Biology Group . 2023 June 13;4(6):1002-1010. doi: 10.37871/jbres1761.
Design and Preparation of a Novel Antibacterial Hydrogel based on Maleimide-Thiol Conjugation
Song Jiang1*, Yue Liu2, Taoqing Wang3, Yuan Gu4 and Yige Luo5
2Shanghai Center for Systems Biomedicine, Shanghai Jiao Tong University, Shanghai 200240, China
3Department of Chemistry, University of New Hampshire, USA
4The Statistics Department, The George Washington University, USA
5College of Biological Sciences, UC Davis, USA
- Hydrogel
- Antibacterial
- Maleimide-Thiol
- Chitosan
- Dextran
Abstract
This article synthesized and characterized a novel antibacterial hydrogel, which is formed using maleimide–thiol conjugation. Two precursors chitosan functionalized thiol groups and dextran functionalized maleimide groups prepared and characterized by Nuclear Magnetic Resonance (NMR). Key features of the hydrogel, such as gelation time, swelling behavior, viscoelastic properties, and degradation rate, were thoroughly examined. From gelation time result, we found that formed hydrogel gelation time could be changed with diffident weight percentage of precursors. Based on references, we found the best formular for the gelation and it was also determined for other studies. The swelling study indicated hydrogel has good flexibility and the degradation test indicated hydrogel is biodegradable. The viscoelastic test indicated hydrogel is an elastic solid. Given these attributes, this novel hydrogel has significant potential for use in various biomedical applications. Finally, colony-counting tests revealed that the hydrogel has an excellent antibacterial ability. Given these attributes, this novel hydrogel has significant potential for use in various biomedical applications.
Introduction
Antibacterial agents have become widely used in various products, such as hand sanitizers, disinfectants, and cleaning solutions [1]. Using antibacterial agents only when appropriate can help reduce the potential negative impacts on traffic and public health [2-4]. In order to maximize the efficiency of antibacterial properties, it is necessary to utilize antibacterial agents that are specifically designed and integrated for the appropriate niche. Hydrogel has been approved as an excellent niche due to its water-swollen, crosslinked polymer network, which that is widely used in various fields, including cancer therapy, antibacterial agents, etc [5-10].
The hydrogel can be made from synthetic or natural polymers, such as polyethylene glycol, polyvinyl alcohol, or hyaluronic acid, among others [11]. One of the most significant advantages of hydrogel is its ability to absorb and retain large amounts of water. This property makes it an excellent material for wound healing, as it helps maintain a moist environment that facilitates faster healing [12]. Additionally, hydrogels have been shown to have excellent biocompatibility and low toxicity, making them an ideal option for use in various medical applications, including tissue engineering and drug delivery systems [13]. Hydrogels are also used in agriculture, where they can be used to improve crop yields and reduce water usage [14]. Hydrogel-based soil amendments can improve soil structure and water retention capacity, which helps plants grow in water-stressed environments. Additionally, hydrogels can be used to release fertilizers and pesticides in a controlled manner, reducing environmental contamination and increasing the effectiveness of these chemicals [15]. In engineering, hydrogels are used to develop smart materials that respond to environmental stimuli such as temperature, pH, or light. These materials have applications in areas such as sensors, actuators, and drug delivery systems [16].
Hydrogel can be created through several methods, depending on the desired properties and applications of the final product. One of the most common methods is free-radical polymerization, which involves crosslinking monomers under the influence of free radicals. This process typically involves the use of a crosslinking agent, such as ethylene glycol dimethacrylate, and a free-radical initiator, such as ammonium persulfate [17]. Another method for creating hydrogel is through physical crosslinking, which involves using physical interactions, such as hydrogen bonding or electrostatic interactions, to form a polymer network. This method typically involves the use of natural polymers, such as chitosan or hyaluronic acid, and requires no chemical crosslinking agents or initiators [18]. A third method for creating hydrogel is through ionotropic gelation, which involves the use of ionic interactions to crosslink polymers. This method typically involves the use of a cationic polymer, such as chitosan, and an anionic crosslinking agent, such as tripolyphosphate, sulfobetaine [19-23].
As we known, maleimide-thiol conjugation is a popular method for crosslinking hydrogels that involves the reaction of a maleimide functional group with a thiol functional group [13,24,25]. This method is widely used in the synthesis of hydrogels for biomedical applications, including drug delivery systems and tissue engineering.
Maleimide functional groups have a high affinity for thiol groups, making them an ideal choice for crosslinking hydrogels. The reaction between the maleimide and thiol groups forms a covalent bond, resulting in a stable crosslinked network. The reaction can be performed under mild conditions, which minimizes the risk of damage to bioactive molecules or cells encapsulated within the hydrogel [26]. One of the advantages of using maleimide-thiol conjugation to crosslink hydrogels is its high specificity. The reaction only occurs between maleimide and thiol groups, which reduces the likelihood of non-specific binding or unwanted reactions [27]. Another advantage of this method is its versatility. Maleimide functional groups can be incorporated into a variety of polymers, including synthetic and natural polymers, to create hydrogels with tailored properties. Additionally, thiol functional groups can be easily introduced into biomolecules, such as peptides or proteins, allowing for the creation of hydrogels with biologically active components [28].
However, previously investigated hydrogels based on maleimide-thiol conjugation have many existing defects, including toxicity and slow gelation time. For instance, a typically example is from Zeng etc., who created a novel chitosan-PEG hydrogels from maleimide-thiol conjugation [29]. However, they utilized initiator UV lamp, which could be harmful to the cells in the biomedical application. And the gelation time at least 30 seconds with very high amount 20% precursors.
To conquer both defects mentioned above, in this paper, we selected two nature materials chitosan and dextran due to their biocompatibility, biodegradability, antimicrobial property, water treatment, etc. Then, we functionalized chitosan and dextran with shorter chain thiol groups and maleimide groups. Also, we didn’t use the initiator due to its toxicity. Meanwhile, we investigated conjugated hydrogel with its gelation speed, swelling behavior, viscoelastic property, degradation rate and antibacterial properties.
