Biology Group . 2023 June 10;4(6):993-998. doi: 10.37871/jbres1759.
Is Anisakis simplex a Trigger for Anaphylactic Reactions in Hereditary Alpha-Tryptasemia?
María Teresa Audicana1*, Paula Ollo1, Natividad Longo1, Marta Velasco1, Ariadna Besga2 and Eduardo Fernández1
2Psychiatry Department, Hospital Universitario de Álava, OSI Araba, Vitoria-Gasteiz Basque Country, Spain
Abstract
The prevalence of fish, seafood and Anisakis allergy varies greatly depending on country-specific eating habits, assuming that the highest consumption corresponds to the highest prevalence. Mediterranean countries are particularly affected.
Hereditary Alpha-Tryptasemia (HaT) is a common autosomal dominant genetic trait recently identified. Although it is estimated to affect approximately 1 in 20 individuals of white European origin, a diagnostic method is not routinely available.
Here by we present the cases of two families with different allergic reaction phenotypes to fish and seafood, IgE and no IgE mediated reactions. The current review focused on the impact of the diagnosis of HaT in families, particularly when the mutation is symptomatic and the affectation from an early age and in both sexes and finally this review tried to warn about the possible involvement of Anisakis in allergic reactions in patients with HaT.
Introduction
Anisakis simplex (AS) is a helminth parasite belonging to the Nematode class. In 1995, our group described the first case of Anisakis-induced anaphylaxis [1]. When humans are infected with AS, they become an occasional host that acquires the parasite through ingestion of sea-fish that contains L3 stage larvae, but these larvae are unable to complete their life cycle in humans. Anisakis simplex larvae in the L3 phase include antigenic components capable of inducing an immune response in individuals after consuming both raw (with risk of infestation) and cooked fish. There are three kinds of antigens: somatic, ES (Excretion-Secretion) and surface antigens [2].
Anaphylaxis is the most serious immediate hypersensitivity reaction since it carries a life-threatening risk. Although drugs are the main allergens involved in anaphylaxis, followed by hymenoptera venoms and some foods, parasites are increasingly responsible for anaphylactic reactions, with AS being the most common in the adult population [3]. Infection and allergy to AS has been described in the 5 continents but the prevalence varies greatly, especially depending on the ingestion of fresh fish and sea food, and relates to the eating habits. It is particularly frequent among the Mediterranean area and Japanese population [4].
Clinical Cases
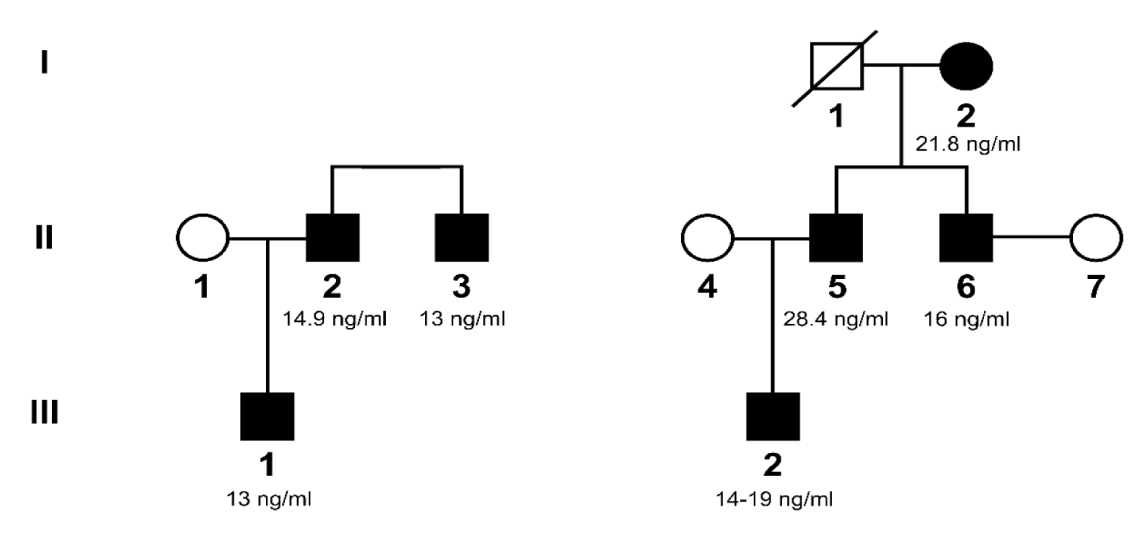
In July 2022, a 10- year old boy (Case 1) suffered from severe anaphylaxis after eating pineapple that the patient had previously tolerated. Despite requiring home treatment with self-injectable adrenaline, the patient needed a new dose of adrenaline along with systemic corticosteroids and antihistamines due to vomiting, odynophagia, pallor, and affected general condition. The allergy study concluded that the patient was sensitized to pineapple, as demonstrated by a positive skin prick test and a high specific IgE level (51.9 KU/l). Previously, the patient had been studied for allergic episodes associated with Basal Serum Tryptase (BST) levels above 11 mg/ml. The patient had presented repeated episodes of digestive and respiratory discomfort with the ingestion of multiple foods including fish and cephalopods, kiwi and legumes but the last pineapple episode had been the most severe. The patient had also required a study by the digestive department due to recurrent abdominal discomfort concluding that he suffered from bacterial overgrowth, ruling out at the present time celiac disease among other digestive pathologies. The father of this child (Case 2) had previously been studied for anaphylactoid reaction with ingestion of fish/sea food without demonstrating an allergy to seafood proteins nor Anisakis. His Basal Serum Tryptase level (BST) was nevertheless elevated (14.9 mg/ml) (Table 1).
| Table 1: Cases studied, mentioning pedigree identification (id), Basal Serum Triptase level (BST), personal history and clinical symptoms. | |||||
| Case/ Pedigree id |
Sex/Age | Basal Triptase Level (ng/ml) | Number of Copies of the GeneTPSAB1 | Personal History | Clinical Symptoms |
| 1 III.1 |
M/10 | 13 | 3α/2β | Incomplete simple syndactyly foot MTHFR homozygous mutated Celiac disease risk due to DQ2+ |
Anaphylactoid reactions with food from 2 years old (IgE and non-IgE mediated) Sudden episodes of digestive symptoms Severe abdominal pain with viral infections Severe episode of anaphylaxis |
| 2 II.2 |
M/51 | 14.9 | 2α/3β | Bicuspid aorta and foramen ovale MTHFR heterozygous mutated C46T mutation in the factor XII gene, heterozygous |
Anaphylactoid reactions induced by fish and sea foods (non-IgE mediated) |
| 3 I.2 |
F/60 | 21.8 | 2α/3β | C46T mutation in the factor XII gene, heterozigous Congenital heart disease with involvement of the great vessels (abnormal pulmonary venous drainage) Pulmonary thromboembolism |
Chronic urticaria, severe asthma, anaphylactoid reactions from drugs and fish/sea food |
| 4 III.2 |
M/5 | 14 (2 year- old) 19 (4 year- old) |
2α/3β | Autism spectrum disorder | Cutaneous mastocytosis Heat induced urticaria |
| 5 II.3 |
M/49 | 13 | 2α/3β | Mild asthma | |
| 6 II.6 |
M/34 | 16 | 2α/3β | Syncope without certified cause | |
| 7 II.5 |
M/41 | 28.4 | 2α/3β | Mild asthma | |
On the other hand, for more than 20 years we have been visiting patient number 3 in the Allergy Department for repeated episodes of chronic urticaria, severe asthma and adverse drug reactions, having observed BST of 20 mg/ml. This patient's dyspnea improved significantly after presenting severe decompensation of his congenital heart disease for which she required surgery at age 50 and after continuously receiving inhaled treatment with Long-Acting Beta Agonists (LABA) and corticosteroids. The patient was treated daily with Acetyl Salicylic Acid (ASA) 100 mg for repeated episodes of thrombosis and antihistamines in addition to the cardiologic treatment. This patient consulted our Allergy Department for having observed episodes of urticaria in her grandson (Case 7) related to bathing the baby at 2 years of age suspecting heat induced urticaria. The meticulous examination of the child's skin raised the suspicion of the presence of mastocitoma, which was confirmed by skin biopsy. The BST as can be seen in table 1, at 2 year-old was elevated (14 mg/ml) and it has been increasing during the last two years to 19 mg/ml.
