Biology Group . 2023 June 24;4(6):1072-1082. doi: 10.37871/jbres1770.
Mushrooms and Lichens the Factory of Important Secondary Metabolites: Review
Waill A Elkhateeb*, Dina E El-Ghwas and Daba GM
- Biological activities
- Wild mushrooms
- Lichens
- Natural products
- Secondary metabolites
Abstract
Mushrooms and lichens are plentiful source of nutritional and medicinal compounds. However, medicinal uses of the mushrooms and lichens still need to be worked out for their biological activities. Mushrooms and Lichens have reserved their position centuries ago as food and medicine. They are rich in nutrients and in biologically active compounds that belong to different chemical classes. Capabilities of different members of mushrooms and Lichens have encouraged researchers to investigate further applications of these macrofungi and lichens in fields of food and pharmaceutical industries and also other than food and pharmaceutical industries. This review, aims to put together Lentinan, schizophyllan, Betulinans A and B, ganoderic acid, cordycepin and usnic acid under light spot through representing their importance as biological activities exerted by ever one. Further studies and investigations are fortified in order to find more about these interesting natural products.
Introduction
Mushrooms are a rich source of nutritional and bioactive compounds; hence were used as food and medicine centuries ago. Mushrooms are used from ancient times and have a long history as nutritious tasty food items with low fattening values and high in proteins, vitamins, iron, zinc, selenium, sodium, chitin, fibres, minerals and other important nutrients [1-5]. Biologically active compounds from species of the phylum Basidiomycota and Ascomycota have been shown a wide range of pharmacological activities and provide a massive reservoir of potential innovational drugs. We show that isolated ganoderic acid and cordycepin from Ganoderma species and Cordyceps species fruit bodies, and other secondary metabolites like polysaccharides that presented antioxidant properties and other bioactivities. Investigating the mechanisms of action of biologically active compounds (Ganoderic acid and cordycepin) extracted form wild medicinal mushrooms (Ganoderma species and Cordyceps species) and other medicinal edible mushrooms will facilitate further efforts to accelerate the discovery of novel therapeutic strategies [1]. Besides high nutritional value mushrooms have well known medicinal usefulness due to well recognized therapeutic potential. Fruit bodies and mycelium of medicinal mushrooms are reported to contain important bioactive compounds (Such as ganoderic acid, cordycepin) with high antioxidant potential, anticancer, antiviral, anti-inflammatory, antidiabetic, anti-cholesterol and other [6].
Extracts from the wild mushroom fruiting body are considered as important medicines for the prevention and cure of many diseases in several parts of the world [7-10]. Wild edible mushrooms have a high nutritional property that has been consumed by people from different parts of the world, producing a wide variety of bioactive compounds such as polysaccharides, peptides, glycoproteins, triterpenoids, lipids, and their derivatives [11-13]. In the world, multidrug-resistant pathogens have been increasing extremely, and it is very urgent to search for alternative solutions to fight against multidrug-resistant pathogens. Moreover, unhealthy foods, ultraviolet radiation, as well as other environmental effects, are responsible for generating free radicals, oxidative stress, and numerous health diseases [14-20]. Hence, the wild edible mushroom could be an alternative source of new antimicrobial potential and possesses antioxidant properties that can play significant roles in preventing various health diseases [21-33].
Lichens are unique structures formed by an association between fungi and algae or cyanobacteria, by developing a unique morphological form that is separate from either component organism [34-36]. They have been used by humans for centuries as food and as a source of dye, and for their therapeutic properties in traditional medicine [37-44]. In this often-mutualistic relationship, the fungus and the algae are referred as mycobiont and the phycobiont [45], respectively. The mycobiont component most commonly belongs to the Ascomycetes, however, some may be Basidiomycetes and may even form mushroom-like spore bearing structures. The phycobiont component belongs to the divisions Chlorophyta and Cyanophyta [46]. Lichens can grow on a range of surfaces from rocks to existing as epiphytes on trees or leaves [47]. The vegetative component of lichen is called the thallus and this can be subdivided into four main categories.
Foliose: a leaf-like thallus, attached to the substrate at various points.
Crustose: a thallus which is flattened against the substrate and its lower surface is entirely attached.
Fruticose: the thallus is mainly composed of pendulous or, less commonly, upright branches and is attached at a single point. Squamulose: In which the thallus begins like a foliose lichen, but subsequently develops erect branches named podetia [48,49].
The lichen has different functional layers and each functional layer accumulates specific secondary metabolites such as upper cortex which is mycobiont layer (Accumulates atranorin, parietin, usnic acid, fungal melanins), medullary layer (accumulates physodic acid, physodalic acid, protocetraric acid), and an algal layer which is having photobionts [50]. Most of the lichen secondary metabolites exhibit significant biological activities. For example, Usnic acid exhibits several biological properties such as antimicrobial, larvicidal, anticancer, and UV absorption etc. Lichens play an important role in many ecosystems and exist as a symbiotic association between fungi and algae or cyanobacteria. This symbiosis results in the production of unique secondary metabolites known as lichen substances, which arise within the thalli and are typically in crystal form on the surface of the fungal hyphae. Recently, lichens and their secondary metabolites have been receiving increased attention due to their nutritional value and pharmaceutical potential [42,43].
