Biology Group . 2023 June 16;4(6):1057-1068. doi: 10.37871/jbres1768.
Development of a New Anti-Rabies Antiviral: Effective Treatment with Beclin-1 and Lifespan Prolongation
Homeira Parizad1, Asghar Abdoli2, Ehsan Mostafavi3 and Farzaneh Sheikholeslami1*
2Department of Hepatitis and AIDS, Pasteur Institute of Iran, Tehran, Iran
3Department of Epidemiology and Biostatistics, Pasteur Institute of Iran, Tehran, Iran
- Beclin-1
- Lifespan prolongation
- Street rabies virus
- Autophagy
Abstract
Autophagy is a cellular mechanism that exerts antiviral effects in some virus-infected cells, while it can have opposite effects on some other cells. Rabies is an infectious disease caused by negative-stranded RNA virus. After the street Rabies Virus (RABV), the vector containing Beclin-1 were injected into the brains of mice. They were sacrificed to collect the samples. Next, the focus forming assay, TUNEL assay and q PCR were performed. The expression of MAP1LC3B, ATG5, and BECN1 genes and the relative mRNA expression of autophagy-related genes increased after in vivo transfection of the pIRES2-EGFP-Beclin-1 vector in test groups (p < 0.01). The number of viruses decreased in the brains of test group compared to the controls. Survival significantly increased in mice treated with the Beclin-1 containing vector compared to controls. The TUNEL assay did not indicate apoptosis. LC3 was notably expressed in the brain samples that were previously transfected with the pIRES-EGFP-Beclin-1 vector.
Abbreviations
ATGs: Autophagy-related Genes; BSR cells: Cells derived from Baby Hamster Kidney (BHK) cells; CVS-11: Challenge Virus Standard 11; DMEM: Dulbecco’s Modified Eagle Medium; FBS: Fetal Bovine Serum; FFA: Focus Forming Assay; hpi: hours post-infection; mTOR: Mammalian Target of Rapamycin; LC3: Microtubule-associated Protein 1A/1B Light Chain 3; PBS: Phosphate-Buffered Saline; RABV: Rabies Virus; SRABV: Street Rabies Virus; TUNEL: Terminal deoxynucleotidyl transferase dUTP Nick End Labeling
Introduction
Rabies is recognized as a public health threat and a well-known infectious disease. It is responsible for the death of nearly all infected patients following the emergence of symptoms. This disease is a major clinical challenge, as no effective medicine has been introduced for its treatment [1]. Rabies Virus (RABV) is a highly neurotropic virus, which belongs to the genus Lyssavirus and the family Rhabdoviridae [2]. The RABV genome is a single negative-stranded RNA of approximately 12 kb, encoding five structural proteins, that is, nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and large protein (L) [3]. Vaccination with dead RABV in conjugation with immunoglobulin injection is the only approved strategy to combat RABV infection [4]. Different strategies have been proposed to develop effective medications or increase the efficacy of treatments, such as vaccination with live genetically modified RABV to induce long-lasting immunity [5] and use of monoclonal antibodies to control rabies [6]. Autophagy (meaning “self-eating”) is a conserved process, implicated in several biological pathways, including the elimination of cancer cells, immunity against infectious diseases, and prevention of nutritional stress [7]. If autophagy is used as a defense mechanism against infection, it is referred to as xenophagy. It is described as a type of programmed cell death, controlled by many genes. This mechanism is involved in the removal or degradation of excess or injured cellular organelles to maintain homeostasis [8]. Different types of degradative autophagy include macroautophagy, chaperone-mediated autophagy, and microautophagy [9]. Macroautophagy is a vital mechanism, leading to the formation of a double-membrane vesicular structure, called autophagosome, which can degrade cytosolic materials and fuse with lysosomes to form autolysosomes. This process involves a set of proteins, encoded by Autophagy-Related Genes (ATGs) [10]. Microtubule-associated protein Light Chain 3 (LC3) is an autophagosome orthologue of ATG8 gene in yeast. LC3-II, a lipidic form of LC3, is an approved autophagosomal marker in neurodegenerative and neuromuscular diseases, tumorigenesis, and bacterial or viral infection [7,8]. Evidence suggests that autophagy is associated with innate and adaptive immunity, metabolic syndrome, infections, some cancers, and life span of some animals [11-13]. The role of autophagy in survival is represented by the complete knockout of some ATGs, such as ATG3, ATG5, and ATG7, resulting in neonatal death in mice [8]. The neuron-specific ATG gene knockout suggests that a basal rate of autophagy is required for normal neuronal survival [14,15]. Different reactions may occur in viral infections. While autophagy may show antiviral activities against some viruses [16-18] it may facilitate the replication of some viruses, such as dengue, hepatitis, and influenza viruses. Many viruses escape the degradation process of autophagy or even exploit autophagy for replication [19-25]. The response of autophagy to viral infection depends on both the host cell and virus type [26]. Beclin-1 (Atg6/Vps30) is a key regulator of autophagy, which belongs to the autophagy class III phosphatidylinositol 3-kinase complex. It plays an essential role in the construction of a lipid signaling molecule (PIP3 on phagophore membrane), utilization of downstream autophagy factors, and autophagosome formation. It is also involved in numerous biological processes, such as adaptation to stress, endocytosis, cytokinesis, immunity, tumorigenesis, aging, and cell death [27,28]. Beclin-1 expression, which regulates ß-amyloid accumulation in the brains of mice, decreases in the early stages of Alzheimer's disease [29] Beclin-1 diminishes α-synuclein accumulation in the limbic system of Parkinson’s models, increases lysosomal activation, and plays a crucial role in intracellular decadence of a-synuclein through the autophagy pathway [30]. Taji F, et al. [31] found that Beclin-1 overexpression in MDCK cells diminished telomerase activity and increased apoptosis. Generally, blockade of Beclin-1 autophagy and inhibition of Fas-mediated apoptosis by γ2-herpes virus Bcl-2 homolog (vBcl-2) are important signals for viral amplification [32].
A new promising approach is to use autophagy to eliminate RABV, as it acts against this obligate intracellular microorganism. The findings of a proteomics study on an animal model revealed that challenge virus standard-11 (CVS-11), a pathogenic laboratory strain, induced less autophagosome accumulation than SRV9 vaccine strain [33]. Moreover, Peng J, et al. [34] estimated the autophagy flux and found that infection with GD-SH-01, a pathogenic street strain, induced a complete autophagic response in humans, while fusion of autophagosomes with lysosomes was prevented in a mouse neuroblastoma cell line. Additionally, the feasibility of autophagy induction through the overexpression of exogenous Beclin-1 (reducing telomerase activity in the HeLa cell line) has been reported in previous research [35]. With this background in mind, the present study aimed to investigate autophagy induction through Beclin-1 overexpression in mice infected with pathogenic street rabies virus (SRABV).
Materials and Methods
Virus titration
In the GenBank, the accession number of SRABV strain used in the present study is KX148186 [36]. For in vitro virus titration, the entire brain was homogenized, and a 10% suspension was prepared from each sample. Next, 100 µL of Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 7% Fetal Bovine Serum (FBS), was added to each well of a 96-well microplate. Subsequently, serial dilutions of 10% suspensions were prepared with a dilution factor of three. Finally, 50 × 103 BSR cell suspensions were added to each well. The plates were incubated at 37ºC in a 5% CO2 atmosphere for 24 hours. Next, the wells were drained and rinsed with 200 µL of Phosphate-Buffered Saline (PBS). After the cells were fixed with 100 µL of cold acetone (80%) for 30 minutes, 40 µL of FITC-conjugated anti-nucleocapsid antibody was added to each well, and incubation was performed for 30 minutes at 37ºC. Next, the cells were rinsed with PBS three times (for 30 seconds, one minute, and five minutes, respectively). PBS containing 50% glycerol was then added to each well, and the microplates were examined under an inverted fluorescence microscope. The number of fluorescent foci per well was counted for each dilution, and titer was calculated in FFU/mL, based on the Reed-Muench method [37].
