Medicine Group . 2023 June 07;4(6):962-971. doi: 10.37871/jbres1757.
Nutritional Comparison of Fish Species from the Bizerte Lagoon (Mediterranean Coasts)
Feriel Ghribi1*, Safa Bejaoui1,2, Rym Ennouri1,2, Dalya Belhassen1, Imene Chetoui1, Chaima Fouzai1, Wafa Trabelsi1, Nejla Soudani1 and Sami Mili2,3
2University of Carthage, Higher Institute of Fisheries and Aquaculture of Bizerte (ISPAB), Errimel, B.P.15. 7080, Bizerte, Tunisia
3Laboratory of Fisheries Sciences, National Institute of Marine Sciences and Technologies (INSTM), 28 Rue du 2 mars 1934, Salammbô 2025, Tunis, Tunisia
- Bizerte lagoon
- Fish
- Proximate composition
- Fatty acids
- Nutritional quality
Abstract
The present study aimed to compare the nutritional quality of three fish species (Sparus aurata, Sarpa salpa, and Mullus barbatus) collected from the Bizerte lagoon (South Mediterranean coasts). Proximate composition, fatty acids and nutritional quality indices were determined. Results showed that the nutritional quality of the three fish follows this trend: S. aurata > S. salpa > M. barbatus. S. aurata had significantly higher protein content (16.02 ± 2.01 mg/g ww), glycogen level (1.08 ± 0.16 mg/g ww), and lipid content (8.47 ± 0.96 mg/g ww) which are rich in omega-3 fatty acids (47.34%). The Total Fatty Acid (TFA) profile of S. aurata was dominated by Polyunsaturated Fatty Acids (PUFA) (70.34 ± 0.02%), followed by monounsaturated (MUFA) (20.72 ± 0.04%) and Saturated Fatty Acids (SFA) (9.03 ± 0.01%). The use of different Nutritional Quality Indices (NQI) (n-3/n-6 ratio, PUFA/SFA, EPA+DHA, Atherogenicity (AI) and Thrombogenicity (TI) indices) confirmed that S. aurata is a good source of beneficial Fatty Acids (FA) and an excellent source of nutrients. Overall, the consumption of S. aurata over S. salpa and M. barbatus is highly recommended and can be considered as a healthy food for human consumption.
Introduction
Coastal lagoons are very rich environments and are ranked among the most productive ecosystems in the world [1]. These ecosystems are reservoirs of biodiversity and ecological productivity. They play a role in maintaining marine fish stocks by providing essential nursery and refuge areas for many migratory species [2]. This functional role of nurseries is essential for the ichthyologic biodiversity of coastal lagoons and also of the related coastal areas. Mediterranean lagoons reveal a great variability of hydrodynamic and sedimentological conditions, a rapid evolution of their geomorphological framework as well as a great vulnerability [3]. These lagoons are ecosystems that are particularly sensitive to eutrophication under the direct influence of the quantity and quality of the effluents that flow into them. The development of these bodies of water of high socio-economic value requires in-depth hydrobiological studies to understand their functioning and to estimate their trophic situation [4,5].
Among these lagoons, the Bizerte Lagoon is one of the most important lagoons in Tunisia. It is characterized by its socio-economic importance at the regional and national scale [6]. It is greatly exploited, mainly in the field of fishing and it constitutes a very important fishing and shellfish production area [4-7]. Bizerte Lagoon is a nursery for several marine species and a privileged feeding ecosystem for other migratory species [8]. The fishing exploitation of the lagoon of Bizerte started for decades, and its production has always been important and varied, because of its geographical position. Shellfish farming activities began around the 1950s with oyster farming (Crassostrea angulata), then with the oyster Ostrea edulis in 1958 followed by Crassostrea gigas in 1972. At the same time, mussel farming (Mytilus galloprovincialis) began in 1963 with clam grow-out activities (Ruditapes decussatus). Unfortunately, in recent decades, a clear decline in fishery resources has been observed. This lagoon receives water loaded with urban waste from neighboring villages as well as industrial waste. The industrial activity in this lagoon is highly developed and diversified (oil, steel, cement...) since it is chronically exposed to xenobiotics such as OCs, PAHs, PCBs and trace metals [9,10].
Fish are considered of good quality products thanks to their richness in lipids, fatty acids and other components such as proteins and vitamins. Several studies have demonstrated that fish lipids have beneficial effects on human health, as they play an essential role in the development and function of certain organs, as well as in the biochemical and physiological responses of the organism [11,12]. They are also important for the defense and prevention of certain diseases [13,14]. The biochemical study of fish tissues is of considerable interest to assess their nutritional value in terms of energy units. Chemical components such as lipids, protein and water content are essential for evaluating the nutritional components, organoleptic quality and physiological state of fish [15]. Thus, the current work aimed to determine and compare the nutritional quality of three commercial fish species: sea bream (Sparus aurata), salema porgy (Sarpa salpa) and mullet (Mullus barbatus) that are available in the Tunisian market. The moisture, Ash, proteins, glycogen, lipids, FAs and Nutritional Quality Indices (NQI) were evaluated.
