective effect of the selected drug plant was consistent with the study of Ahsan R, et al. [56] tested the hepatoprotective effect of several drug plants against CCl4-induced toxicity and results showed its effectiveness. Hepatoprotective effects over time and in dose-dependent forms. Current studies are also supported by various literature where different studies demonstrating the protective effect of plant and plant on CCl4 -induced hepatotoxicity in animal models [26,57].
Conclusion
Plants under investigation are less harmful than manufactured chemicals. Analyzing products derived from plant sources is the most effective way to discover novel medications. The major goal of this study was to investigate the hepatoprotective properties of plant extracts while also examining the various biochemical impacts on the liver. The following conclusions were reached based on the findings: Selected plants had hepatoprotective effects against CCl4 in toxins in experimental animals. Biochemical tests of the liver and liver tissue compared to exposed to mice show that the effect is batter in combination of different plants [58].
The hepatoprotective effects of plant extracts and compounds were determined by reducing the biochemical potential of liver damage induced by CCl4 toxicity. These findings support the therapeutic potential of using herbal teas and blends to increase hepatotoxicity. The liver parenchyma in one treatment at the end of the cycle became normal as the control treatment. Liver histopathology and pretreated animals showed protective effects of medicinal plants and mixtures. In addition to the hepatoprotective properties mentioned above, this plant is used in many herbal medicines to treat various diseases. Plants can also be obtained in this mixture, to separate the active ingredient from its potency. The scientific evidence thus obtained contributes to the preparation of herbal medicines to produce medicines with modern medical concepts for the treatment of liver diseases.
Acknowledgment
This work was supported by the Philosophical and Social Sciences Research Project of the Hubei Education Department (19Y049), and the Staring Research Foundation for the Ph.D. of Hubei University of Technology (BSQD2019054), Hubei Province, China.
Conflict of Interest
We have no conflict of interests to disclose and the manuscript has been read and approved by all named authors.
References
- Maheswari C, Maryammal R, Venkatanarayanan R. Hepatoprotective activity of Orthosiphon stamineus on liver damage caused by paracetamol in rats. Jordan J Biol Sci. 2008 sept;1(3):105-108.
- Opoku AR, Ndlovu IM, Terblanche SE, Hutchings A. In vivo hepatoprotective effects of Rhoicissus tridentata subsp. cuneifolia, a traditional Zulu medicinal plant, against CCl4-induced acute liver injury in rats. South African Journal of Botany. 2007;73(3):372-377.
- Arhoghro EM, Ekpo EK, Anosike OE, Ibeh OG. Effect of aqueous extract of bitter leaf (Vernonia amygdalina Del) on Carbon tetrachloride (CCl4) induced liver damage in albino Wistar rats. Eur J Sci Res. 2009 January;26(1):12-30.
- Soni J, Ansari U, Sharma D, Soni S. Predictive data mining for medical diagnosis: An overview of heart disease prediction. International Journal of Computer Applications. 2011;17:43-48.
- Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007 Aug 1;76(3):391-6. PMID: 17708140.
- Blazka ME, Wilmer JL, Holladay SD, Wilson RE, Luster MI. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 1995 Jul;133(1):43-52. doi: 10.1006/taap.1995.1125. PMID: 7597709.
- Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, Shah AG, Pohl LR. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology. 2002 Feb;35(2):289-98. doi: 10.1053/jhep.2002.30956. PMID: 11826401.
- Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology. 2007 May;132(5):1925-36. doi: 10.1053/j.gastro.2007.02.038. Epub 2007 Feb 21. PMID: 17484885.
- Cornella J, Larrosa I. Decarboxylative carbon-carbon bond-forming transformations of (hetero) aromatic carboxylic acids. Synthesis. 2012;44(5):653-676.
- El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011 Jan;4(1):5-10. doi: 10.1177/1756283X10385964. PMID: 21317990; PMCID: PMC3036965.
- Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010 Aug;7(8):448-58. doi: 10.1038/nrgastro.2010.100. Epub 2010 Jul 13. PMID: 20628345; PMCID: PMC3926946.
- Nazeema TH, Brindha V. Antihepatotoxic and antioxidant defense potential of Mimosa pudica. Int J Drug Discov. 2009 December;1(2):1-4. doi: 10.9735/0975-4423.1.2.1-4.
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005 Sep;5(9):558-67. doi: 10.1016/S1473-3099(05)70216-4. PMID: 16122679.