Plasmid construction
The pIRES2-EGFP vector (Clontech, Takara, Japan) was used to determine the transient expression of BECN1 gene in the mouse brain. The BECN1 gene, cloned on the pcDNA3.1 vector [38], was amplified using PCR-specific primers (Table 1). Next, an EcoRI restriction site was added to the forward primer, and the His-tag sequence and BamHI were added to the reverse primer. The PCR product was then sub-cloned on the pIRES2-EGFP vector, using a T4 DNA ligase kit (Thermo Scientific, USA). The cloning accuracy was confirmed by sequencing the cloned fragment in Mega 7 software [39].
| Table1: Primers used throughout this study. | |||
| Gene | Primer | Product Size | Used for |
| Beclin1 | F: 5′ GTTGAATTCGCCACCATGGAAGGGTCTAAG 3′ R: 5′ GCGGATCCTCAGTGATGGTGATGGTGATGTTTGTTATAAA 3′ |
1431bp | Cloning- PCR |
| Map1lc3b | F: 5′ GACGGCTTCCTGTACATGGTTT 3′ R: 5′ TGGAGTCTTACACAGCCATTGC 3′ |
70bp | RT-PCR |
| Atg5 | F: 5′ GAGCGGCCTTTCATCCA 3′ R: 5′ CCTGGCTCCTCTTCTCTCCA 3′ |
130bp | RT-PCR |
| Beclin1 | F: 5′ TGCAGGTGAGCTTCGTGTG 3′ R: 5′ CTGGGCTGTGGTAAGTAATGGAG 3′ |
119bp | RT-PCR |
| Hprt | F: 5′ CTCAACTTTAACTGGAAAGAATGT 3′ R: 5′ GGGCTGTACTGCTTAACC 3′ |
99bp | RT-PCR |
| gfp | F: 5′ GACGGCTTCCTGTACATGGTTTGG 3′ R: 5′ TGGAGTCTTACACAGCCATTGCG 3′ |
712bp | PCR |
In vivo and in vitro assessments of gene expression in the pIRES2-EGFP-Beclin-1 vector
The in vitro and in vivo assessments were performed based on the function of pIRES2-EGFP-Beclin-1 expression vector. The Chinese Hamster Ovary (CHO) cells were cultured in DMEM (Thermo Fisher Scientific, USA) supplemented with fetal calf serum (10%, v/v) and transfected with the pIRES2-EGFP-Beclin-1 vector using Lipofectamine 2000 (Thermo Scientific, USA). The transfection efficiency was more than 60% .The in vitro functional expression of the transfected vector was examined after 48 hours. The post-transfection examination was performed under a UV microscope. To evaluate Beclin-1 protein expression, Western blotting and a His-tagged antibody were used. The in vivo-jetPEI™ reagent is a cationic transfection polymer used for effective and reproducible gene delivery in vivo, without cell toxicity. In the next stage, 3 µg of the vector (pIRES2-EGFP or pIRES2-EGFP-Beclin-1) was injected into the brains of mice, using a Polyplus Transfection Kit (Polyplus, France) [40-42]. The mice were then scarified, and brain samples were collected from the injection site at 24 hours post-vector transfection in vivo. Finally, RNA was extracted using a Trizol reagent, and PCR assay was carried out using specific primers.
Groups
Male NMRI mice, weighing 20 ± 5 g, were used in this study. To insert a cannula, a stereotaxic device was used to drill 0.6 mm behind the Bregma, 1 mm to the right, on the cortex of the brain to a depth of 0.5 mm. All injections were performed intracerebroventricularly (ICV), and all samples were obtained from the same site. Seven groups of NMRI mice (eight animals per group) were used to evaluate the effects of pIRES-EGFP-Beclin-1 vector injection on autophagy [42]. The control group received no treatment other than saline, while the second and third groups received 3 µg of pIRES-EGFP and pIRES-EGFP-Beclin-1 vectors via unilateral ICV injections, respectively. Of eight mice per group, six were used for a real-time PCR assay, and two were used for a histological analysis. The fourth group received SRABV (LD50 10-8.398/0.03mL) without any medications, while the fifth group received SRABV (LD50 10-8.398/0.03mL), as well as 3 µg of pIRES-EGFP-Beclin-1 vector at one hour post-virus inoculation (hpi). For survival analysis, both groups were maintained until death. Two other groups (groups 6 and 7), similar to the fourth and fifth groups, were also included, but all mice were sacrificed five days after injection for a titration assay.
Gene expression according to real-time PCR assay
Total RNA was extracted from the prefrontal cortex tissue of mice, using a Trizol reagent (Bio-Basic, Canada) in each group. For cDNA synthesis, the Thermo Scientific RevertAid First-Strand cDNA Synthesis Kit (K1622, Thermo Scientific, USA) was used according to the manufacturer’s instructions. Next, real-time PCR was conducted with SYBR Green Master Mix (Takara, Japan) on a Corbet-600 system. Table 1 presents the PCR primers and sequences. Real-time PCR was performed for MAP1LC3b, ATG5, and BECN1 genes. The Hprt gene was used as the housekeeping gene to normalize the gene expression and its stability checked by Best keeper program [43]. For all real-time PCR runs, melting curves were plotted, and the PCR products were run on 2% agarose gel.
Immunohistochemistry
For a histological analysis, transcardiac perfusion was performed with 10% paraformaldehyde, and the animals’ brain cortex samples were placed in 10% formalin for 72 hours after anesthesia induction with a combination of ketamine (100 mg/kg) and xylene (10 mg/kg). Next, the samples were cut into 4-µm sections after collecting paraffin-embedded specimens. The slides were first incubated with LC3B polyclonal antibody (1:200, Novus Biotech, USA) and then, with a goat HRP-conjugated antibody (1:500, Novus Biotech, USA). A normal brain sample was used as a negative control. The chromogenic reaction was developed using 3, 3-diaminobenzidine (DAB) tetrahydrochloride. Finally, the sections were counterstained with Mayer’s hematoxylin solution, mounted, and observed under a light microscope.
TUNEL assay
The TUNEL assay was conducted on tissue slides obtained from the paraffin-embedded brain tissues of mice at 24 and 72 hours post-infection (hpi). A positive control (mouse brain cancer cells) and a negative control (normal mouse brain cells) were included by the manufacturer in the TUNEL assay kit. This assay was performed according to the manufacturer’s protocol (TUNEL Assay Kit-HRP-DAB, ab206386, Abcam, UK). Counterstaining was also performed using methyl green to evaluate and characterize normal and apoptotic cells morphologically.
Statistical analysis
A real-time PCR assay was conducted using LinRegPCR and REST 2009. GraphPad Prism version 8.0 was also used for statistical analysis, and ImageJ version 1.51 (2018) was used to convert images into quantifiable data. Besides, Mann-Whitney and Kruskal-Wallis tests were performed to calculate fold change differences between the groups. Additionally, Kaplan-Meier test was employed for survival analysis. The level of statistical significance was set at p < 0.05.