Experimental Section
Materials
Chitosan (MW = 80 kDa), dextran (MW = 80 kDa), ethanol, dichloromethane, N,N’-dicyclohexyl-carbodiimide (DCC), cystamine dihydrochloride, 1-(3-Dimethylamino-propyl)-3-ethylcarbodiimide hydrochloride (EDC), hydroxybenzotriazole (HOBT), tris(2-carboxyethyl)phosphine (TCEP), dimethylsulfoxide (DMSO), hydrochloric acid (HCl) and carboxyl end group maleimide (GMI) were purchased from Sigma-Aldrich.
Synthesis
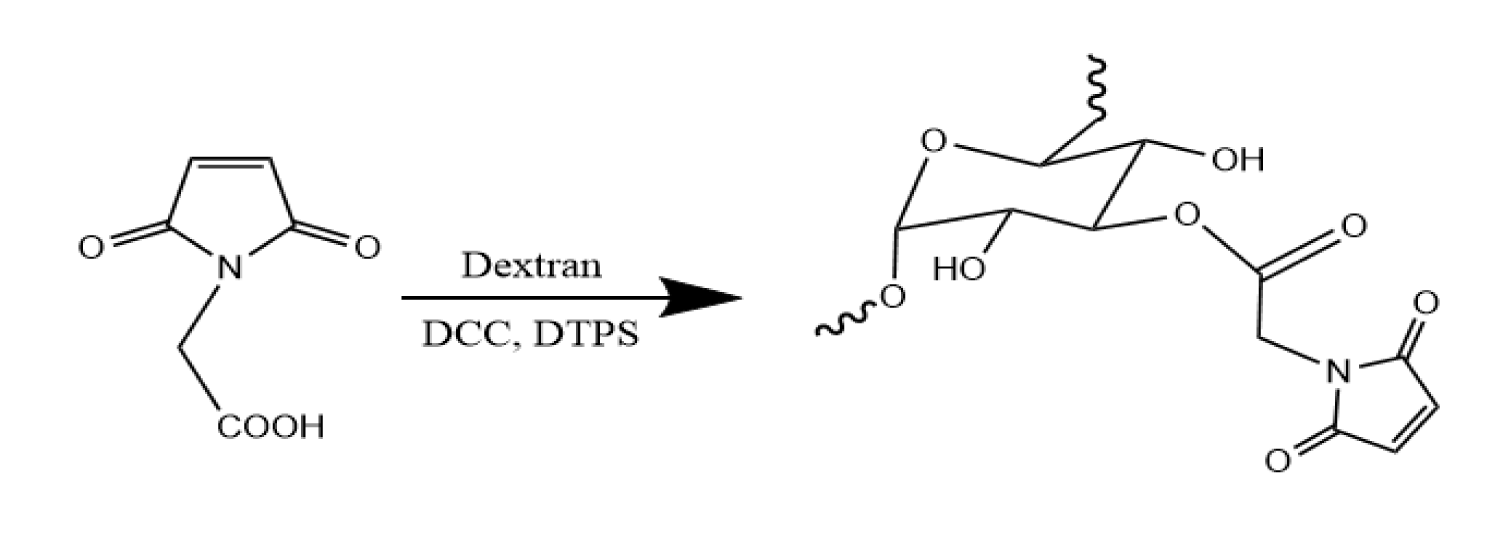
Synthesis of dextran with maleimide groups: Scheme 1 outlines the process for synthesizing dextran with maleimide groups (dextran-Mal). To create dextran-Mal, the hydroxyl group of dextran was esterified with N-maleoylamino acid using DCC mediation. The N-maleoylamino acid was prepared using a previously reported procedure. In this process, 1.0 mmol N-maleoylamino acids were dissolved in 10 mL DMSO, followed by the addition of 0.22 mmol DPTS and 2.3mmol DCC. 2.5 mmol Dextran was dissolved in 5 mL DMSO and slowly added to the reaction mixture. After stirring at room temperature for 24 hours, the N,N’-dicyclohexylurea salt was removed by filtration, and the crude product was precipitated in cold ethanol. The precipitate was then collected by filtration, washed with ethanol, dissolved in water, and purified through ultrafiltration.
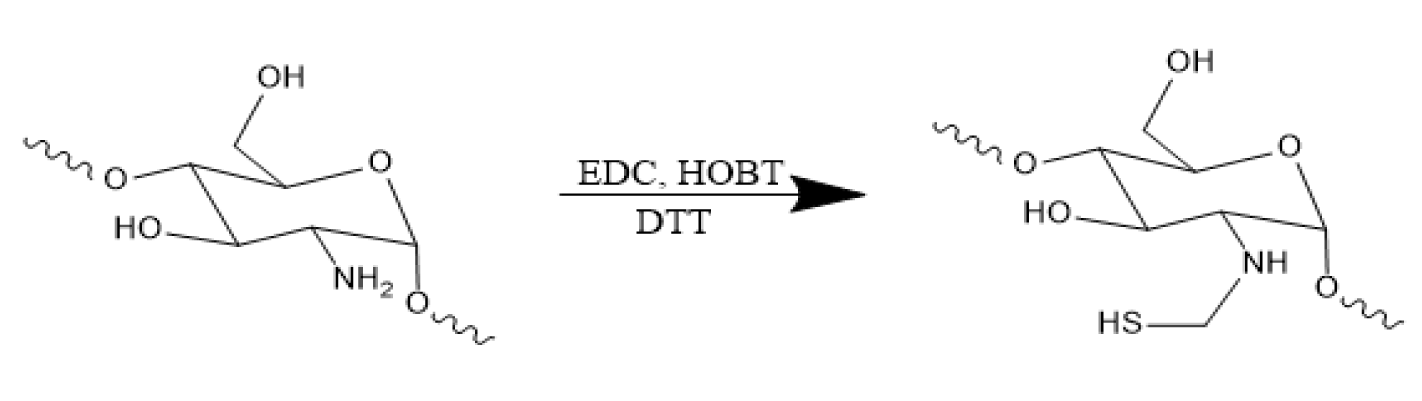
Synthesis of chitosan with thiol groups: Chitosan with thiol groups (chitosan-SH) was produced and characterized using scheme 2. To synthesize this compound, 5 mg chitosan was dissolved in 5 ml DI water and combined with three molar equivalents of cystamine dihydrochloride at pH 4.8 [30]. The conjugation process took place overnight after activation of the carboxyl group of chitosan using EDC and HOBT at 3 equivalents for two hours. The resulting product was purified using dialysis tubing with a molecular weight cutoff of 8,000 kDa against purified water for three days. To cleave the disulfide linkage of the cystamine component, the solution was treated with TCEP at 5 equivalents and stirred for two hours. The pH of the solution was then adjusted to 3.5 with HCl. Finally, chitosan-SH was precipitated in ethanol, re-dissolved in DI water, lyophilized, and stored at -20ºC.