- McKillop IH, Moran DM, Jin X, Koniaris LG. Molecular pathogenesis of hepatocellular carcinoma. J Surg Res. 2006 Nov;136(1):125-35. doi: 10.1016/j.jss.2006.04.013. Epub 2006 Oct 3. PMID: 17023002.
- Mckillop IH, Moran DM, Jin X, Koniaris LG. Molecular pathogenesis of hepatocellular carcinoma. J Surg Res. 2006 Nov;136(1):125-35. doi: 10.1016/j.jss.2006.04.013. Epub 2006 Oct 3. PMID: 17023002.
- World Health Organization (WHO). The world health report 2003: Shaping the future. World Health Organization. 2003.
- Pandey S, Seth A, Tiwari R, Singh S, Behl HM, Singh S. Development and evaluation of antimicrobial herbal cosmetic preparation. African Journal of Pharmacy and Pharmacology. 29 May 2014;8(20):514-528. doi: 10.5897/AJPP2013.3967.
- Hakemi-Vala M, Makhmor M, Kobarfar F, Kamalinejad M, Heidary M, Khoshnood S. Investigation of antimicrobial effect of Tribulus terrestris L. against some gram positive and negative bacteria and candida spp. Novelty Biomed. 2014 January;2(3):85-90.
- Eze JN, Umeora OU, Obuna JA, Egwuatu VE, Ejikeme BN. Cervical cancer awareness and cervical screening uptake at the Mater Misericordiae Hospital, Afikpo, Southeast Nigeria. Ann Afr Med. 2012 Oct-Dec;11(4):238-43. doi: 10.4103/1596-3519.102856. PMID: 23103924.
- Bhat CR. The Multiple Discrete-Continuous Extreme Value (MDCEV) model: Role of utility function parameters, identification considerations, and model extensions. Transportation Research Part B: Methodological. 2008;42(3):274-303.
- Rasool Hassan BA. Medicinal plants (importance and uses). Pharmaceut Anal Acta. 2012;3(10):2153-2435. doi: 10.4172/2153-2435.1000e139.
- Kensa VM, Yasmin SS. Phytochemical screening and antimicrobial activity on Ricinus communis. Plant Sciences Feed. 2011;1:167-173.
- Jamil SS, Nizami Q, Salam M. Centella asiatica (Linn.) Urban: A review. Natural product Radiance. 2007;6(2):158-170.
- Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine. 2001 Sep;8(5):401-9. doi: 10.1078/0944-7113-00060. PMID: 11695885.
- Tatiya AU, Surana SJ, Sutar MP, Gamit NH. Hepatoprotective effect of poly herbal formulation against various hepatotoxic agents in rats. Pharmacognosy Res. 2012 Jan;4(1):50-6. doi: 10.4103/0974-8490.91040. PMID: 22224062; PMCID: PMC3250040.
- Ashoush IS, El-Batawy OI, Gehan A. Antioxidant activity and hepatoprotective effect of pomegranate pell and whey powder in rats. Annals of Agricultural Science. 2013 June;58 (1):27-32.
- Asirvatham R, Akhil J. Morinda reticulata Gamble Tubers: A potent hepatoprotective agent against CCl4 and paracetamol induced hepatotoxicity in Wistar albino rats. International Journal of Nature and Life Sciences. 2020 June;4(1):26-39.
- Kiran PM, Raju AV, Rao BG. Investigation of hepatoprotective activity of Cyathea gigantea (Wall. ex. Hook.) leaves against paracetamol-induced hepatotoxicity in rats. Asian Pac J Trop Biomed. 2012 May;2(5):352-6. doi: 10.1016/S2221-1691(12)60055-0. PMID: 23569929; PMCID: PMC3609311.
- Hurkadale PJ, Shelar PA, Palled SG, Mandavkar YD, Khedkar AS. Hepatoprotective activity of Amorphophallus paeoniifolius tubers against paracetamol induced liver damage in rats. Asian Pac J Trop Biomed. 2012;2(1):238-242.
- Venkatachalam U, Muthukrishnan S. Hepatoprotective activity of Desmodium gangeticum in paracetamol induced liver damage in rats. Biomedicine & Preventive Nutrition. 2013 July;3(3):273-277. doi: 10.1016/j.bionut.2012.12.003.