Results
Virus titration
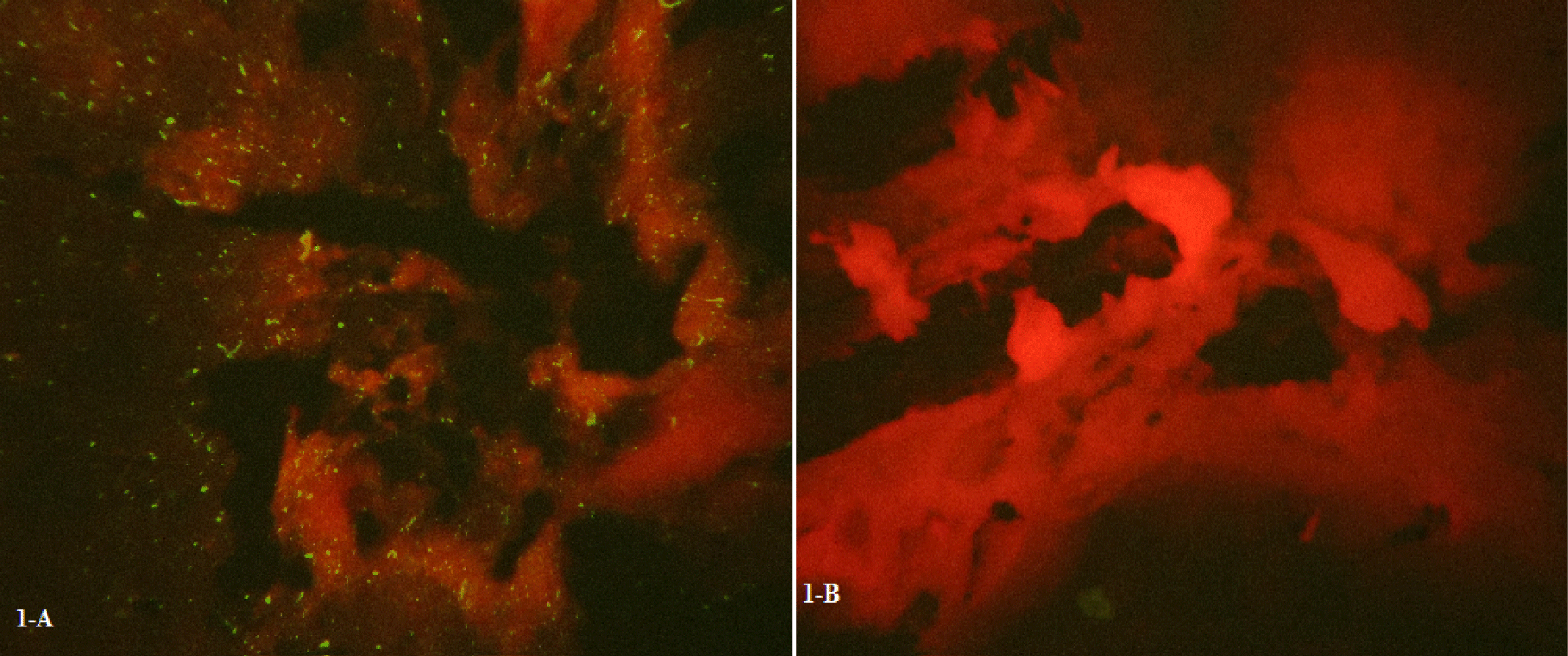
The brains of NMRI mice were inoculated with 10% SRABV-infected brain homogenates to evaluate SRABV pathogenicity. The Focus Forming Assay (FFA) was then conducted by infecting mouse brain cells with SRABV. Bright green spots of Negri bodies were clearly observed under UV light using a fluorescence microscope. Figures 1A,B represent in vivo samples which have prepared using a fluorescent antibody test.
Expression of exogenous BECN1 gene
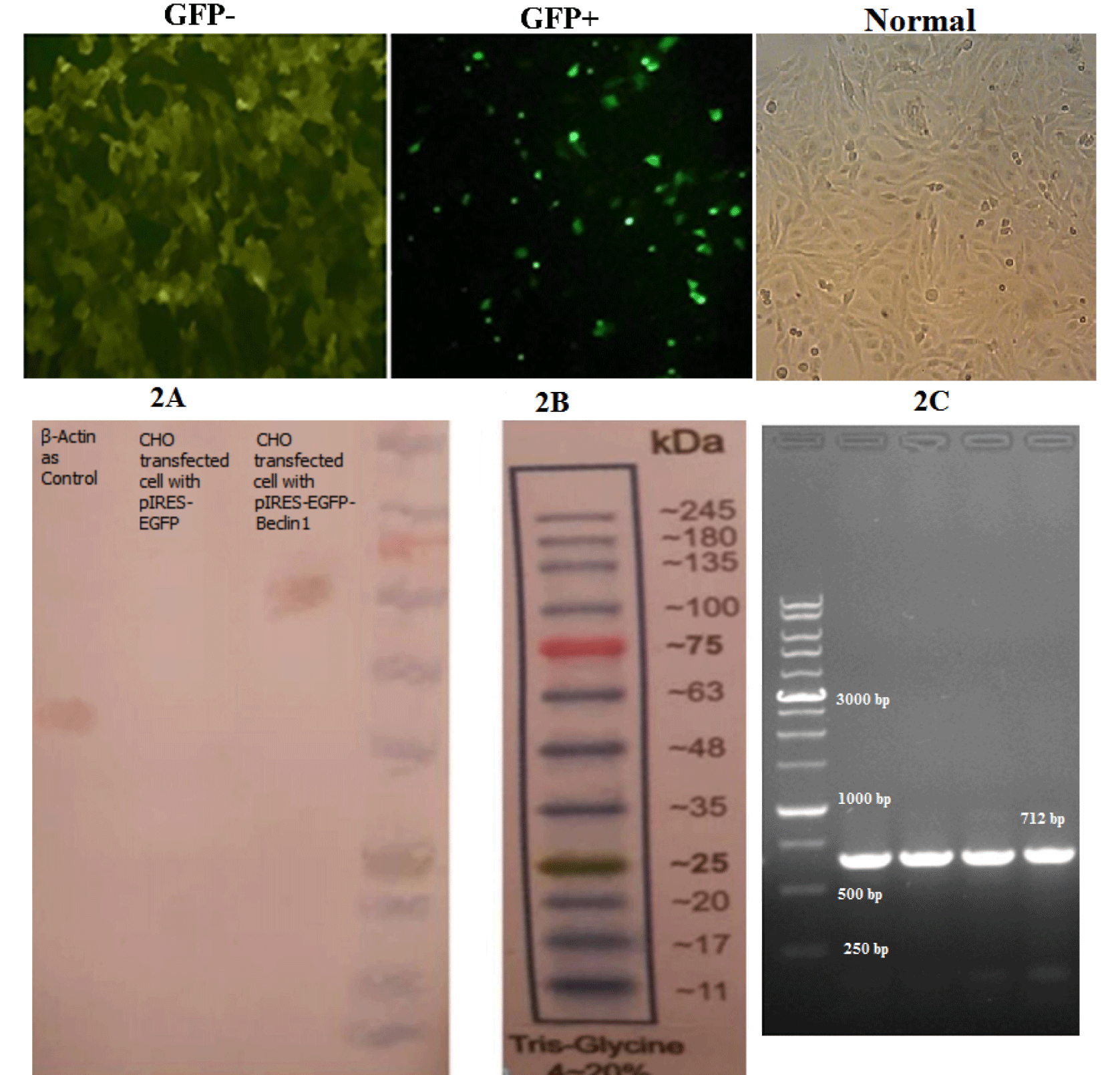
In vitro and in vivo experiments were conducted to confirm the exogenous expression of BECN1 gene. Based on the in vitro test, the CHO cells (Figure 2C) were transfected with the pIRES-EGFP-Beclin-1 vector using Lipofectamine, and the cells were evaluated via UV microscopy, which indicated the remarkable expression of Green Fluorescent Protein (GFP) compared to uninfected or normal CHO cells (Figures 2A,B). Protein was then extracted from transfected CHO cells, and Western blotting was carried out. The results indicated the acceptable expression of recombinant Beclin-1 protein (Figure 2D). After in vivo transfection of the vector, a PCR assay was carried out using specific primers and brain cortex samples, collected from the injection site to confirm the expression of GFP in mice (Figure 2E).