Material and Methods
Sampling and preparation
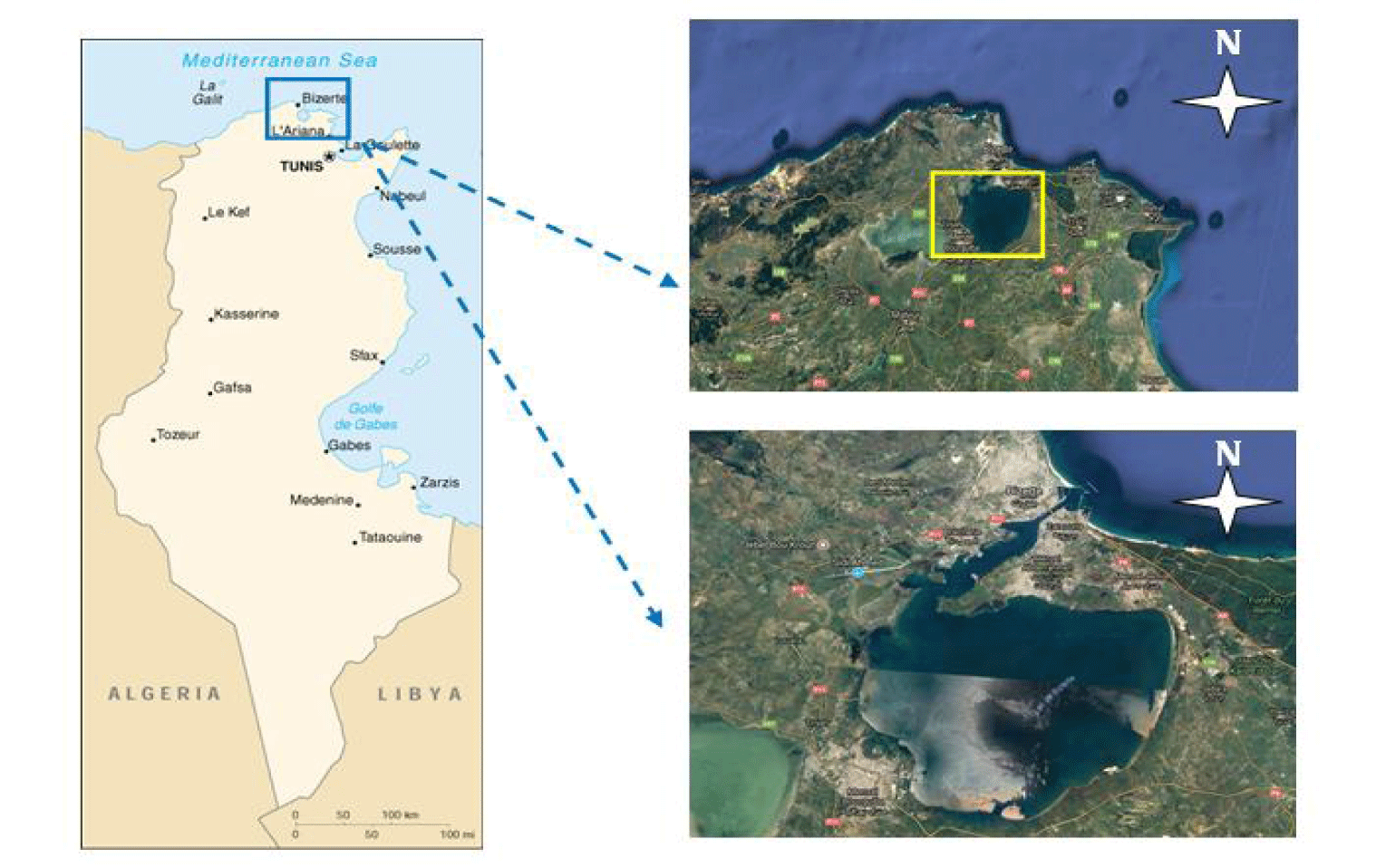
In the Bizerte lagoon, three species of economically valuable fish, namely Sparus aurata (total weight = 98.81 ± 8.39 g; total length = 18.39 ± 0.76 cm), Sarpa salpa (total weight = 76.05 ± 2.22 g; total = 15.19 ± 1.15 cm) and Mullus barbatus (total weight = 81.22 ± 1.09 g; total length = 17.29 ± 1.75 cm) were selected for the current study. These fish species are widely consumed in Tunisia and their prices are convenient for consumers compared to other fish species. In November 2021, for each species, a total of 30 mature individuals were purchased from local fishermen. The samples were immediately brought to the laboratory of the Higher Institute of Fisheries and Aquaculture of Bizerte (ISPAB) inside a cooler container. After cleaning with distilled water to remove sediment debris, the fish specimens were dissected and gutted. The flesh of each batch was removed and stored at -20°C for subsequent biochemical analyses.
Proximate composition
Moisture: Moisture content (%) was determined following the method of AOAC [16] by weight difference after heating the sample for 24 h at 105 ± 2°C.
Ash: Ash content (%) was determined based on the method of AOAC [17] by dry-ashing the samples at 550 °C for 24 h.
Protein: Protein content (mg/g ww) was determined based on the bovine serum albumin used as standard according to the method of Lowry OH, et al. [18].
Glycogen: Glycogen level (mg/g ww) was estimated according to the colometric method described by Dubois M, et al. [19].
Lipid analysis
Extraction: Lipid content was determined according to the method of Folch J, et al. [20] using a chloroform-methanol mixture (2:1, v/v) containing 0.01% Butylhydroxytoluene (BHT). Then, a volume of 2 mL of Sodium chloride (NaCl; 15%) was added to facilitates the separation of the aqueous phase from the organic one. The mixture undergoes centrifugation for 10 min at 15,000 rpm then the organic phase containing the lipids was collected in 5 mL vials and stored at -30°C until the methylation step.
Methylation: 1 ml of hexane is added to 20 µL of lipids to solubilize the fatty acids in the organic phase according to the method of Cecchi G, et al. [21]. 500 µL of nonadecanoic acid (C 19) was used as the internal standard and 500 µL of sodium methylate were added. Then, 200 µL of sulfuric acid (H2SO4) and 1.5 mL of sodium chloride (15% solution) are added to separate the different phases. After centrifugation at 4000 rpm for 10 min, the supernatant which contains esterified fatty acids is stored at -30°C in vials.
Gas chromatographic characterization of FAMEs fraction
The Fatty Acid Methyl Esters (FAMEs) were qualitatively and quantitatively analysed under GC-FID conditions on Agilent Technologies GC (CA, USA), equipped with a split/splitless injector equipped with a flame ionization detector and a 30 m HP Innowax capillary column with an internal diameter of 250 um and a 0.25 um film thickness. Fatty acids were identified through comparison of their retention times with those of a mixture of methyl esters (Supelco PUFA-3, Bellefonte, PA, USA) then integrated and analyzed using HP Chemstation software. The single fatty acids are expressed as a percentage (%).