These two family histories put us on the brink of suspecting HaT and we decided to perform a study of both families. Currently, in our country the genetic study of HaT cannot be carried out routinely, in fact it can only be carried out in a private center authorized for research purposes. A family pedigree is represented in figure 1 and table 1 shows the most important data of the first two families studied. We were pleasantly surprised to find that the HaT mutation was present in all the patients treated in our Department (Cases 1 to 4) and also in their direct relatives with elevated BST (Cases 5, 6 and 7). Regarding sensitization to Anisakis, although it has not been demonstrated in these specific cases, some episodes (Cases 1, 2, 3 and possibly 6) were nevertheless related to the ingestion of fish or shellfish and we can suspect that this parasite may favor the episodes of tryptase release. Tryptase gene genotyping of the TPSAB1 gene was performed in our cases by quantitative real-time digital PCR using the Biomark HD system by Citometry Laboratory nucleus from Salamanca (Spain). HaT mutation was defined as three or more alpha-tryptase copies or two alpha-tryptase copies in the presence of three beta-tryptase copies.
HaT is a newly described entity, reported in 2014 and recognized in 2016, that affects 5.7 percent of the general population in the United States, the United Kingdom, and the European Union [5].
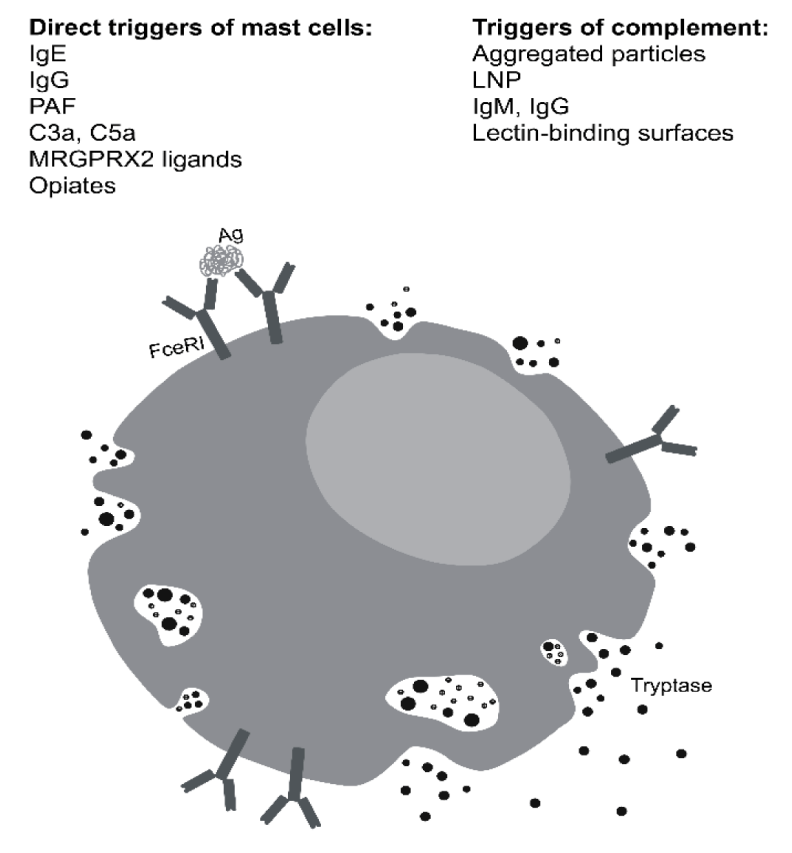
HaT is defined by a increased TPSAB1 gene copy number encoding alpha-tryptase and characterized by elevated basal serum levels of total tryptase (BST). Tryptase is the most abundant protease present in tissue-resident mature mast cells. The best known function of mast cells is their activation when the antigen binds to the IgE present on its surface, giving rise to type I immediate hypersensitivity allergic reactions. However, there are also other ways of activation of mast cells, not dependent on IgE. Once the antigen binds to the high-affinity IgE receptor (FceRI), membrane events occur that release the mediators. Tryptase is precisely the mediator that is routinely measured in this type of reaction, with values below 8 ng/ml being considered normal (Figure 2).