Changes in the expression of ATGs after SRABV and vector inoculation
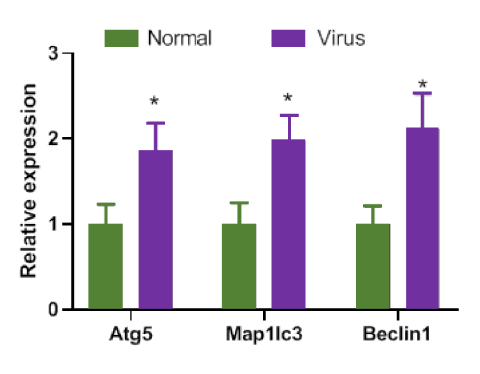
The expression of ATGs in response to SRABV inoculation changed at mRNA levels compared to basal levels. The MAP1LC3b, ATG5, and especially BECN1 genes showed significant changes in mice compared to the control group (*p < 0.05, Figure 3). To evaluate the effect of treatment with exogenous Beclin-1 on the expression of ATGs, samples were collected 24 hours after injecting the saline in normal mice (control), 24 hours after injecting the pIRES2-EGFP vector in the sham group, and 24 hours after transfection with the pIRES-EGFP-Beclin-1 vector in the test group.
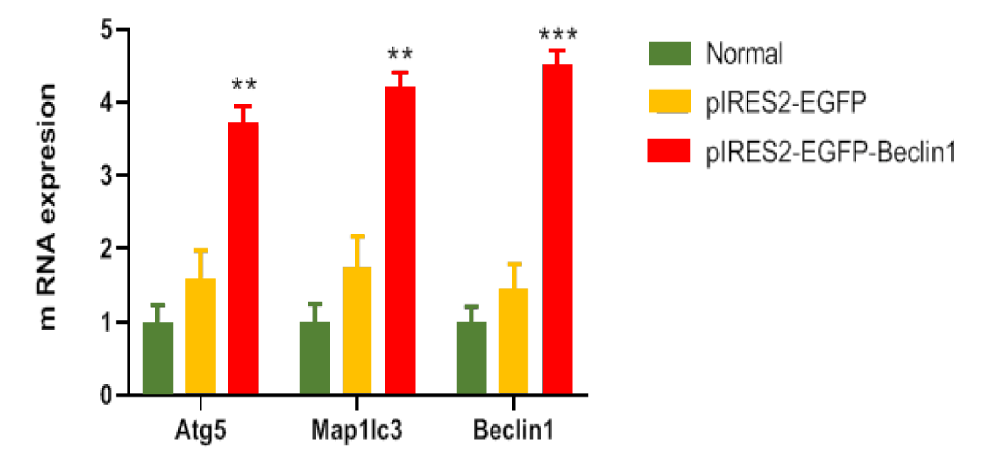
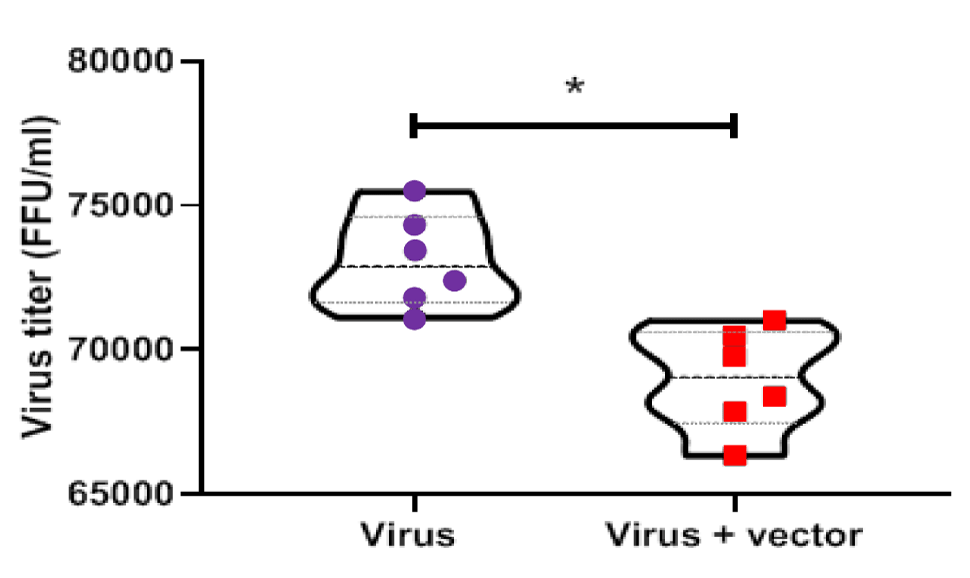
Based on the gene expression analysis, there was a significant increase in the expression of ATGs in the test group (**p < 0.01 and ***p < 0.001); in other words, gene expression was upregulated in the test group (Figure 4). Moreover, the virus titer was calculated five days after inoculation with SRABV in two groups of mice, that is, mice inoculated with 8.396 LD50 of SRABV without treatment and mice inoculated with (LD50 10-8.398/0.03mL) of SRABV and transfected with the pIRES-EGFP-Beclin-1 vector at 1 hpi. Based on the results, a low virus titer was observed in the test group compared to the control group (*p < 0.05) (Figure 5).