Hydrogel preparation: Dextran-Mal and chitosan-SH hydrogel precursors were dissolved in in pH 7.4 PBS, then followed by mixing dextran-Mal and chitosan-SH solutions. The maleimide groups “clicked” thiol groups to create the three-dimensional structure to form the gel.
Characterization
Characterization of GMI, maleimide and thiol hydrogel precursors: Proton nuclear magnetic resonance was utilized to verify the molecular structures of maleimide and thiol hydrogel precursors. The NMR spectra were recorded using an Avance Bruker with a BBO z-gradient probe. The experimental parameters included scanning each sample 256 times. The solvent employed was D2O for dextran-Mal and chitosan-SH.
Gelation time: The gelation time is determined using the vial tilting method. Briefly, dextran-Mal and chitosan-SH hydrogel precursors were dissolved in pH 7.4 PBS solution, respectively. The hydrogels were formed by mixing both solutions and vibrating quickly at room temperature. No flow within 1 min upon inverting the vial is regarded as the gel state.
Swelling studies: Swelling studies of freeze-dried hydrogels were tested by a general gravimetric method. Samples were incubated at 37°C in pH 7.4 PBS until their weight was balanced. The swollen hydrogels were removed from the excess water, absorbed with filter paper, and weighed. The samples were then dried until constant weight. The swelling ratio was calculated using the following Eq:
Percentage of swelling (%) = (Wa-Wb)/Wb × 100,
where Wa and Wb refer to the weight of wet and dry hydrogels respectively.
Degradation: For degradation studies, weighted hydrogel freeze-dried samples (1 ~ 5 grams) were immersed in pH 7.4 PBS and incubated at 37°C at selected time intervals. At each selected time interval, the samples were removed from PBS, dried and weighted. The hydrogel degradation was calculated using following Eq:
Degradation (%) = (ma/mb) × 100,
where ma and mb refer to weight of freeze-dried hydrogels at selected time interval and before immersing in PBS respectively.
Rheological analysis: Dynamic rheological behaviors of hydrogels are analyzed with a discovery hybrid rheometer, using parallel plate geometry (10 mm diameter). Frequency sweep measurements are performed at 37°C from 0.1 to 100 rad s−1 at a fixed strain in the linear viscoelastic region.
Antibacterial test
The hydrogel’s antibacterial activities were evaluated quantitatively. E. coli and S. aureus were cultured on LB plates at 37°C. To prepare the bacterial solution, the aforementioned bacteria were mixed homogeneously in a volume of 500 μL. Sterilized LB medium (30 mL) was then combined with the bacterial solution. Separately, sterilized hydrogels (10 g) were soaked in 30 mL of LB medium and incubated in a shaker at 120 rpm and 37°C for 24 hours. Following incubation, the bacterial suspensions were diluted to a concentration of 10-6 times the original bacterial solution. Subsequently, 10 μL of these diluted bacterial suspensions were spread onto the solid surface of the respective medium. Incubation was carried out at 37°C for 24 hours, depending on the bacteria being tested. The Colony-Forming Units (CFUs) were counted after the incubation period. Each colony assay was repeated independently at least three times. Controls were prepared using bacterial suspensions without hydrogels.
Results and Discussion
NMR analysis
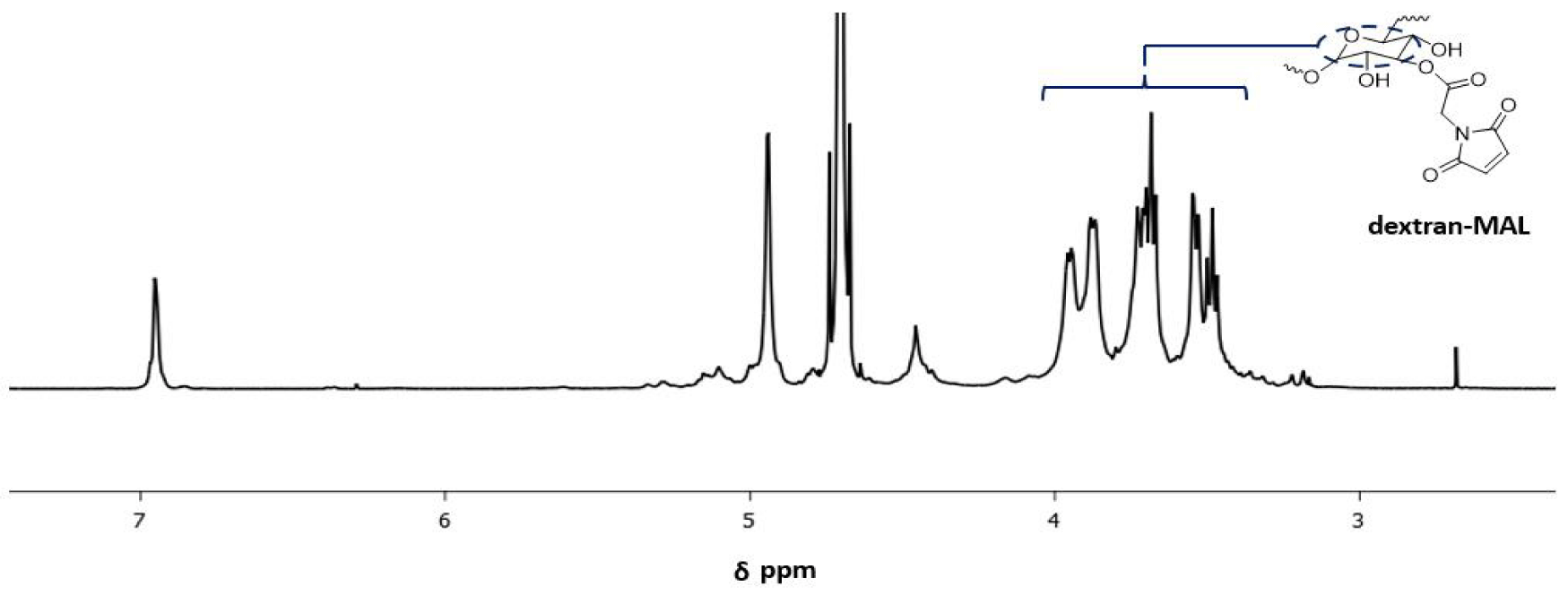
Dextran-Mal: The confirmation of the dextran-Mal structure has been established through the analysis of the 1H NMR spectrum. As illustrated in figure 1, the peak observed at 6.9 ppm is indicative of the protons in the maleimide group, while the peaks detected at 4.0-3.4 ppm are attributed to the protons in the dextran backbone. Based on a comparison of the integrals of the resonance signals at 6.9 ppm and 4.0-3.4 ppm, the degree of functionality of the Dextran-Mal is estimated to be approximately 45%.