Alpha tryptase is inactive but -if it is elevated- it generates inactive homo-tetramers (alpha-tryptase) and hetero-tetramers with beta triptase that make mast cells respond more easily to certain stimuli. HaT is defined by an increase in the number of copies of the TPSAB1 gene encoding alpha-tryptase and is characterized by elevated Basal Serum Total Tryptase (BST) levels [6].
HaT occurs with high levels of tryptase, depending on the number of copies of the gene, if it is duplicated (around 13.6-36.2 ng/ml) or triplicate (26.4-62.2 mg/ml). It is an entity with autosomal dominant inheritance and, however, it is striking that female are the majority of symptomatic individuals reported [7]. Among our cases, it is not possible to extract prevalence data, but the presence of males with symptoms and the involvement of both children from a very early age is novel.
The majority of individuals with HaT (2/3) have been reported to have few symptoms and thus do not require medical intervention. However, among individuals with allergic symptoms who attend specialized medical attention, the finding of elevated basal tryptase should lead to suspicion of HaT because 90% of individuals with the basal levels > 11.4 mg/ml have HaT as the cause. It is accepted today that the presence of HaT increases the frequency and severity of immediate hypersensitivity reactions at least in patient with three disorders: systemic mastocytosis, anaphylaxis due to hymenoptera and idiopathic anaphylaxis [8]. Some histological changes consistent with increased mast cells were found in the digestive tract of patients with HaT, which may explain the prevalent digestive manifestations observed among individuals with this genetic trait [6]. We propose that seafood-induced symptoms should also be taken into account, especially at the expense of Anisakis, at least in regions with a high consumption of fresh fish. In fact, the rules relating to prevention of parasitization by Anisakis, are included in the protocols for action in mastocytosis in our country.
It is not understood that almost a decade after HaT was reported, nowadays it has to be studied in an investigative and non-routine manner. Several author suggest that HaT is a useful biomarker for determining the individual patient´s risk of developing severe anaphylaxis [9]. We propose that the technique should be routinely available, since HaT must be taken into account in the differential diagnoses of anaphylaxis and also in the prognosis and prevention recommendations for our patients and their affected relatives.
Outside of specific clinical presentations (eg, hymenoptera venom anaphylaxis), there are few current expert recommendations for changes in treatment or monitoring, specifically. Treatment strategies for symptomatic pacients with HaT are currently identical to those used in clonal mast cell disorders such indolent mastocytosis: H1 and H2 -antihistamines as well as other mast cell stabilizers and at least two epinephrine autoinjectors [10]. Given our experience and the knowledge that Anisakis can be a stimulus for the release of tryptase by mast cells, we propose adding recommendations regarding this parasite, not in the form of a vaccine as exists for hymenoptera venom allergy but in the form of dietary guidelines for prevention.
Conclusion
- We report some cases of two families with HaT, with different levels of elevated BST and mRNA gene expression in the absence of MC clonality. Unlike most of the authors reported so far, the majority of symptomatic patients were women both sexes are represented in our families.
- One in 20 white Europeans have the HaT mutation and one in 3 of them can develop allergic reactions. For the reasons discussed in the article, we propose that the technique should be available to perform rutinely, since HaT must be taken into account in the differential diagnoses of anaphylaxis and also in the prognosis and recommendations for those patients.
- Regardless of IgE sensitization, exposure to Anisakis should be studied in countries where fresh sea foods are involved in the usual diet as a possible trigger for anaphylaxis in HaT mutation cases in addition to hymenoptera venoms.
Acknowledgment
The authors would like to thank Maialen Mendiguchia for the elaboration of the figures.
Informed consent statement
Informed consent was obtained from each of the patients/guardians for the determination of HaT and granted the corresponding permission to publish the results anonymously.