Relationship between survival and Beclin-1 overexpression
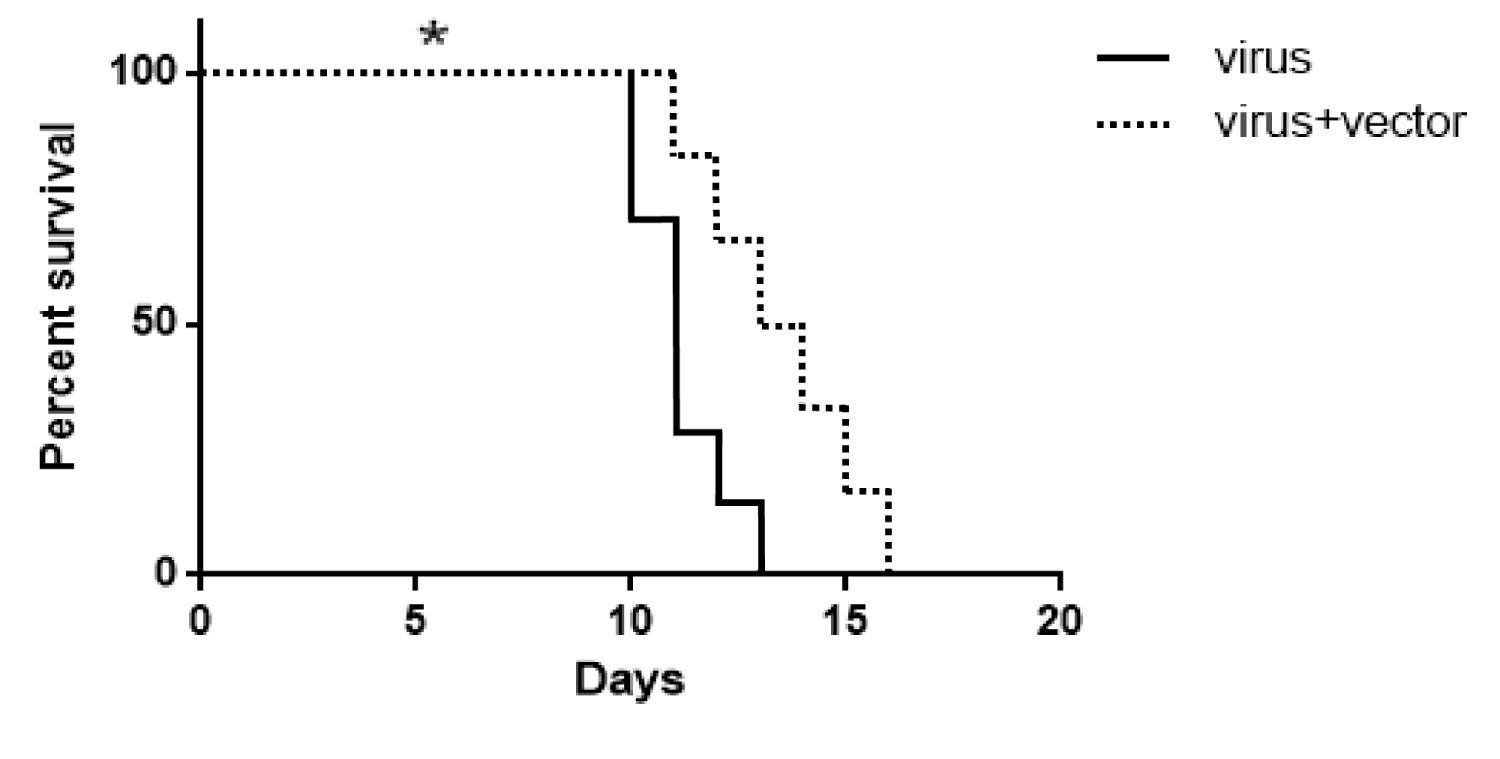
The effect of exogenous Beclin-1 expression on the survival of rabid mice was evaluated in the present study. The control group (n = 7) was inoculated with SRABV and maintained until death, while another group of mice was inoculated with SRABV and transfected with Beclin-1-containing vector at 1 hpi. The Kaplan-Meier method was used for survival analysis. Based on the results, the lifespan increased significantly in rabid mice treated with the pIRES-EGFP-Beclin-1 vector compared to the control group (*p < 0.05) (Figure 6).
Apoptosis in pIRES-EGFP-Beclin-1 vector-treated mice
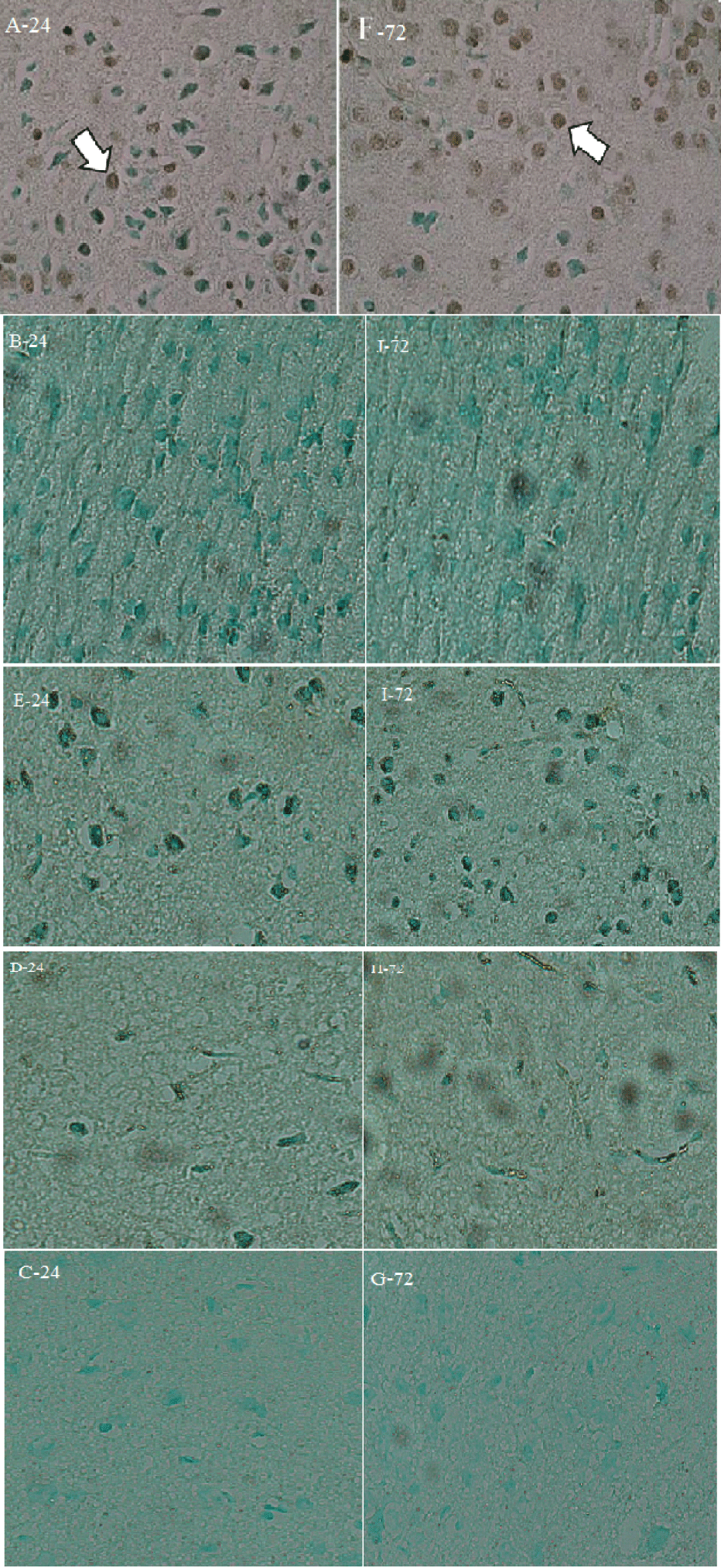
A TUNEL assay was carried out at 24 and 72 hpi to examine apoptosis in all groups. The assay images clearly indicated the presence of apoptosis only in A and F (Figure 7). In the comparison of findings and images, no significant difference was observed between the groups. All images were acquired at 40× magnification, and two mice were studied in each group.