- Nurrochmad A, Margono SA, Sardjiman, Hakim AR, Ernawati, Kurniawati E, Fatmawati E. Hepatoprotective and antioxidant activity of pentagamavunon-0 against carbon tetrachloride-induced hepatic injury in rats. Asian Pac J Trop Med. 2013 Jun;6(6):438-42. doi: 10.1016/S1995-7645(13)60070-X. PMID: 23711702.
- Li L, Li W, Kim YH, Lee YW. Chlorella vulgaris extract ameliorates carbon tetrachloride-induced acute hepatic injury in mice. Exp Toxicol Pathol. 2013 Jan;65(1-2):73-80. doi: 10.1016/j.etp.2011.06.003. Epub 2011 Jul 8. PMID: 21741806.
- Borkham-Kamphorst E, van de Leur E, Zimmermann HW, Karlmark KR, Tihaa L, Haas U, Tacke F, Berger T, Mak TW, Weiskirchen R. Protective effects of lipocalin-2 (LCN2) in acute liver injury suggest a novel function in liver homeostasis. Biochim Biophys Acta. 2013 May;1832(5):660-73. doi: 10.1016/j.bbadis.2013.01.014. Epub 2013 Jan 31. PMID: 23376114.
- Saleem M, Ahmed B, Karim M, Ahmed S, Ahmad M, Qadir MI. Hepatoprotective effect of aqueous methanolic extract of Rumex dentatus in paracetamol- induced hepatotoxicity in mice. Bangladesh Journal of Pharmacology. 2014;9(3):284-289.
- Chander TR, Reddy YN, Bollikunta V, Warangal. Evaluation of hepatoprotective and antihepatotoxic activity of ethanolic extract of Evolvulus alsinoides Linn on CCl4 induced rats. Asian Journal of Pharmacology and Toxicology. 2014 August;02(04):01-06.
- Reddy VR, Kumar V, Reddy MK. Hepatoprotective activity of Elytraria acaulis plant extracts in albino rats. International Journal of Pharmacognosy and Phytochemical Research. 2014 January;6(4):925-929.
- Desplaces A, Choppin J, Vogel G, Trost W. The effects of silymarin on experimental phalloidine poisoning. Arzneimittelforschung. 1975 Jan;25(1):89-96. PMID: 125090.
- Vogel G, Tuchweber B, Trost W, Mengs U. Protection by silibinin against Amanita phalloides intoxication in beagles. Toxicol Appl Pharmacol. 1984 May;73(3):355-62. doi: 10.1016/0041-008x(84)90087-5. PMID: 6719456.
- De Vizcaya-Ruiz A, Barbier O, Ruiz-Ramos R, Cebrian ME. Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mutat Res. 2009 Mar 31;674(1-2):85-92. doi: 10.1016/j.mrgentox.2008.09.020. Epub 2008 Oct 15. PMID: 18984063.
- Akkaya H, Yilmaz O. Antioxidant capacity and radical scavenging activity of Silybum marianum and Ceratonia siliqua. Ekoloji. 2012;21(82):9-16.
- Yang CC, Fang JY, Hong TL, Wang TC, Zhou YE, Lin TC. Potential antioxidant properties and hepatoprotective effects of an aqueous extract formula derived from three Chinese medicinal herbs against CCl4-induced liver injury in rats. Int Immunopharmacol. 2013 Jan;15(1):106-13. doi: 10.1016/j.intimp.2012.10.017. Epub 2012 Nov 8. PMID: 23142091.
- Yang CC, Fang JY, Hong TL, Wang TC, Zhou YE, Lin TC. Potential antioxidant properties and hepatoprotective effects of an aqueous extract formula derived from three Chinese medicinal herbs against CCl4-induced liver injury in rats. Int Immunopharmacol. 2013 Jan;15(1):106-13. doi: 10.1016/j.intimp.2012.10.017. Epub 2012 Nov 8. PMID: 23142091.
- Fiamegos YC, Kastritis PL, Exarchou V, Han H, Bonvin AM, Vervoort J, Lewis K, Hamblin MR, Tegos GP. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS One. 2011 Apr 4;6(4):e18127. doi: 10.1371/journal.pone.0018127. PMID: 21483731; PMCID: PMC3070693.