Nutritional quality indices
In the current study, ratios and the sum of particular Fatty Acids (FA) were calculated to determine the Nutritional Quality Indices (NQI) of fish species: n-3/n-6 PUFA ratio, PUFA/SFA, DHA+EPA sum, Atherogenicity (AI) and Thrombogenicity (TI) indices were determined according to Marques A, et al. [22], Unusan N [23] and Ulbricht TL, et al. [24].
Statistical analysis
Data are presented as means ± Standard Deviation (SD) and were performed using the software R (package version 4.1.1). Each value was a mean of 10 replications for moisture, Ash, proteins, glycogen, lipids and fatty acids. The homogeneity and normality of variables were checked then the one-way of variance (ANOVA) was used to determine the significant differences between variables. The obtained results were considered significant at p < 0.05. The Principal Components Analysis (PCA) was used to explore relationships between the whole dataset variables based on all biochemical compounds within different fish species.
Results
Moisture (%)
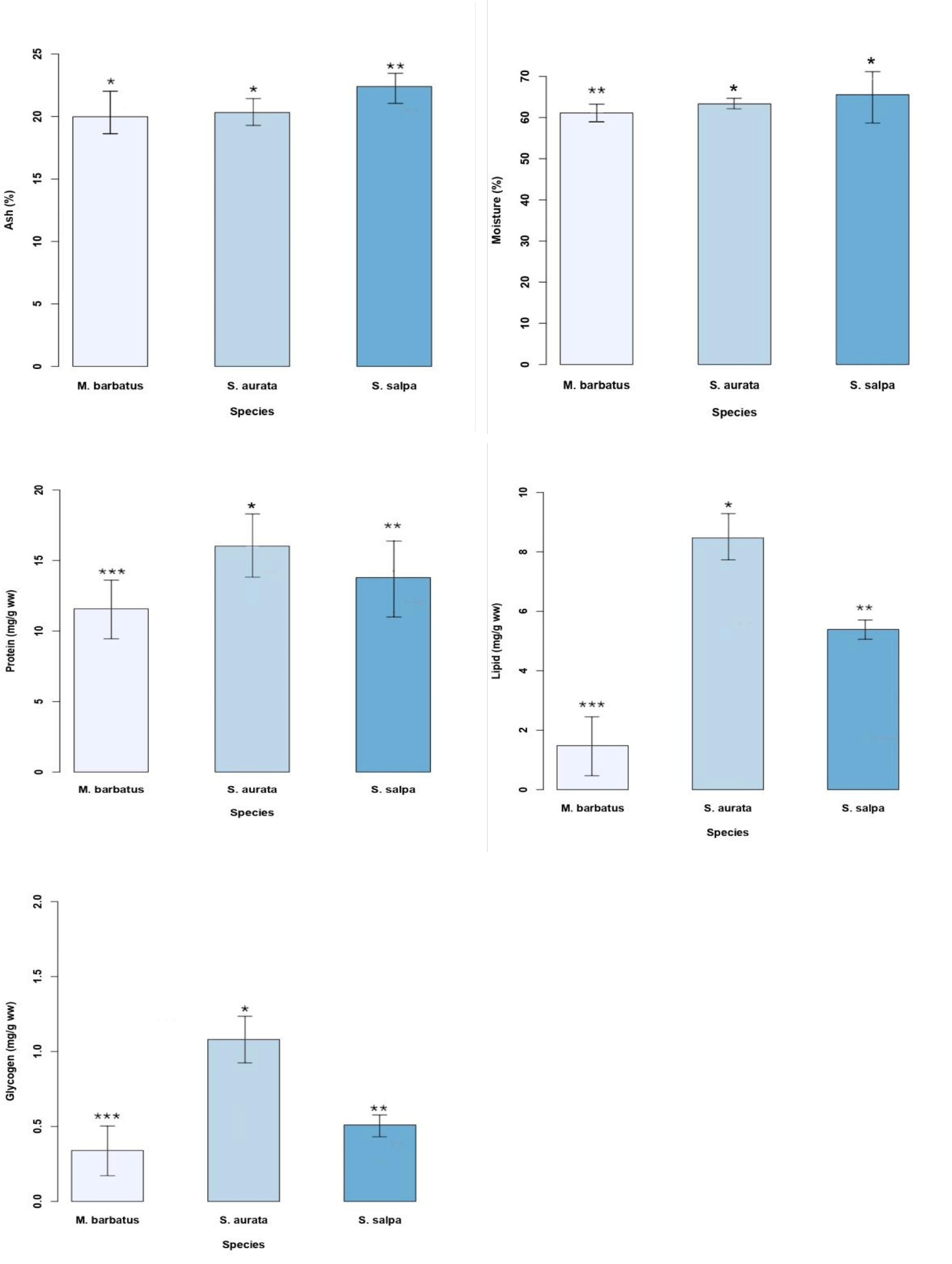
A significant variation in moisture between the studied fish was observed (Figure 1). S. salpa has the highest levels (65.55 ± 1.23%) compared to S. aurata (63.33 ± 2.17%, p < 0.01) and M. barbatus (61.11 ± 6.02%, p < 0.001).
Ash (%)
Our results showed a clear and significant variation in ash content between the three fish species. S. salpa specimens have the highest ash content (22.40 ± 1.04%) compared to S. aurata (20.32 ± 1.70%, p < 0.01) and M. barbatus specimens (19.98 ± 1.16%, p < 0.05) (Figure 2).
Biochemical composition
The fish S. aurata showed the highest glycogen content (1.08 ± 0.16 mg/g) compared to S. salpa (0.51 ± 0.15 mg/g, p < 0.05) and M. barbatus (0.34 ± 0.07 mg/g, p < 0.001) (Figure 2). Similarly, the highest protein content with an average of 16.02 ± 2.01 mg/g was observed in S. aurata when compared to S. salpa (13.79 ± 2.16 mg/g, p < 0.05) and M. barbatus (11.57 ± 2.16 mg/g, p < 0.01). The same trend was observed for lipids, S. aurata showed the highest level (8.47 ± 0.96 mg/g, p < 0.001) followed by S. salpa (5.93 ± 0.75 mg/g, p < 0.001) and M. barbatus (1.48 ± 0.31 mg/g, p < 0.001) (Figure 2).