LC3 and Beclin-1 overexpression
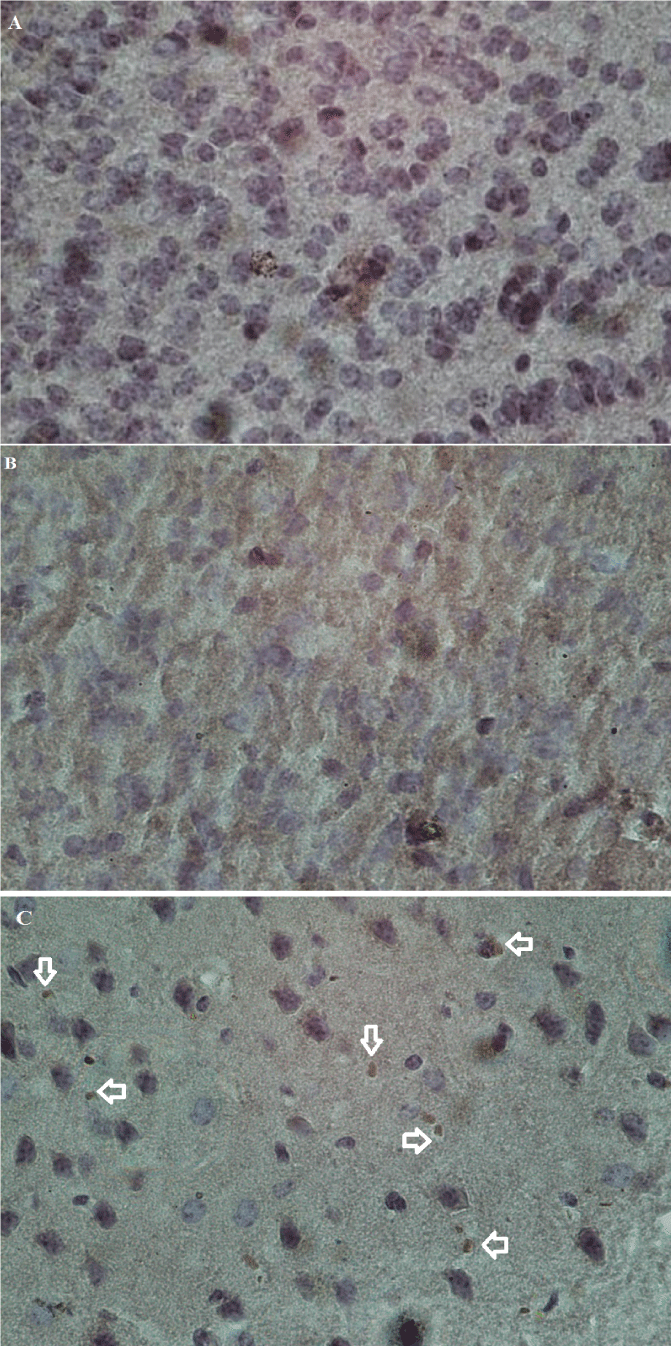
As mentioned earlier, two mice were studied in each group to confirm the presence of LC3 immunohistochemically. All images were acquired at 40× magnification (Figure 8). The results quantified by ImageJ revealed autophagy-related proteins. LC3, as a marker of autophagosome formation, was expressed in the brain samples transfected with the pIRES-EGFP-Beclin-1 vector. There was a slight LC3 expression in samples transfected with the pIRES2-EGFP vector (**p < 0.01) (Figure 9).
Discussion
Rabies is a zoonotic disease which cannot be treated after the emergence of its clinical signs. Considering the high fatality of this disease, finding an effective treatment for infected individuals is a major challenge for researchers. Autophagy involves various pathways, which are used by cells to provide lysosomes with cytoplasmic components for demolition [44]. It plays a crucial role in preventing necrosis, inflammation, neuropathies, ischemia, infections, and many other diseases. Researchers hope to increase the lifespan of these patients by reducing the complications. Some studies have reported the in vitro antiviral activity of autophagy in humans, as well as mouse neuroblastoma cell lines [31]. They also suggested that autophagy is induced by binding of the virus to Hsp90AA1 and inactivation of AKT-mTOR pathway [45], while the opposite occurs in some viruses [46]. The function of autophagy depends on the type of host cells and the virus [47]. Some viruses use the autophagy system components for their growth and replication, while autophagic proteins are mostly involved in a process, called xenophagy, by targeting viruses for destruction by lysosomes [48]. Besides, they initiate innate or adaptive immune responses against viral infections [49,50]. In line with previous research [51], the present study aimed to increase the survival of SRBV-infected mice by activating autophagy. Autophagy was induced by administering exogenous Beclin-1 into the brains of rabid mice. The results confirmed that autophagy induction led to an increase in the lifespan of rabid mice, which might be related to the antiviral activity of autophagy. Also, exogenous Beclin-1 could induce autophagy through antiviral activity. The increased expression of LC3II-dependent genes confirmed autophagosome formation. Additionally, the titer of SRABV decreased during autophagy induction in infected mice. These findings highlight the capacity of autophagy to fight against SRABV in infected mice. However, the mechanism of autophagy induction by SRABV has not been identified yet.
Therefore, the overexpression of Beclin-1 in the present study might have contributed to the degradation of SRABV by autophagy. In this regard, Orvedahl A, et al. [52] reported that lack of ATG5 function increases the risk of infection in mice with fatal Sindbis virus. They found that autophagy contributes to the clearance of this virus from the central nervous system. Moreover, Pyo JO, fig. [53] found that moderate overexpression of ATG5 genes in ATG5 transgenic mice intensified autophagy, and mice showed anti-aging phenotypes (e.g., weight loss, enhanced insulin sensitivity, and improved motor activity). Moreover, the increased lifespan of transgenic mice could be related to an enhanced autophagic activity.
The current results showed that an increase in the expression of exogenous Beclin-1 in the brain cells of SRABV-infected mice prevented apoptosis. Also, a TUNEL assay was performed to observe the effects of Beclin-1 and other vectors on apoptosis in the brain cells of mice. The results failed to represent any significant increase in apoptosis in the groups. Neither the vectors, nor Beclin-1 caused apoptosis in the brain cells of mice. This study presented important findings about the behavior of SRABV in autophagy and apoptosis in mouse models. Although there is a large body of in vivo studies, only few studies have focused on this type of RABV.
Conclusion
By in vivo transfection of Beclin-1 containing vector into the brain of RABV infected mice, significant changes were observed in the relative mRNA expression of autophagy-related genes compared to the normal mice. In addition, a decrease in virus titer and an increase in the lifespan of test group were seen compared to controls. The authors believe that extensive studies are necessary to survey autophagic response of a pathogenic wild strain such as KX148186 in humans.
Ethical Approval
The animals were maintained according to the National Institutes of Health (NIH) guidelines for animal care and control. They were kept in separate cages in a room with controlled temperature in a 12:12 h light/dark cycle at humidity of 40-60% with free access to food and water during the study. All experimental protocols in the present study were approved by the Institutional Animal Ethics Committee of Pasteur Institute of Iran (certificate No.: IR.PII.REC.1395.49).
Funding
This work was financially supported by a grant (No. 565) by Pasteur Institute of Iran.
Credit Authorship Contribution Statement
Homeira Parizad: Performed the experiments; Gathered data. Asghar Abdoli: Carried out the methodology. Ehsan Mostafavi: Performed the statistical analysis. Farzaneh Sheikholeslami: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
CCompeting Interest
All authors declare that they have no competing financial interests.
Availability of Data
The datasets used and/or analyzed in the current study are available from the corresponding authors upon reasonable request.
References
- Bourhy H, Reynes JM, Dunham EJ, Dacheux L, Larrous F, Huong VTQ, Xu G, Yan J, Miranda MEG, Holmes EC. The origin and phylogeography of dog rabies virus. J Gen Virol. 2008 Nov;89(Pt 11):2673-2681. doi: 10.1099/vir.0.2008/003913-0. PMID: 18931062; PMCID: PMC3326349.
- Yamaoka S, Ito N, Ohka S, Kaneda S, Nakamura H, Agari T, Masatani T, Nakagawa K, Okada K, Okadera K, Mitake H, Fujii T, Sugiyama M. Involvement of the rabies virus phosphoprotein gene in neuroinvasiveness. J Virol. 2013 Nov;87(22):12327-38. doi: 10.1128/JVI.02132-13. Epub 2013 Sep 11. PMID: 24027304; PMCID: PMC3807887.