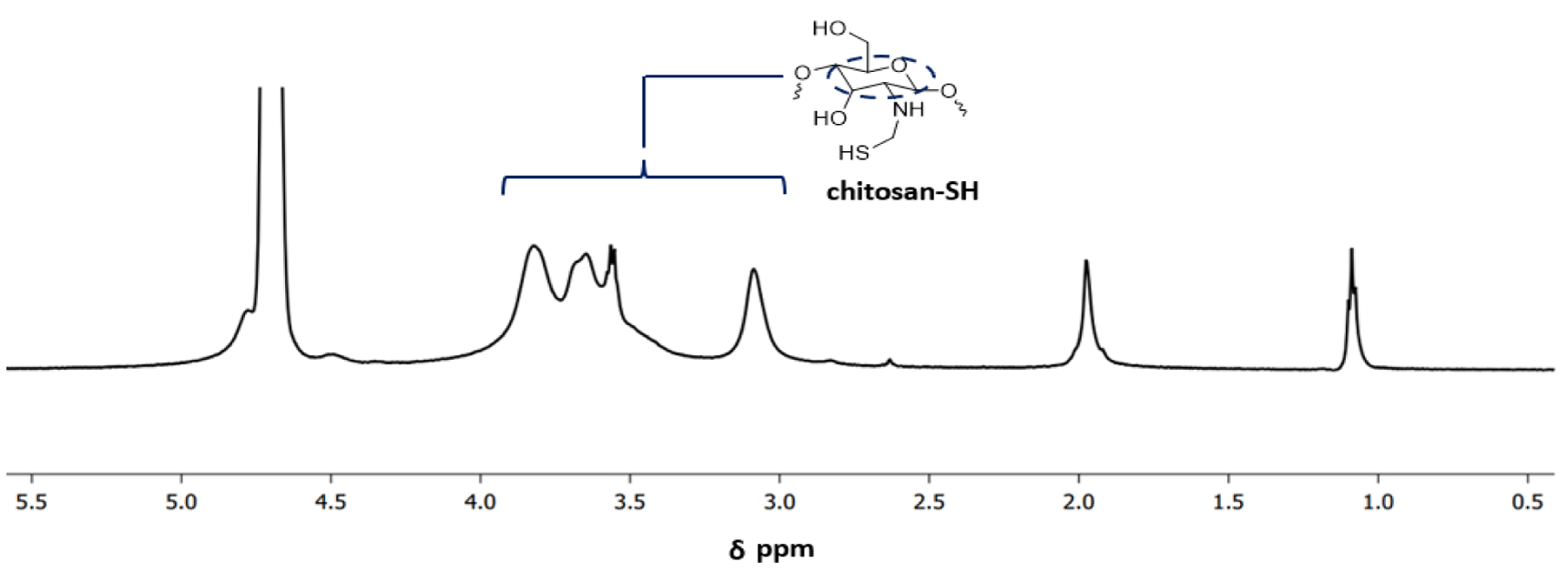
Chitosan-SH: The confirmation of the chitosan-SH structure was achieved through 1H NMR analysis (Figure 2). The modification of chitosan was determined by utilizing the resonance of the acetamido group of chitosan as an internal standard at δ = 1.15 ppm. The peaks observed at δ = 1.95 ppm indicate the presence of cysteamine in chitosan-SH. Meanwhile, the peaks detected at 4.0-3.0 ppm are attributed to the protons in the chitosan backbone. Based on a comparison of the integrals of the resonance signals at 1.95 ppm and 4.0-3.0 ppm, the degree of functionality of the chitosan-SH is estimated to be approximately 67%.
Gelation time analysis
Table 1 summarizes the gelation time of hydrogels, with the weight percent of hydrogel precursors ranging from 1% to 2%. Gelation time ranges from ∞ (no gelation) to ≤1 second (ultrafast). The only feasible formula is 1.5% chitosan-SH and 1.5% Dextran-Mal because longer or shorter times could lead to the failure of cell encapsulation. For example, longer times may result in lower weight percent of hydrogel precursors used, leading to the formation of an amorphous gel. Conversely, shorter times could result in incomplete "clicking" of the maleimide and thiol groups, which may negatively impact cell growth. Consequently, this formula was used for the remaining experiments and analysis.