Fatty acid profile
The FA profile of the three fish is shown in table 1. In M. barbatus, the Total Fatty Acid (TFA) profile was dominated by Polyunsaturated Fatty Acids (PUFA) (54.82 ± 0.39%) followed by saturated (SFA) (33.21 ± 0.04%) and monounsaturated (MUFA) (11.47 ± 0.04%) fatty acids, while in S. aurata and S. salpa, SFA represented the lowest proportion of TFA (9.03 ± 0.01% and 14.97 ± 0.04%, respectively). However, the fish S. aurata showed high PUFA levels (70.34 ± 0.02%) compared to S. salpa (58.33 ± 0.33%). In S. aurata, S. salpa and M. barbatus, the main PUFA were Docosahexaenoic acid (DHA, C22:6n-3) and Eicosapentaenoic acid (EPA, C20:5n-3). However, their levels were significantly elevated in S. aurata (20.45 ± 0.001% and 13.50 ± 0.002%, respectively) compared to S. salpa (5.93 ± 0.003 % and 7.78 ± 0.01%, respectively) and M. barbatus (8.47 ± 0.02% and 4.89 ± 0.01%, respectively). In general, n-3 PUFAs were the highest in the flesh of S. aurata (47.34 ± 0.002%) and lowest in M. barbatus (15.14 ± 0.03%), while the opposite was observed for the level of n-6 PUFA with high levels in M. barbatus (40.06 ± 0.003%) and low levels in S. aurata (22.88 ± 0.004%).
| Table 1: Fatty acids composition (%) of S. aurata, S. salpa and M. barbatus flesh. | |||
| FA | S. aurata | S. salpa | M. barbatus |
| C16:0 | 0.691 ± 0.004 | 9.491 ± 0.007 | 21.381 ± 0.012 |
| C16:1 | 2.090 ± 0.001 | 0.712 ± 0.047 | 4.642 ± 0.009 |
| C18:0 | 1.253 ± 0.002 | 1.495 ± 0.042 | 0.693 ± 0.011 |
| C18:1 | 9.421 ± 0.001 | 22.394 ± 0.086 | 6.743 ± 0.001 |
| C18:2n-6 | 7.439 ± 0.003 | 28.716 ± 0.124 | 20.321 ± 0.002 |
| C18:3n-6 | 9.565 ± 0.003 | 4.945 ± 0. 021 | 18.304 ± 0.008 |
| C18:3n-3 | 13.373 ± 0.002 | 8.360 ± 0.001 | 1.775 ± 0.002 |
| C20:0 | 7.085 ± 0.001 | 3.971 ± 0.027 | 11.183 ± 0.001 |
| C20:1 | 9.203 ± 0.004 | 3.591 ± 0.029 | 0.121 ± 0.004 |
| C20:4n-6 | 5.882 ± 0.002 | 2.594 ± 0.035 | 1.447 ± 0.008 |
| C20:5n-3 | 13.501 ± 0.002 | 7.782 ± 0.003 | 4.897 ± 0.001 |
| C22:6n-3 | 20.460 ± 0.001 | 5.934 ± 0.015 | 8.473 ± 0.002 |
| SFA | 9.033 ± 0.003a | 14.973 ± 0.061b | 33.261 ± 0.024c |
| MUFA | 20.728 ± 0.005ba | 26.698 ± 0.010c | 11.525 ± 0.006a |
| PUFA | 70.234 ± 0.002b | 58.339 ± 0.051a | 55.214 ± 0.031a |
| PUFA n-3 | 47.341 ± 0.002c | 22.081 ± 0.018b | 15.147 ± 0.034a |
| PUFA n-6 | 22.880 ± 0.004a | 36.252 ± 0.069b | 40.068 ± 0.003b |
| n-3/n-6 PUFA | 2.062 ± 0.001b | 0.601 ± 0.001a | 0.379 ± 0.006a |
| PUFA/SFA | 7.771 ± 0.017c | 3.892 ± 0.013b | 1.653 ± 0.009a |
| DHA+EPA | 33.973 ± 0.018b | 13.714 ± 0.012a | 13.082 ± 0.087a |
| AI | 0.022 ± 0.0002a | 0.125 ± 0.0004b | 0.334 ± 0.001ca |
| TI | 0.010 ± 0.0001a | 0.117 ± 0.0002b | 0.311 ± 0.003c |
| a,bandc letters are significantly different at p < 0.05. | |||
Nutritional Quality Indices (NQI)
The NQI studied during this work are presented in table 1. The highest n-3/n-6 ratio was observed in S. aurata (2.06 ± 0.001) and the lowest in M. barbatus (0.37 ± 0.006). The PUFA/SFA ratio varied between the fish species with a minimum value observed in M. barbatus (1.65 ± 0.009) and a maximum value in S. aurata (7.76 ± 0.01). The EPA+DHA sum was maximal (33.97 ± 0.01) in S. aurata followed by S. salpa (13.71 ± 0.01) and M. barbatus (13.08 ± 0.28). The lowest values of AI and TI were observed in S. aurata (0.02 ± 0.0002 and 0.01 ± 0.0003, respectively) (Table 1).