- Finke S, Mueller-Waldeck R, Conzelmann KK. Rabies virus matrix protein regulates the balance of virus transcription and replication. J Gen Virol. 2003 Jun;84(Pt 6):1613-1621. doi: 10.1099/vir.0.19128-0. PMID: 12771432.
- Zhu S, Guo C. Rabies Control and Treatment: From Prophylaxis to Strategies with Curative Potential. Viruses. 2016 Oct 28;8(11):279. doi: 10.3390/v8110279. PMID: 27801824; PMCID: PMC5127009.
- Shuai L, Feng N, Wang X, Ge J, Wen Z, Chen W, Qin L, Xia X, Bu Z. Genetically modified rabies virus ERA strain is safe and induces long-lasting protective immune response in dogs after oral vaccination. Antiviral Res. 2015 Sep;121:9-15. doi: 10.1016/j.antiviral.2015.06.011. Epub 2015 Jun 18. PMID: 26093157.
- Sparrow E, Torvaldsen S, Newall AT, Wood JG, Sheikh M, Kieny MP, Abela-Ridder B. Recent advances in the development of monoclonal antibodies for rabies post exposure prophylaxis: A review of the current status of the clinical development pipeline. Vaccine. 2019 Oct 3;37 Suppl 1:A132-A139. doi: 10.1016/j.vaccine.2018.11.004. Epub 2018 Nov 29. PMID: 30503659.
- Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, Cuervo AM, Debnath J, Deretic V, Dikic I, Eskelinen EL, Fimia GM, Fulda S, Gewirtz DA, Green DR, Hansen M, Harper JW, Jäättelä M, Johansen T, Juhasz G, Kimmelman AC, Kraft C, Ktistakis NT, Kumar S, Levine B, Lopez-Otin C, Madeo F, Martens S, Martinez J, Melendez A, Mizushima N, Münz C, Murphy LO, Penninger JM, Piacentini M, Reggiori F, Rubinsztein DC, Ryan KM, Santambrogio L, Scorrano L, Simon AK, Simon HU, Simonsen A, Tavernarakis N, Tooze SA, Yoshimori T, Yuan J, Yue Z, Zhong Q, Kroemer G. Molecular definitions of autophagy and related processes. EMBO J. 2017 Jul 3;36(13):1811-1836. doi: 10.15252/embj.201796697. Epub 2017 Jun 8. PMID: 28596378; PMCID: PMC5494474.
- Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010 Sep;12(9):823-30. doi: 10.1038/ncb0910-823. PMID: 20811354; PMCID: PMC3127249.
- Tamargo-Gómez I, Mariño G. AMPK: Regulation of Metabolic Dynamics in the Context of Autophagy. Int J Mol Sci. 2018 Nov 29;19(12):3812. doi: 10.3390/ijms19123812. PMID: 30501132; PMCID: PMC6321489.
- Dong X, Levine B. Autophagy and viruses: adversaries or allies? J Innate Immun. 2013;5(5):480-93. doi: 10.1159/000346388. Epub 2013 Jan 31. PMID: 23391695; PMCID: PMC3790331.
- Madeo F, Zimmermann A, Maiuri MC, Kroemer G. Essential role for autophagy in life span extension. J Clin Invest. 2015 Jan;125(1):85-93. doi: 10.1172/JCI73946. Epub 2015 Jan 2. PMID: 25654554; PMCID: PMC4382258.
- Nakamura S, Yoshimori T. Autophagy and Longevity. Mol Cells. 2018 Jan 31;41(1):65-72. doi: 10.14348/molcells.2018.2333. Epub 2018 Jan 23. PMID: 29370695; PMCID: PMC5792715.
- Seah NE, de Magalhaes Filho CD, Petrashen AP, Henderson HR, Laguer J, Gonzalez J, Dillin A, Hansen M, Lapierre LR. Autophagy-mediated longevity is modulated by lipoprotein biogenesis. Autophagy. 2016;12(2):261-72. doi: 10.1080/15548627.2015.1127464. PMID: 26671266; PMCID: PMC4836030.
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006 Jun 15;441(7095):885-9. doi: 10.1038/nature04724. Epub 2006 Apr 19. PMID: 16625204.
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006 Jun 15;441(7095):880-4. doi: 10.1038/nature04723. Epub 2006 Apr 19. PMID: 16625205.
- Derrien B, Baumberger N, Schepetilnikov M, Viotti C, De Cillia J, Ziegler-Graff V, Isono E, Schumacher K, Genschik P. Degradation of the antiviral component ARGONAUTE1 by the autophagy pathway. Proc Natl Acad Sci U S A. 2012 Sep 25;109(39):15942-6. doi: 10.1073/pnas.1209487109. Epub 2012 Sep 10. Erratum in: Proc Natl Acad Sci U S A. 2019 Jul 2;116(27):13703-13705. PMID: 23019378; PMCID: PMC3465452.
- Lee HK, Iwasaki A. Autophagy and antiviral immunity. Curr Opin Immunol. 2008 Feb;20(1):23-9. doi: 10.1016/j.coi.2008.01.001. Epub 2008 Feb 8. PMID: 18262399; PMCID: PMC2271118.
- Nakamoto M, Moy RH, Xu J, Bambina S, Yasunaga A, Shelly SS, Gold B, Cherry S. Virus recognition by Toll-7 activates antiviral autophagy in Drosophila. Immunity. 2012 Apr 20;36(4):658-67. doi: 10.1016/j.immuni.2012.03.003. Epub 2012 Mar 29. PMID: 22464169; PMCID: PMC3334418.
- Dreux M, Gastaminza P, Wieland SF, Chisari FV. The autophagy machinery is required to initiate hepatitis C virus replication. Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):14046-51. doi: 10.1073/pnas.0907344106. Epub 2009 Aug 3. PMID: 19666601; PMCID: PMC2729017.
- Khandia R, Dadar M, Munjal A, Dhama K, Karthik K, Tiwari R, Yatoo MI, Iqbal HMN, Singh KP, Joshi SK, Chaicumpa W. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells. 2019 Jul 3;8(7):674. doi: 10.3390/cells8070674. PMID: 31277291; PMCID: PMC6678135.
- Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C, Jung JU. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol. 2008 Jul;10(7):776-87. doi: 10.1038/ncb1740. Epub 2008 Jun 15. PMID: 18552835; PMCID: PMC2878716.
- McLean JE, Wudzinska A, Datan E, Quaglino D, Zakeri Z. Flavivirus NS4A-induced autophagy protects cells against death and enhances virus replication. J Biol Chem. 2011 Jun 24;286(25):22147-59. doi: 10.1074/jbc.M110.192500. Epub 2011 Apr 21. PMID: 21511946; PMCID: PMC3121359.
- Sir D, Tian Y, Chen WL, Ann DK, Yen TS, Ou JH. The early autophagic pathway is activated by hepatitis B virus and required for viral DNA replication. Proc Natl Acad Sci U S A. 2010 Mar 2;107(9):4383-8. doi: 10.1073/pnas.0911373107. Epub 2010 Feb 8. PMID: 20142477; PMCID: PMC2840127.
- Wileman T. Aggresomes and autophagy generate sites for virus replication. Science. 2006 May 12;312(5775):875-8. doi: 10.1126/science.1126766. PMID: 16690857.
- Zhou Z, Jiang X, Liu D, Fan Z, Hu X, Yan J, Wang M, Gao GF. Autophagy is involved in influenza A virus replication. Autophagy. 2009 Apr;5(3):321-8. doi: 10.4161/auto.5.3.7406. Epub 2009 Apr 14. PMID: 19066474.