| Table 1: Summary of four hydrogels’ gelation time. | |||
| Dextran-Mal/Chitosan-SH | 1% | 1.5% | 2% |
| 1% | No gelation | No gelation | x |
| 1.50% | No gelation | ≈ 10s | ≤1s |
| 2% | x | ≤1s | ≤1s |
Degradation analysis
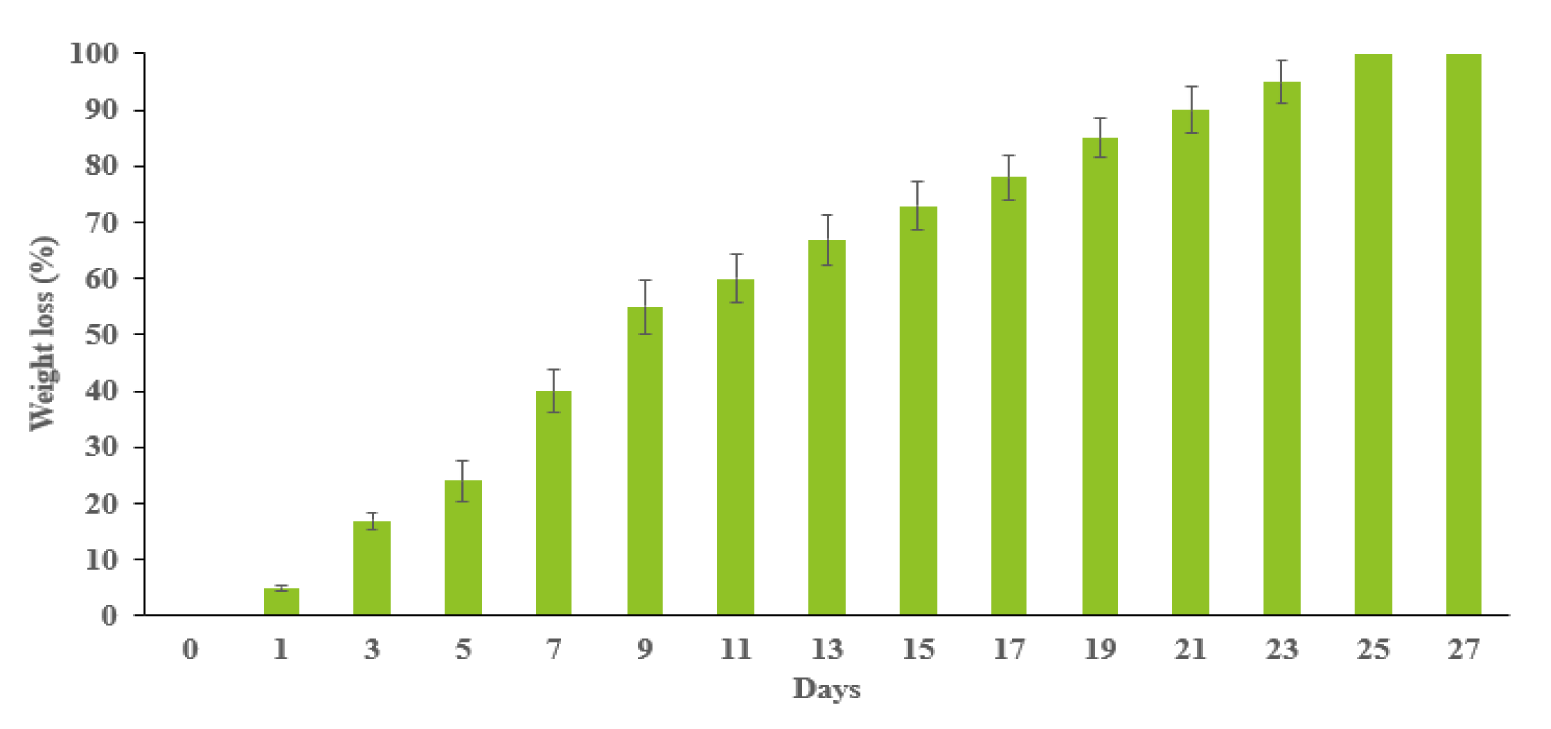
The weight loss of the hydrogels was plotted in figure 3. It exhibited the weight loss speed of hydrogels. The initial weight loss of hydrogels was rapid and reached 40% in the first week. After day 7, the hydrogels degraded steadily until the 23rd day. Then, the hydrogels were degraded completely.
Swelling analysis
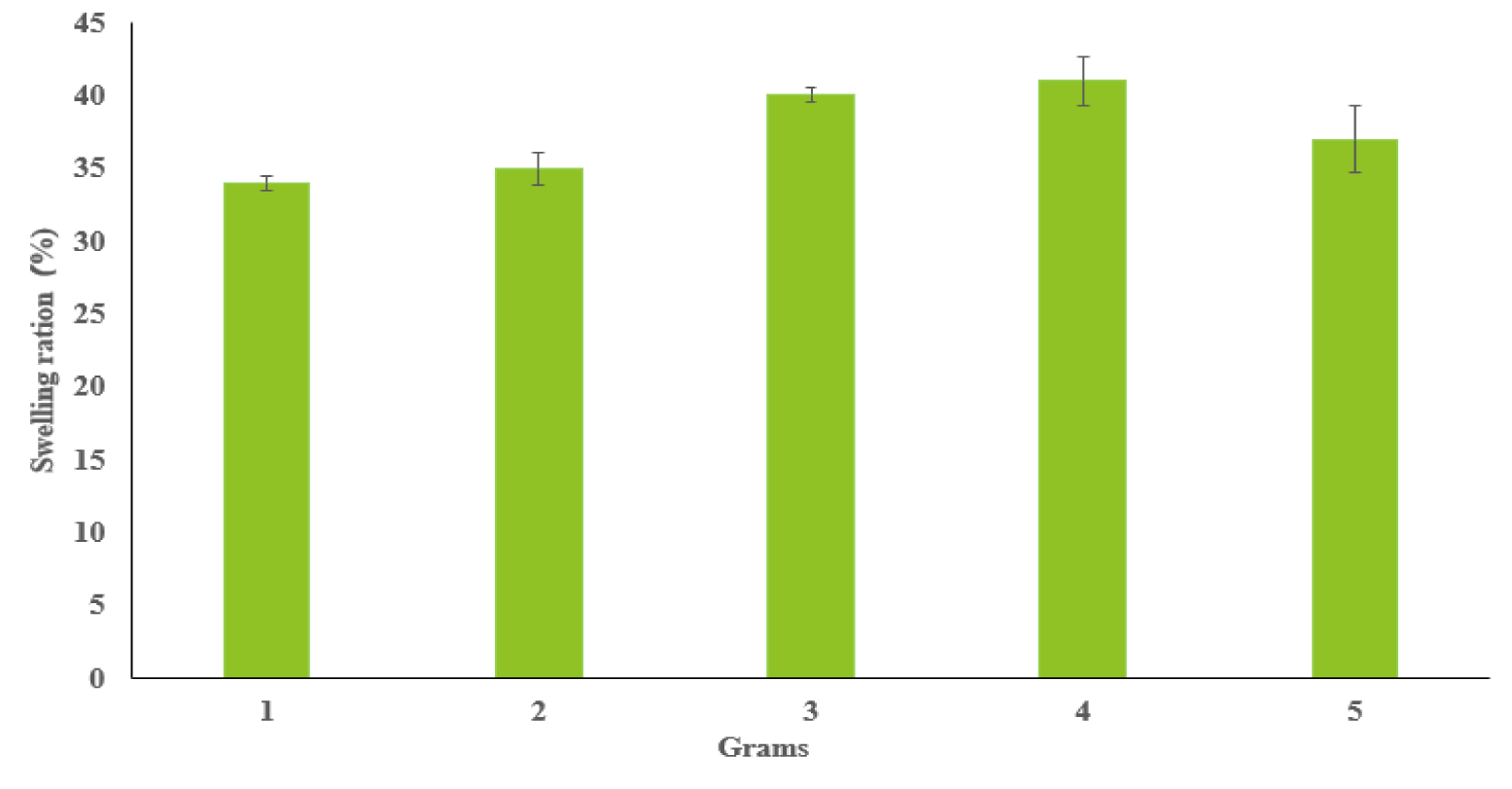
Figure 4 presents the swelling analysis of synthesized dextran-Mal and chitosan-SH hydrogels with xx replicates in each sample. The weight of freeze-dried hydrogel samples are ranged from 1 to 5 grams. Our analysis revealed a relatively consistent swelling ratio of approximately 37% across all samples, regardless of their weights, suggesting a uniform swelling behavior intrinsic to these hydrogels. The data suggest that these hydrogels, when submerged in Phosphate-Buffered Saline (PBS), can absorb water approximately half their own weight. This result underscores the hydrophilic nature of the 1.5% dextran-Mal and 