Principal Components Analysis (PCA)
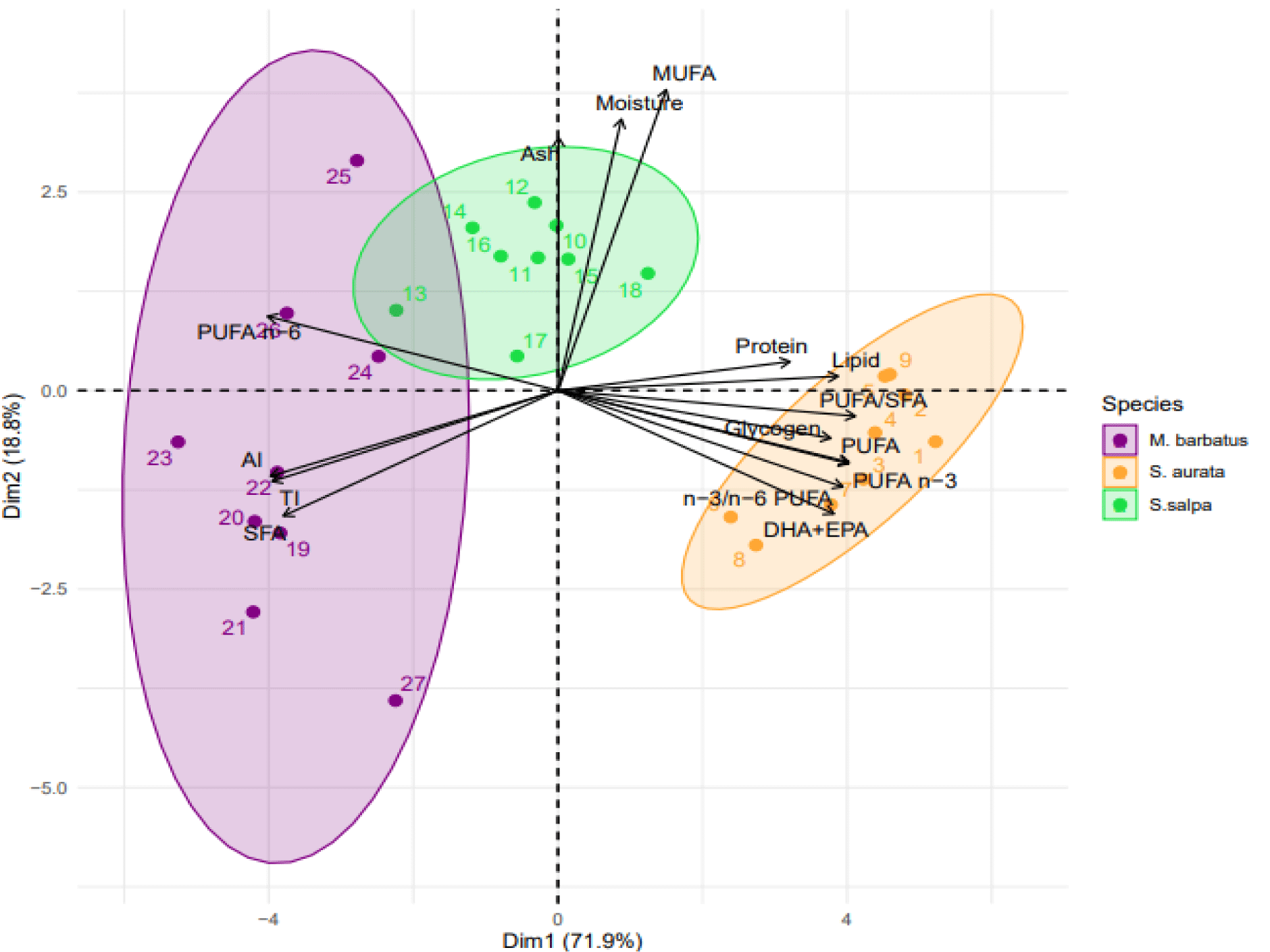
The data on protein, glycogen, lipids, FA and NQI were used in the principal components analysis (Figure 3). The first two factorial axes explained 90.7% of the total variance as sum of PC1 (71.9%) and PC2 (18.8%). A high positive contribution of protein, glycogen, lipid, PUFA, PUFA n-3, n-3/n-6 PUFA, PUFA/SFA, DHA+EPA and a negative contribution of SFA, PUFA n-6, AI and TI were noted for the first component (PC1). The projection of individuals on the factorial plane (1:2) showed a clear separation between fish species. The S. aurata group showed the highest nutritional characteristics with high levels of protein, glycogen, lipid, PUFA, PUFA n-3 and NQI. S. salpa and M. barbatus specimens were separated from the S. aurata group, especially M. barbatus showed low nutritional value which was characterized by high levels of SFA, PUFA n-6, AI and TI (Figure 3).
Discussion
Fish are important nutritious foods that are essential in the human diet. They are considered healthy seafood and have gained increased interest from nutritionists and pharmacologists due to their health benefits [25,26]. For the good functioning of the human body, a healthy diet which involves the consumption of fish is highly recommended [27]. Generally, fish have a high nutritional value as they are rich in proteins, and fatty acids, in particular, ecosapenteinoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), vitamins (A, C, B1, B2 and B3), amino acids and essential elements such as calcium, magnesium and zinc [28]. Mainly, lipids and proteins have many imperative roles in human health [29].
In the current study, the water content in the studied fish was above 60 % which corroborates with previous findings [30]. Based on previous works, moisture content can reflect the relative energy in the fish which is mainly provided by known several reserves like lipids and proteins [31]. It has been reported that lower percentages of moisture are closely related to higher levels of proteins and lipids [32]. In the current study, the ash content which represents the total inorganic content of fish was important and varied in the studied species. It has to be noted that ash was considered a reliable tool for the detection of mineral compounds in fish samples [33]. The obtained results showed that all three fish have a high protein content followed by lipids and then glycogen. These high protein levels can be explained by the fact that fish have a high protein requirement because they use a large proportion of dietary protein to meet their energy needs (e.g. growth, reproduction etc.) [34]. High levels of protein were reported previously in S. aurata, S. salpa and M. barbatus [30,35,36]. However, the observed low glycogen levels can be probably related to the conditions of the autumn season in the Bizerte lagoon since these reserves could be the alternative primary energy resource in unfavorable conditions [37]. The lipids content also varied between the three species as the highest level was observed in S. aurata and the lowest in M. barbatus. In general, lipid contents are essential for providing metabolic energy for fish that are mainly implicated in several physiological functions (e.g. reproduction, cell membrane formation…) [38]. One of the common classifications of fish based on their fat content was established by Stansby ME, et al. [39]. This latter considered fish with less than 5% of fat as low oil fish, between 5% and 15% as medium oil fish, while fish with more than 15% have been considered as high oil fish. Overall, the comparison of the three species revealed that S. aurata has the highest nutritional value followed by S. salpa and M. barbatus. This difference may be related to the availability of food in the Bizerte lagoon, to environmental conditions and also to the reproductive cycle of each species. It has been reported previously that the biochemical compounds of marine organisms can be closely related to endogenous (reproduction, etc.) and/or exogenous (temperature, food availability, etc.) factors [40,41]. Our results are in accordance with the findings of Basto-Silva C, et al. [42] confirming the richness of S. aurata flesh in nutritional compounds.