References
- Audicana MT, Fernández de Corres L, Muñoz D, Fernández E, Navarro JA, del Pozo MD. Recurrent anaphylaxis caused by Anisakis simplex parasitizing fish. J Allergy Clin Immunol. 1995 Oct;96(4):558-60. doi: 10.1016/s0091-6749(95)70301-2. PMID: 7560669.
- Audicana MT, Kennedy MW. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev. 2008 Apr;21(2):360-79, table of contents. doi: 10.1128/CMR.00012-07. PMID: 18400801; PMCID: PMC2292572.
- Audicana MT. Anisakis, Something Is Moving inside the Fish. Pathogens. 2022 Mar 7;11(3):326. doi: 10.3390/pathogens11030326. PMID: 35335650; PMCID: PMC8950136.
- EFSA BIOHAZ Panel, 2010. Scientific opinion on risk assessment of parasites in fishery products. EFSA Journal 2010;8(4):1543. doi: 10.2903/j.efsa.2010.1543.
- Lyons JJ. Hereditary alpha-tryptasemia. 2022.
- Hamilton MJ, Zhao M, Giannetti MP, Weller E, Hufdhi R, Novak P, Mendoza-Alvarez LB, Hornick J, Lyons JJ, Glover SC, Castells MC, Pozdnyakova O. Distinct Small Intestine Mast Cell Histologic Changes in Patients With Hereditary Alpha-tryptasemia and Mast Cell Activation Syndrome. Am J Surg Pathol. 2021 Jul 1;45(7):997-1004. doi: 10.1097/PAS.0000000000001676. PMID: 33481382; PMCID: PMC8192345.
- Le QT, Lyons JJ, Naranjo AN, Olivera A, Lazarus RA, Metcalfe DD, Milner JD, Schwartz LB. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019 Oct 7;216(10):2348-2361. doi: 10.1084/jem.20190701. Epub 2019 Jul 23. PMID: 31337736; PMCID: PMC6780998.
- Lyons JJ, Greiner G, Hoermann G, Metcalfe DD. Incorporating Tryptase Genotyping Into the Workup and Diagnosis of Mast Cell Diseases and Reactions. J Allergy Clin Immunol Pract. 2022 Aug;10(8):1964-1973. doi: 10.1016/j.jaip.2022.05.003. Epub 2022 May 18. PMID: 35597543.
- Sordi B, Vanderwert F, Crupi F, Gesullo F, Zanotti R, Bonadonna P, Crosera L, Elena C, Fiorelli N, Ferrari J, Grifoni F, Sciumè M, Parente R, Triggiani M, Palterer B, Mecheri V, Almerigogna F, Santi R, Di Medio L, Brandi ML, Iorno ML, Ciardetti I, Bencini S, Annunziato F, Mannarelli C, Pieri L, Guglielmelli P, Mannelli F, Vannucchi AM. Disease correlates and clinical relevance of hereditary α-tryptasemia in patients with systemic mastocytosis. J Allergy Clin Immunol. 2023 Feb;151(2):485-493.e11. doi: 10.1016/j.jaci.2022.09.038. Epub 2022 Oct 27. PMID: 36309122.
- Pyatilova P, Akin C, Alvarez-Twose I, Arock M, Bonadonna P, Brockow K, Butterfield JH, Broesby-Olsen S, Carter MC, Castells M, George TI, Gotlib J, Greiner G, Gülen T, Hartmann K, Hermine O, Horny HP, Jawhar M, Lange M, Lyons JJ, Maurer M, Metcalfe DD, Nedoszytko B, Niedoszytko M, Orfao A, Reiter A, Schwaab J, Sotlar K, Sperr WR, Triggiani M, Valent P, Siebenhaar F. Refined Treatment Response Criteria for Indolent Systemic Mastocytosis Proposed by the ECNM-AIM Consortium. J Allergy Clin Immunol Pract. 2022 Aug;10(8):2015-2024. doi: 10.1016/j.jaip.2022.05.037. Epub 2022 Jun 18. PMID: 35724950.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.