1.5% chitosan-SH hydrogels and their potential to mimic the flexibility of the extracellular matrix. This consistent swelling property across different sample weights enhances the potential of these hydrogels for a broad spectrum of biomedical applications.
Rheological analysis
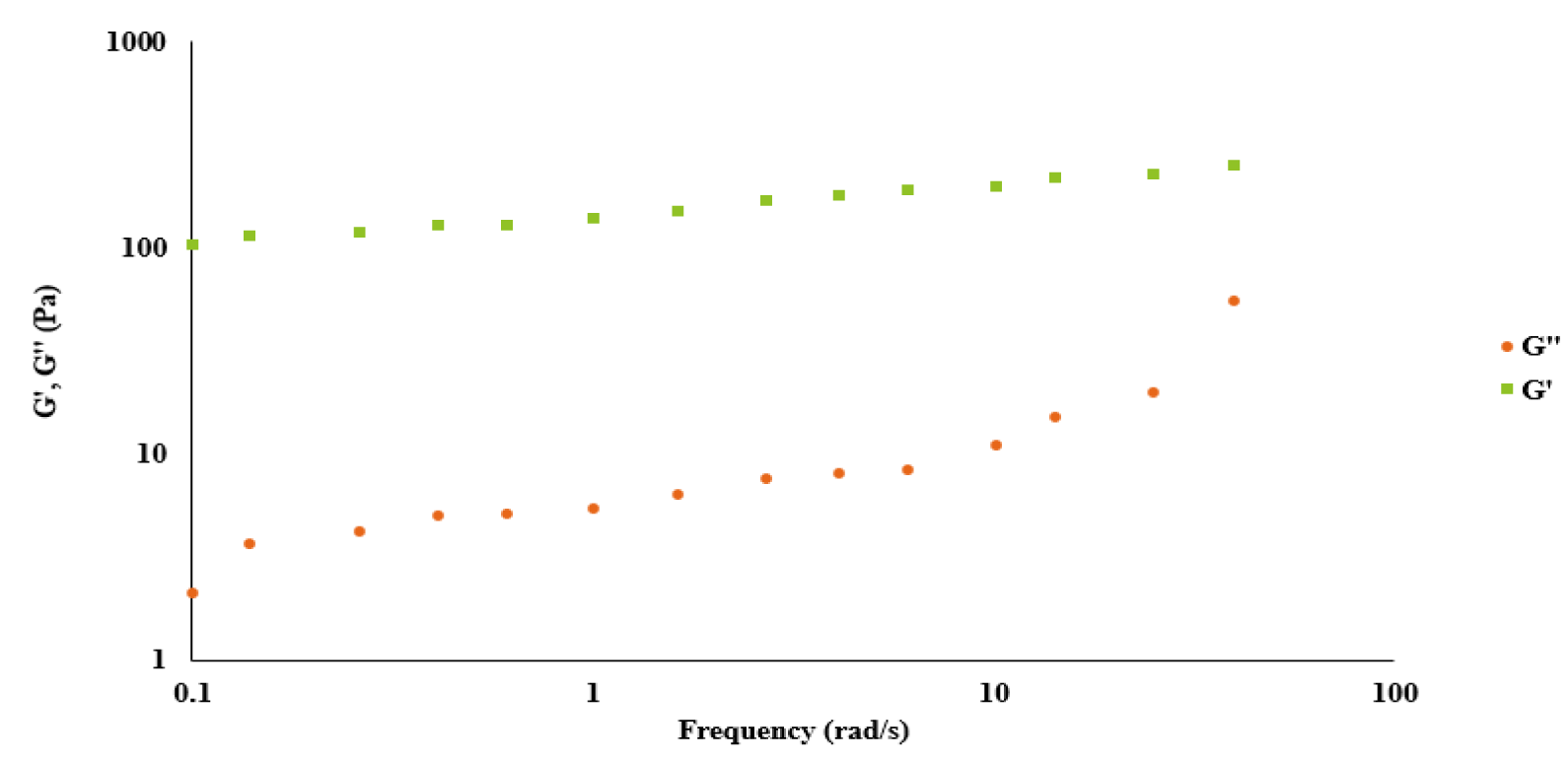
Figure 5 presents a comprehensive rheological analysis of the hydrogels, in which the storage modulus (G') and the loss modulus (G'') are plotted against the angular frequency. This analysis underscores the unique viscoelastic behavior of the hydrogels. Specifically, the storage modulus G' persistently exceeded the loss modulus G'' across the entire frequency spectrum investigated. This characteristic pattern of the G' values outpacing the G'' values at every frequency point suggests that the elasticity of the hydrogels is dominant over their viscosity. Given that these hydrogels are formulated through maleimide-thiol coupling chemistry, this result is indicative of their predominant behavior as elastic solids. These findings hold significant implications for the mechanical understanding of these hydrogels and lend support to their potential for a variety of biomedical applications where such elastic properties are desirable.