The FA profile of the three fish species from the Bizerte lagoon showed a predominance of Polyunsaturated Fatty Acids (PUFA) during the autumn season. The main fatty acids identified in S. aurata, S. salpa and M. barbatus are palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1), EPA (C20:5n 3) and DHA (C22:6n 3). Our results are in agreement with those reported for S. aurata in the Adriatic [43], for S. salpa on the Tunisian coasts [30] and for M. barbatus in the Adriatic Sea [44]. Among PUFA mainly EPA and DHA were the most abundant FA. These n-3 PUFAs have been widely associated with a good healthy diet since they are essential FA for the human body and may be effective in the prevention of inflammatory and cardiovascular diseases [45]. Our results showed that S. aurata, S. salpa and M. barbatus are rich in PUFA. S. aurata showed the highest omega-3 levels in particular of EPA and DHA, followed by S. salpa and M. barbatus. These results are in accordance with those found by Mnari A, et al. [46] from the Tunisian coasts revealing that S. aurata is very rich in PUFA. Meanwhile, PUFA n-6 dominated the PUFA fraction of S. salpa and M. barbatus. The consumption of n-3 PUFA has been widely recommended over n-6 PUFA to reduce the development of several diseases [47]. The SFA levels varied between species with high levels reported in M. barbatus. It has been reported that the consumption of SFA is closely related to heart attacks and increased blood LDL [48]. Kocatepe D, et al. [49] showed that M. barbatus collected from the Turkish coasts had higher SFA contents than PUFA. Based on the SFA levels, S. aurata seems to be healthier than S. salpa and M. barbatus. Overall, the observed FA composition variation in the studied fish species may be related to the diet of each species.
The nutritional value of fish meat depends on its lipid quality indices, which indicate the relative proportions of saturated and unsaturated fatty acids and their potential impact on the development of coronary heart disease [24]. Our study found that S. aurata had a high n-3/n-6 PUFA ratio, while M. barbatus had the lowest. Sargent [50] suggested that the optimal n-3/n-6 PUFA ratio should be 1:5 (0.2). The n-3/n-6 ratio has been used as a reliable index for interspecies comparisons of nutritional values in previous studies [51]. Thus, all the studied fish showed n-3/n-6 ratios above the recommended values. The sum of EPA+DHA was higher in S. aurata, followed by S. sarpa and M. barbatus. The American Heart Association [52] recommends consuming two servings of fish per week, particularly oily fish, to meet the recommended EPA and DHA dietary requirements. This would provide approximately 500 mg of EPA and DHA combined daily, which is the recommended intake for optimal overall health and reduced cardiovascular risk by most organizations [53]. The World Health Organization recommended a PUFA/SFA ratio above 0.40 to minimize the risk of cardiovascular diseases [53]. The recommended value was observed in all the fish species since the PUFA/SFA ratio ranged from 1.65 in M. barbatus to 7.77 in S. aurata. The Atherogenic (AI) and Thrombogenic (TI) indices have been used to evaluate the impact of different fatty acids on human health and their effects on the incidence of pathogenic phenomena, such as atheroma and thrombus formation, as reported by Rossano R, et al. [54]. The AI and TI indices were found to be lowest in S. aurata and highest in M. barbatus. However, all studied fish presented AI and TI levels below 1 which is beneficial for human health [55] and proves that the consumption of these species carries the lowest risk for potential coronary diseases. Overall, our findings highlight the superior nutritional value of S. aurata compared to S. salpa and M. barbatus.
Conclusion
This study highlights the significant differences in the biochemical composition among the studied species. The gilthead seabream (S. aurata) had the highest nutritional value when compared to the salema porgy (S. salpa) and the red mullet (M. barbatus). S. aurata was found to be a rich source of omega-3 fatty acids, particularly in DHA and EPA. Overall, consuming EPA and DHA through fish can be beneficial for maintaining overall health and reducing the risk of chronic diseases. Likewise, all the studied fish species met the recommended n-3/n-6, PUFA/SFA, AI and TI values. In general, consumers are encouraged to eat a diverse range of fish species. To sum up, the nutritional characteristics of gilthead seabream, as determined by established NQI, make it a highly valuable and recommended food source for maintaining human health.
References
- Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007 Nov 1;450(7166):98-101. doi: 10.1038/nature06252. PMID: 17972885.
- Barletta M, Jaureguizar AJ, Baigun C, Fontoura NF, Agostinho AA, Almeida-Val VM, Val AL, Torres RA, Jimenes-Segura LF, Giarrizzo T, Fabré NN, Batista VS, Lasso C, Taphorn DC, Costa MF, Chaves PT, Vieira JP, Corrêa MF. Fish and aquatic habitat conservation in South America: a continental overview with emphasis on neotropical systems. J Fish Biol. 2010 Jun;76(9):2118-76. doi: 10.1111/j.1095-8649.2010.02684.x. PMID: 20557657.
- Hammami J, Brahim M, Gueddari M. Essai d’évaluation de la qualité des eaux de ruissellement du bassin versant de la lagune de Bizerte. Bulletin de l'Institut national des sciences et technologies de la Mer de Salammbô. 2005;32:69-77.
- Bejaoui B, Ferjani D, Zaaboub N, Chapelle A, Moussa M. Caractérisation hydrobiologique saisonnière de la lagune de Bizerte (Tunisie). Revue des sciences de l'eau. 2010;216-219.
- Wilcock DN. River and inland water environments. In: Environmental Management in Practice. Volume 3. Routledge; 2013. p.38-58.
- Beji O. Les ressources vivantes exploitables du lac de Bizerte: Etat actuel et potentialités. Bulletin de l'Institut National des Sciences et Technologies de la Mer (INSTM). 2000;27:45-60.