- Ali H, Kabir N, Muhammad A, Shah MR, Musharraf SG, Iqbal N, Nadeem S. Hautriwaic acid as one of the hepatoprotective constituent of Dodonaea viscosa. Phytomedicine. 2014 Jan 15;21(2):131-40. doi: 10.1016/j.phymed.2013.08.019. Epub 2013 Sep 24. PMID: 24075215.
- Kanbur M, Eraslan G, Beyaz L, Silici S, Liman BC, Altinordulu S, Atasever A. The effects of royal jelly on liver damage induced by paracetamol in mice. Exp Toxicol Pathol. 2009 Mar;61(2):123-32. doi: 10.1016/j.etp.2008.06.003. Epub 2008 Aug 6. PMID: 18693095.
- Wang X, Xu X, Tao W, Li Y, Wang Y, Yang L. A systems biology approach to uncovering pharmacological synergy in herbal medicines with applications to cardiovascular disease. Evid Based Complement Alternat Med. 2012;2012:519031. doi: 10.1155/2012/519031. Epub 2012 Nov 29. PMID: 23243453; PMCID: PMC3518963.
- Rao ChV, Rawat AK, Singh AP, Singh A, Verma N. Hepatoprotective potential of ethanolic extract of Ziziphus oenoplia (L.) Mill roots against antitubercular drugs induced hepatotoxicity in experimental models. Asian Pac J Trop Med. 2012 Apr;5(4):283-8. doi: 10.1016/S1995-7645(12)60040-6. PMID: 22449519.
- Tang X, Gao J, Wang Y, Fan YM, Xu LZ, Zhao XN, Xu Q, Qian ZM. Effective protection of Terminalia catappa L. leaves from damage induced by carbon tetrachloride in liver mitochondria. J Nutr Biochem. 2006 Mar;17(3):177-82. doi: 10.1016/j.jnutbio.2005.06.008. Epub 2005 Aug 10. PMID: 16169207.
- Yue SM, Kang WY. Lowering blood lipid and hepatoprotective activity of amentoflavone from Selaginella tamariscina in vivo. J Med Plant Res. 2011 July 18;5(14):3007-3014.
- Gong F, Yin ZH, Xu QT and Kang WY. Hepatoprotective effect of Mitragyna rotundifolia Kuntze on CCl4-induced acute liver injury in mice. Afr J Pharm Pharmacol. 2012;6(5):330-335. doi: 10.5897/AJPP11.766.
- Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000 Jun;17(3):215-34. doi: 10.1039/a902202c. PMID: 10888010.
- Aghel N, Rashidi I, Mombeini A. Hepatoprotective Activity of Capparis spinosa root bark against CCl4 induced hepatic damage in mice. Iran J Pharm Res. 2007 Sempt;6(4):285-290.
- Ritesh KR, Suganya A, Dileepkumar HV, Rajashekar Y, Shivanandappa T. A single acute hepatotoxic dose of CCl4 causes oxidative stress in the rat brain. Toxicol Rep. 2015 Jun 11;2:891-895. doi: 10.1016/j.toxrep.2015.05.012. PMID: 28962426; PMCID: PMC5598138.
- Kim EY, Kim EK, Lee HS, Sohn Y, Soh Y, Jung HS, Sohn NW. Protective effects of Cuscutae semen against dimethylnitrosamine-induced acute liver injury in Sprague-Dawley rats. Biol Pharm Bull. 2007 Aug;30(8):1427-31. doi: 10.1248/bpb.30.1427. PMID: 17666798.
- Ghosh A, Ghosh T, Jain S. Silymarin: A review on the pharmacodynamics and bioavailability enhancement approaches. Journal of Pharmaceutical Science and Technology. 2010;2(10):348-355.
- Ahsan R, Islam KM, Balbul IJ, Musaddik MA, Haque E. Hepatoprotective activity of methanol extract of some medicinal plants against carbon tetrachloride-induced hepatotoxicity in rats. European Journal of Scientific Research. 2009;37(2):302-310.
- Krishna KI, Mruthunjaya K, Patel JA. Antioxidant and hepatoprotective potential of stem methanolic extracts of Juticia gendarussa Burn Int J Pharm. 2010;6(2):72-80. doi: 10.3923/ijp.2010.72.80.
- Hurkadale PJ, Shelar PA, Palled SG, Mandavkar YD, Khedkar AS. Hepatoprotective activity of Amorphophallus paeoniifolius tubers against paracetamol induced liver damage in rats. Asian Pac J Trop Biomed. 2012;1:238-242.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.