- Jackson WT. Viruses and the autophagy pathway. Virology. 2015 May;479-480:450-6. doi: 10.1016/j.virol.2015.03.042. Epub 2015 Apr 6. PMID: 25858140; PMCID: PMC5917100.
- Houtman J, Freitag K, Gimber N, Schmoranzer J, Heppner FL, Jendrach M. Beclin1-driven autophagy modulates the inflammatory response of microglia via NLRP3. EMBO J. 2019 Feb 15;38(4):e99430. doi: 10.15252/embj.201899430. Epub 2019 Jan 7. PMID: 30617086; PMCID: PMC6376276.
- Kaur S, Changotra H. The beclin 1 interactome: Modification and roles in the pathology of autophagy-related disorders. Biochimie. 2020 Aug;175:34-49. doi: 10.1016/j.biochi.2020.04.025. Epub 2020 May 16. PMID: 32428566.
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008 Jun;118(6):2190-9. doi: 10.1172/JCI33585. PMID: 18497889; PMCID: PMC2391284.
- Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci. 2009 Oct 28;29(43):13578-88. doi: 10.1523/JNEUROSCI.4390-09.2009. PMID: 19864570; PMCID: PMC2812014.
- Taji F, Abdoli A, Baesi K, Sheikholeslami F, Kouchesfahani HM. Overexpression of Beclin1 gene leads to reduction of telomerase activity in MDCK cells and enhances apoptosis. J Cancer Res Ther. 2021 Jan-Mar;17(1):225-230. doi: 10.4103/jcrt.JCRT_265_17. PMID: 33723159.
- Liang C, E X, Jung JU. Downregulation of autophagy by herpesvirus Bcl-2 homologs. Autophagy. 2008 Apr;4(3):268-72. doi: 10.4161/auto.5210. Epub 2007 Oct 29. PMID: 17993780.
- Li L, Jin H, Wang H, Cao Z, Feng N, Wang J, Zhao Y, Zheng X, Hou P, Li N, Chi H, Huang P, Jiao C, Li Q, Wang L, Wang T, Sun W, Gao Y, Tu C, Hu G, Yang S, Xia X. Autophagy is highly targeted among host comparative proteomes during infection with different virulent RABV strains. Oncotarget. 2017 Mar 28;8(13):21336-21350. doi: 10.18632/oncotarget.15184. PMID: 28186992; PMCID: PMC5400588.
- Peng J, Zhu S, Hu L, Ye P, Wang Y, Tian Q, Mei M, Chen H, Guo X. Wild-type rabies virus induces autophagy in human and mouse neuroblastoma cell lines. Autophagy. 2016 Oct 2;12(10):1704-1720. doi: 10.1080/15548627.2016.1196315. Epub 2016 Jul 27. PMID: 27463027; PMCID: PMC5079669.
- Taji F, Kouchesfahani HM, Sheikholeslami F, Romani B, Baesi K, Vahabpour R, Edalati M, Teimoori-Toolabi L, Jazaeri EO, Abdoli A. Autophagy induction reduces telomerase activity in HeLa cells. Mech Ageing Dev. 2017 Apr;163:40-45. doi: 10.1016/j.mad.2016.12.011. Epub 2016 Dec 30. PMID: 28043814.
- Hosseini Heydarabadi F, Baessi K, Bashar R, Fazeli M, Sheikholeslami F. A phylogenetic study of new rabies virus strains in different regions of Iran. Virus Genes. 2020 Jun;56(3):361-368. doi: 10.1007/s11262-020-01752-6. Epub 2020 Mar 31. PMID: 32236772.
- Bourhy H, Sureau P. Laboratory methods for rabies diagnosis (Institut Pasteur). 1990.
- Prizad H, Sheikholeslami F, Mahmoudi M, Fazeli M, Fadajan Z. The role of assaying recombinant Beclin1 by in vitro and in vivo tests. Gene Reports. 2021;24:101221. doi: 10.1016/j.genrep.2021.101221.
- Goula D, Remy JS, Erbacher P, Wasowicz M, Levi G, Abdallah B, Demeneix BA. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Ther. 1998 May;5(5):712-7. doi: 10.1038/sj.gt.3300635. PMID: 9797878.
- Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013 Oct;4(4):303-6. doi: 10.4103/0976-500X.119726. PMID: 24250214; PMCID: PMC3826013.
- Demeneix BA, Ghorbel M, Goula D. Optimizing polyethylenimine-based gene transfer into mammalian brain for analysis of promoter regulation and protein function. Methods Mol Biol. 2000;133:21-35. doi: 10.1385/1-59259-215-5:21. PMID: 10561828.
- Demeneix B, Behr J, Boussif O, Zanta MA, Abdallah B, Remy J. Gene transfer with lipospermines and polyethylenimines. Adv Drug Deliv Rev. 1998 Mar 2;30(1-3):85-95. doi: 10.1016/s0169-409x(97)00109-9. PMID: 10837604.
- Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004 Mar;26(6):509-15. doi: 10.1023/b:bile.0000019559.84305.47. PMID: 15127793.
- Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011 Sep 2;146(5):682-95. doi: 10.1016/j.cell.2011.07.030. PMID: 21884931.
- Hu B, Zhang Y, Jia L, Wu H, Fan C, Sun Y, Ye C, Liao M, Zhou J. Binding of the pathogen receptor HSP90AA1 to avibirnavirus VP2 induces autophagy by inactivating the AKT-MTOR pathway. Autophagy. 2015;11(3):503-15. doi: 10.1080/15548627.2015.1017184. PMID: 25714412; PMCID: PMC4502722.
- Surviladze Z, Sterk RT, DeHaro SA, Ozbun MA. Cellular entry of human papillomavirus type 16 involves activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway and inhibition of autophagy. J Virol. 2013 Mar;87(5):2508-17. doi: 10.1128/JVI.02319-12. Epub 2012 Dec 19. PMID: 23255786; PMCID: PMC3571372.
- Kudchodkar SB, Levine B. Viruses and autophagy. Rev Med Virol. 2009 Nov;19(6):359-78. doi: 10.1002/rmv.630. PMID: 19750559; PMCID: PMC2852112.
- Tallóczy Z, Virgin HW 4th, Levine B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy. 2006 Jan-Mar;2(1):24-9. doi: 10.4161/auto.2176. Epub 2006 Jan 15. PMID: 16874088.
- Chiramel AI, Brady NR, Bartenschlager R. Divergent roles of autophagy in virus infection. Cells. 2013 Jan 25;2(1):83-104. doi: 10.3390/cells2010083. PMID: 24709646; PMCID: PMC3972664.
- Espert L, Codogno P, Biard-Piechaczyk M. Involvement of autophagy in viral infections: antiviral function and subversion by viruses. J Mol Med (Berl). 2007 Aug;85(8):811-23. doi: 10.1007/s00109-007-0173-6. Epub 2007 Mar 6. PMID: 17340132; PMCID: PMC7080067.
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010 May;221(1):3-12. doi: 10.1002/path.2697. PMID: 20225336; PMCID: PMC2990190.
- Orvedahl A, MacPherson S, Sumpter R Jr, Tallóczy Z, Zou Z, Levine B. Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe. 2010 Feb 18;7(2):115-27. doi: 10.1016/j.chom.2010.01.007. PMID: 20159618; PMCID: PMC2860265.
- Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, Jung S, Jung YK. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun. 2013;4:2300. doi: 10.1038/ncomms3300. PMID: 23939249; PMCID: PMC3753544.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.