Antibacterial ability analysis
The antibacterial properties of hydrogel were also characterized using colony formation counting. As shown in table 2, the control samples had no significant antibacterial activity against all strains, whereas hydrogel with our optimal formular was resistant to E. coli and S. aureus, indicating their antibacterial activity. Notably, after contacting with this hydrogel for 24 h, 100% of the bacteria were killed.
| Table 2: Summary of antibacterial ability of hydrogel against E. coli and S. aureus. | ||||
| Test Strain | Test Sample | Early Conc. (cfu/mL) | After 24h, Conc. (cfu/mL) | Reduction Rate of Bacteria (%) |
| E. coli | Blank | 1.2 × 105 | 6.1 × 106 | 99.99 |
| Hydrogel | 1.2 × 105 | <10 | ||
| S. aureus | Blank | 3.3 × 105 | 6.3 × 106 | 99.99 |
| Hydrogel | 3.3 × 105 | <10 | ||
Conclusion
In this study, our objective was to develop a novel antibacterial hydrogel by utilizing dextran-Mal and chitosan-SH functionalized precursors through the Michael Addition reaction and investigate its antibacterial properties. The successful synthesis of both precursors was confirmed through NMR analysis. Subsequently, the hydrogels underwent a series of comprehensive investigations, including gelation time assessment, swelling behavior analysis, evaluation of viscoelastic properties, and determination of the degradation rate. Based on our experimental results, we identified that the optimal composition for the hydrogels was achieved using 1.5% dextran-Mal and 1.5% chitosan-SH. This formulation was employed for all the subsequent investigations. Our findings revealed that the hydrogels exhibited remarkable flexibility, as demonstrated by their ability to mimic the characteristics of the extracellular matrix during the swelling study. Furthermore, the degradation study showed that the hydrogels completely degraded after a period of 9 days. Moreover, the viscoelastic study indicated that the hydrogels formed through the Michael Addition reaction possessed elastic solid properties. Collectively, our research provides compelling evidence supporting the potential biomedical applications of this novel biocompatible hydrogel. Finally, we measure the antibacterial activities of this hydrogel quantitively. We approved that this hydrogel with optimal formular can successfully kill the bacteria E. coli and S. aureus.
References
- Kabiri K, Omidian H, Zohuriaan‐Mehr M, Doroudiani S. Superabsorbent hydrogel composites and nanocomposites: A review. Polymer Composites. 2011;32(2):277-289. doi: 10.1002/pc.21046.
- Kang D, Zhao J, Tyler Dick C, Liu X, Bian Z, Kirkpatrick SW, Lin CY. Probabilistic risk analysis of unit trains versus manifest trains for transporting hazardous materials. Accid Anal Prev. 2023 Mar;181:106950. doi: 10.1016/j.aap.2022.106950. Epub 2022 Dec 31. PMID: 36592490.
- Kang D, Levin MW. Maximum-stability dispatch policy for shared autonomous vehicles. Transportation Research Part B: Methodological. 2021;148:132-151. doi: 10.1016/j.trb.2021.04.011.
- Kang D, Hu F, Levin M. Impact of automated vehicles on traffic assignment, mode split, and parking behavior. Transportation Research Part D: Transport and Environment. 2022;104:103200. doi: 10.1016/j.trd.2022.103200.
- Ni L, Tang C, Wang Y, Wan J, Charles MG, Zhang Z, Li C, Zeng R, Jin Y, Song P, Wei M, Li B, Zhang J, Wu Z. Construction of a miRNA-Based Nomogram Model to Predict the Prognosis of Endometrial Cancer. J Pers Med. 2022 Jul 17;12(7):1154. doi: 10.3390/jpm12071154. PMID: 35887651; PMCID: PMC9318842.
- Liu Y, Sun Y, Guo Y, Shi X, Chen X, Feng W, Wu LL, Zhang J, Yu S, Wang Y, Shi Y. An Overview: The Diversified Role of Mitochondria in Cancer Metabolism. Int J Biol Sci. 2023 Jan 16;19(3):897-915. doi: 10.7150/ijbs.81609. PMID: 36778129; PMCID: PMC9910000.
- Chen Y, Li C, Wang N, Wu Z, Zhang J, Yan J, Wei Y, Peng Q, Qi J. Identification of LINC00654-NINL Regulatory Axis in Diffuse Large B-Cell Lymphoma In Silico Analysis. Front Oncol. 2022 May 26;12:883301. doi: 10.3389/fonc.2022.883301. PMID: 35719990; PMCID: PMC9204339.
- Liu Y, Wang Y, Yang Y, Weng L, Wu Q, Zhang J, Zhao P, Fang L, Shi Y, Wang P. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct Target Ther. 2023 Mar 7;8(1):104. doi: 10.1038/s41392-023-01365-z. PMID: 36882399; PMCID: PMC9990587.
- Liu J, Zhang J, Beeraka NM, Chen K, Sinelnikov MY, Manogaran P, Bannimath G, Nikolenko VN, Fan R. Perspectives on the nanocarriers with miRNAs for targeting melanoma stemness through epigenetic regulation. Pigment Cell Melanoma Res. 2023 May-Jul;36(3-4):268-287. doi: 10.1111/pcmr.13081. Epub 2023 Feb 2. PMID: 36691113.