- FAO. The State of World Fisheries and Aquaculture 2016. Contributing to food security and nutrition for all. Rome: Food and Agriculture Organization of the United Nations. 2017.
- Hamdaoui B, Ennouri R, Fatnassi M, Zarrouk H, Romdhane N, Chalghaf M, Mili S. Review of the Situation of Tunisian Lagoon of Bizerta Using Marine Spatial Planning as a Key to Sustainable Blue Growth. 2022.
- Ben Mna H, Alsubih M, Helali MA, Oueslati W, Added A, Aleya L. Early diagenetic behavior of trace metals along with estimation of their diffusive fluxes: Ecological risk assessment in pore water and sediment of Bizerte Lagoon, Tunisia. Mar Pollut Bull. 2022 Dec;185(Pt A):114139. doi: 10.1016/j.marpolbul.2022.114139. Epub 2022 Nov 3. PMID: 36335688.
- Barhoumi B, Maalej S, Budzinski H. Polycyclic Aromatic Hydrocarbons (PAHs) in water, sediment and mussels of Bizerte Lagoon, Tunisia. Medit Mar Sci. 2014;15(3):563-571.
- Arab-Tehrany E, Jacquot M, Gaiani C, Imran M, Desobry S, Linder M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trend Food Sci Tech. 2012;25:24-33.
- Alabdulkarim B, Bakeet ZAN, Arzoo S. Role of some functional lipids in preventing diseases and promoting health. J King Saud Uni-Sci. 2012;24(4):319-329.
- Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002 Aug;61(3):345-58. doi: 10.1079/pns2002166. PMID: 12296294.
- Turner R, McLean CH, Silvers KM. Are the health benefits of fish oils limited by products of oxidation? Nutr Res Rev. 2006 Jun;19(1):53-62. doi: 10.1079/NRR2006117. PMID: 19079875.
- Bjerkeng B, Refstie S, Fjalestad KT, Storebakken T, Rødbotten M, Roem AJ. Quality parameters of the flesh of Atlantic salmon (Salmo salar) as affected by dietary fat content and full-fat soybean meal as a partial substitute for fish meal in the diet. Aquaculture. 1997;157(3-4):297-309.
- Association of Official Analytical Chemists (AOAC). Official methods of analysis. 18th ed. AOAC International. 2005
- Association of Official Analytical Chemists (AOAC). Official methods of analysis. 15th ed. AOAC International. 1990.
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265-75. PMID: 14907713.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350-356. doi: 10.1021/ac60111a017.
- FOLCH J, LEES M, SLOANE STANLEY GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497-509. PMID: 13428781.
- Cecchi G, Basini S, Castano C. Methanolyse rapide des huiles en solvants. Note de laboratoire [Rapid methanolysis of oils in solvant medium. Note of laboratory]. Rev Franc Corps Gras. 1985;32:163-164.
- Marques A, Teixeira B, Barrento S, Anacleto P, Carvalho ML, Nunes ML. Chemical composition of atlantic spider crab Maja brachydactyla: Human Health Implication. J Food Comp Anal. 2010;23:230-237.
- Unusan N. Change in proximate amino acid and fatty acid contents in muscle tissue of rainbow trout (Oncorhychus mykiss) after cooking. Int J Food Sci Technol. 2007;42:1087-1093.
- Ulbricht TL, Southgate DA. Coronary heart disease: seven dietary factors. Lancet. 1991 Oct 19;338(8773):985-92. doi: 10.1016/0140-6736(91)91846-m. PMID: 1681350.
- Kwasek K, Thorne-Lyman AL, Phillips M. Can human nutrition be improved through better fish feeding practices? a review paper. Crit Rev Food Sci Nutr. 2020;60(22):3822-3835. doi: 10.1080/10408398.2019.1708698. Epub 2020 Jan 25. PMID: 31983214.
- Lunn J, Theobald HE. The health effects of dietary unsaturated fatty acids. Nutr Bull. 2006;31(3):178-224.
- Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006 Oct 18;296(15):1885-99. doi: 10.1001/jama.296.15.1885. Erratum in: JAMA. 2007 Feb 14;297(6):590. PMID: 17047219.
- Balami S, Sharma A, Karn R. Significance of nutritional value of fish for human health. Malaysian J Hal Res. 2019;2(2):32-34. doi: 10.2478/mjhr-2019-0012.
- Helkar PB, Sahoo AK, Patil NJ. Review: Food industry by-products used as a functional food ingredients. Inter J waste res. 2016;6(3):1-6. doi: 10.4172/2252-5211.1000248.
- Kacem M, Sellami M, Kammoun W, Frikha F, Miled N, Ben Rebah F. Seasonal variations in proximate and fatty acid composition of viscera of Sardinella aurita, Sarpa salpa, and Sepia officinalis from Tunisia. J Aqua Food Prod Tech. 2011;20(2):233-246. doi: 10.1080/10498850.2011.560365.
- Jonsson N, Jonsson B. Body composition and energy allocation in life‐history stages of brown trout. J Fish Biol. 1998 53(6):1306-1316. doi: 10.1111/j.1095-8649.1998.tb00250.x.
- Fountoulaki E, Pyrzanowski K, Grigorakis K, Noble C. Effect of early feed restriction on long-term growth, condition and fillet composition of gilthead sea bream (Sparus aurata). Aquaculture. 2009;296(3-4):243-251. doi: 10.1016/j.aquaculture.2009.08.002
- Page JW, Andrews JW. Interactions of dietary levels of protein and energy on channel catfish (Ictalurus punctatus). J Nutr. 1973 Sep;103(9):1339-46. doi: 10.1093/jn/103.9.1339. PMID: 4725721.
- Kaushik SJ, Medale F. Kaushik SJ, Médale F. Energy requirements, utilization and dietary supply to salmonids. Aquaculture. 1994;124(1-4):81-97. doi: 10.1016/0044-8486(94)90364-6.