- Ren X, Wang N, Zhou Y, Song A, Jin G, Li Z, Luan Y. An injectable hydrogel using an immunomodulating gelator for amplified tumor immunotherapy by blocking the arginase pathway. Acta Biomater. 2021 Apr 1;124:179-190. doi: 10.1016/j.actbio.2021.01.041. Epub 2021 Jan 30. PMID: 33524560.
- Kodavaty J. Poly (vinyl alcohol) and hyaluronic acid hydrogels as potential biomaterial systems-A comprehensive review. Journal of Drug Delivery Science and Technology. 2022;103298. doi: 10.1016/j.jddst.2022.103298.
- Wang H, Xu Z, Zhao M, Liu G, Wu J. Advances of hydrogel dressings in diabetic wounds. Biomater Sci. 2021 Mar 10;9(5):1530-1546. doi: 10.1039/d0bm01747g. PMID: 33433534.
- Jiang S, Liu Y, Wang T. Investigation of a novel biocompatible fast-gelling hydrogel for neurodegenerative diseases. Biomedical Journal of Scientific & Technical Research. 2023;50(3):1149-1157. doi: 10.26717/BJSTR.2023.50.007951.
- Kalhapure A, Kumar R, Singh VP, Pandey D. Hydrogels: A boon for increasing agricultural productivity in water-stressed environment. Current Science. 2016;1773-1779.
- Patra SK, Poddar R, Brestic M, Acharjee PU, Bhattacharya P, Sengupta S, Pal P, Bam N, Biswas B, Barek V. Prospects of hydrogels in agriculture for enhancing crop and water productivity under water deficit condition. International Journal of Polymer Science. 2022;2022. doi: 10.1155/2022/4914836.
- Qiu X, Hu S. "Smart" Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials (Basel). 2013 Feb 28;6(3):738-781. doi: 10.3390/ma6030738. PMID: 28809338; PMCID: PMC5512797.
- Maitra J, Shukla VK. Cross-linking in hydrogels: A review. Am J Polym Sci. 2014;4(2):25-31. doi: 10.5923/j.ajps.20140402.01.
- Hu W, Wang Z, Xiao Y, Zhang S, Wang J. Advances in crosslinking strategies of biomedical hydrogels. Biomater Sci. 2019 Feb 26;7(3):843-855. doi: 10.1039/c8bm01246f. PMID: 30648168.
- Bhatia S. Natural polymers vs. synthetic polymer. Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae. 2016. p.95-118.
- Chen S, Chen S, Jiang S, Mo Y, Tang J, Ge Z. Synthesis and characterization of siloxane sulfobetaine antimicrobial agents. Surface Science. 2011;605(11-12):L25-L28. doi: 10.1016/j.susc.2011.03.013.
- Chen S, Chen S, Jiang S, Mo Y, Luo J, Tang J, Ge Z. Study of zwitterionic sulfopropylbetaine containing reactive siloxanes for application in antibacterial materials. Colloids Surf B Biointerfaces. 2011 Jul 1;85(2):323-9. doi: 10.1016/j.colsurfb.2011.03.004. Epub 2011 Mar 29. PMID: 21450443.
- Chen S, Chen S, Jiang S, Xiong M, Luo J, Tang J, Ge Z. Environmentally friendly antibacterial cotton textiles finished with siloxane sulfopropylbetaine. ACS Appl Mater Interfaces. 2011 Apr;3(4):1154-62. doi: 10.1021/am101275d. Epub 2011 Mar 28. PMID: 21417413.
- Chen S, Chen S, Jiang S, Xiong M, Luo J, Tang J, Ge Z. Environmentally friendly antibacterial cotton textiles finished with siloxane sulfopropylbetaine. ACS Appl Mater Interfaces. 2011 Apr;3(4):1154-62. doi: 10.1021/am101275d. Epub 2011 Mar 28. PMID: 21417413.
- Oz Y, Sanyal A. The Taming of the Maleimide: Fabrication of Maleimide-Containing 'Clickable' Polymeric Materials. Chem Rec. 2018 Jun;18(6):570-586. doi: 10.1002/tcr.201700060. Epub 2017 Dec 29. PMID: 29286198.
- Jiang S, Zhang T. Studies of a biocompatible maleimide-modified dextran and hyaluronic acid hydrogel system. Fine Chemical Engineering. 2023;100-109. doi: 10.37256/fce.4220232705.
- Hall DJ, Van Den Berghe HM, Dove AP. Synthesis and post‐polymerization modification of maleimide‐containing polymers by ‘thiol‐ene’click and Diels-Alder chemistries. Polymer International. 2011;60(8):1149-1157.
- Guo X, Wang L, Wang S, Li Y, Zhang F, Song B, Zhao W. Syntheses of new chlorin derivatives containing maleimide functional group and their photodynamic activity evaluation. Bioorg Med Chem Lett. 2015 Oct 1;25(19):4078-81. doi: 10.1016/j.bmcl.2015.08.036. Epub 2015 Aug 18. PMID: 26306981.
- Oz Y, Barras A, Sanyal R, Boukherroub R, Szunerits S, Sanyal A. Functionalization of Reduced Graphene Oxide via Thiol-Maleimide "Click" Chemistry: Facile Fabrication of Targeted Drug Delivery Vehicles. ACS Appl Mater Interfaces. 2017 Oct 4;9(39):34194-34203. doi: 10.1021/acsami.7b08433. Epub 2017 Sep 21. PMID: 28905618.
- Zeng Z , Mo XM , He C , Morsi Y , El-Hamshary H , El-Newehy M . An in situ forming tissue adhesive based on poly(ethylene glycol)-dimethacrylate and thiolated chitosan through the Michael reaction. J Mater Chem B. 2016 Sep 7;4(33):5585-5592. doi: 10.1039/c6tb01475e. Epub 2016 Aug 8. PMID: 32263355.
- Smyth DG, Nagamatsu A, Fruton JS. Some reactions of N-ethylmaleimide1. Journal of the American Chemical Society. 1960;82(17):4600-4604.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.