- Di Lena G, Nevigato T, Rampacci M, Casini I, Caproni R, Orban E. Proximate composition and lipid profile of red mullet (Mullus barbatus) from two sites of the Tyrrhenian and Adriatic seas (Italy): A seasonal differentiation. J Food Comp Anal. 2016;45:121-129. doi: 10.1016/j.jfca.2015.10.003.
- Tulgar A, Berik N. Effect of seasonal changes on proximate composition of red mullet (Mullus barbatus) and hake (Merluccius merluccius) were catched from Saroz Bay. Res J Biol. 2012;2(2):45-50.
- Bejoui S, Boussefa D, Telahigue K, Chetoui I, Ghribi F, Rabeh I, El Cafsi M. Geographic variation in fatty acid composition and food source of the commercial clam (Venerupis decussata, Linnaeus, 1758), from the Tunisian coast: trophic links. Grasas y Aceites. 2019;70(1):e289. doi: 10.3989/gya.0580181.
- Mejri SC, Tremblay R, Audet C, Wills PS, Riche M. Essential fatty acid requirements in tropical and cold-water marine fish larvae and juveniles. Front Mar Sci. 2021;557.
- Stansby ME, Olcott HS. Composition of fish. In: Stansby ME, editors. Industrial Fishery Technology. New York: Reinhold Publishing Co; p.339-349. Stockemer J. 1982. Determination of amino acids and biogenic amines in the light and dark muscle meat of tuna by means of the amino acid analyzer and HPLC (Summary). Z Lebensm-Untersuch Forsch. 1963;174:108-113.
- Dridi S, Romdhane MS, El Cafsi M. Seasonal variation in weight and biochemical composition of the Pacific oyster, Crassostrea gigas in relation to the gametogenic cycle and environmental conditions of the Bizert lagoon, Tunisia. Aquaculture. 2007;263(1-4):238-248. doi: 10.1016/j.aquaculture.2006.10.028.
- Ghribi, F, Boussoufa D, Aouini F, Bejaoui S, Chetoui I, Rabeh I. El Cafsi M. Seasonal variation of biochemical composition of Noah's ark shells (Arca noae L. 1758) in a Tunisian coastal lagoon in relation to its reproductive cycle and environmental conditions. Aqua Liv Res. 2018;31:14. doi: 10.1051/alr/2018002.
- Basto-Silva C, Guerreiro I, Oliva-Teles A, Neto B. Life cycle assessment of diets for gilthead seabream (Sparus aurata) with different protein/carbohydrate ratios and fishmeal or plant feedstuffs as main protein sources. Inter J Life Cycle Assess. 2019;24:2023-2034. doi: 10.1007/s11367-019-01625-7.
- Grigorakis K, Alexis MN. Effects of fasting on the meat quality and fat deposition of commercial‐size farmed gilthead sea bream (Sparus aurata, L.) fed different dietary regimes. Aquaculture Nut. 2005;11(5):341-344. doi: 10.1111/j.1365-2095.2005.00351.x.
- Roncarati A, Brambill, G, Meluzzi A, Iamiceli AL, Fanelli R, Moret I, Ubaldi A, Miniero R, Sirri F, Melotti P, Di Domenico A. Fatty acid profile and proximate composition of fillets from Engraulis encrasicholus, Mullus barbatus, Merluccius merluccius and Sarda sarda caught in Tyrrhenian, Adriatic and Ionian seas. J App Ichth. 2012;28(4):545-552. doi: 10.1111/j.1439-0426.2012.01948.x.
- Zhang X, Ning X, He X, Sun X, Yu X, Cheng Y, Yu RQ, Wu Y. Fatty acid composition analyses of commercially important fish species from the Pearl River Estuary, China. PLoS One. 2020 Jan 30;15(1):e0228276. doi: 10.1371/journal.pone.0228276. PMID: 31999793; PMCID: PMC6992182.
- Mnari A, Bouhlel I, Chraief I, Hammami M, Romdhane MS, El Cafsi M, Chaouch A. Fatty acids in muscles and liver of Tunisian wild and farmed gilthead sea bream, Sparus aurata. Food Chem. 2007;100(4):1393-1397. doi: 10.1016/j.foodchem.2005.11.030.
- Mozaffarian D, Wu JHY. Flavonoids, Dairy Foods, and Cardiovascular and Metabolic Health: A Review of Emerging Biologic Pathways. Circ Res. 2018 Jan 19;122(2):369-384. doi: 10.1161/CIRCRESAHA.117.309008. PMID: 29348256; PMCID: PMC5781235.
- Kris-Etherton PM, Petersen K, Van Horn L. Convincing evidence supports reducing saturated fat to decrease cardiovascular disease risk. BMJ Nutr Prev Health. 2018 Nov 17;1(1):23-26. doi: 10.1136/bmjnph-2018-000009. PMID: 33245724; PMCID: PMC7678478.
- Kocatepe D, Turan H. Proximate and fatty acid composition of some commercially important fish species from the Sinop region of the Black Sea. Lipids. 2012 Jun;47(6):635-41. doi: 10.1007/s11745-012-3658-1. Epub 2012 Feb 11. PMID: 22322400.
- Sargent JR. Fish oils and human diet. Br J Nutr. 1997 Jul;78 Suppl 1:S5-13. doi: 10.1079/bjn19970131. PMID: 9292771.
- Pigott GM, Tucker B. Seafood: Effects of technology on nutrition. Volume 39. CRC Press; 1990.
- American Heart Association. 2015.
- WHO. Joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases. WHO Technical Report Series 916. Geneva, Switzerland: World Health Organization. 2003.
- Rossano R, Caggiano MA, Mastrangelo L, Di Lauro R, Ungaro N, Ettorre M, Riccio P. Proteins, fatty acids and nutritional value in the muscle of the fish species Mora moro (Risso, 1810). Mol Nutr Food Res. 2005 Oct;49(10):926-31. doi: 10.1002/mnfr.200500096. PMID: 16189794.
- Pladin J, Lešić T, Krešić G, Barić R, Bogdanović T, Oraić D, Vulić A, Legac A, ZrnčićS. Nutritional quality of different fish species farmed in the Adriatic Sea. Ital J Food Sci. 2017;29(4):537-550.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.