Ganoderic Acids
Mushrooms are rich sources of biologically active compounds and cosmetic ingredients [51]. Ganoderma lucidum, a traditional medicinal mushroom, has been used to treat and prevent various diseases for two millennia in Asian countries [52]. Ganoderic acids, a type of tetracyclic triterpenoid, are one of the major ingredients of G. lucidum and have diverse pharmacological activities, including antitumor, anti-metastasis, anti-HIV, antiviral, hepatoprotective, hypocholesterolemic antioxidant, and antiaging effects.
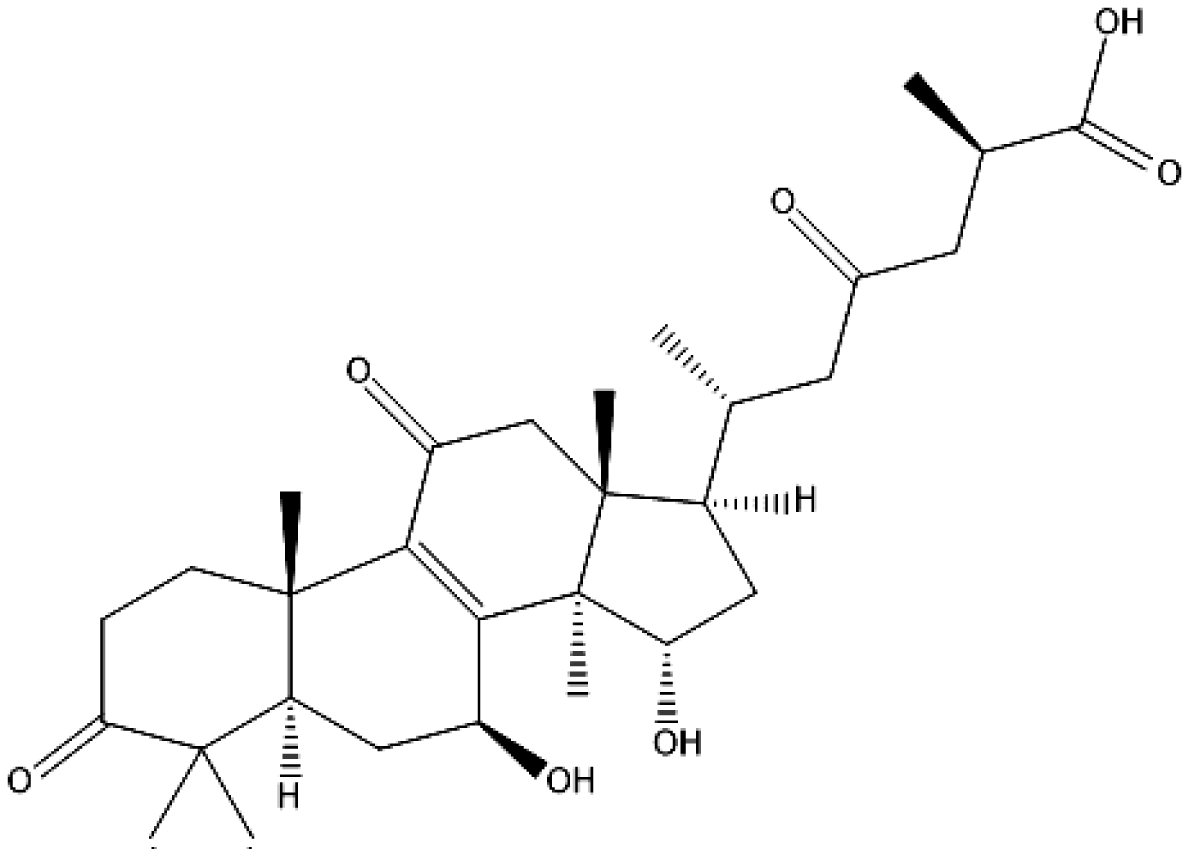
Ganoderma lucidum is a mushroom that has a long history of medicinal use in the Far East countries as this mushroom is revered for its supposed miracle cures and life improving properties. Recently, this mushroom has come under scientific scrutiny to examine the possibility of finding biologically active compounds that may have an impact on human physiology. The main category of biologically active compounds produced in the G. lucidum, are the triterpenoids, which are known as ganoderic acids [53]. G. lucidum contains a wide variety of bioactive compounds, such as, terpenoids, steroids, phenols, and nucleotides and their derivatives, glycoproteins, and polysaccharides. The biologically active molecules that may have a particular interest in the clinical setting are the terpenes and more specifically ganoderic acids. Terpenes are a class of compounds produced by the G. lucidum which are carbon structures composed of one or more isoprene C5 units. Ganoderic acids (Figure 1) are classified as triterpenes, a subtype of triterpenoids, as they are composed of six isoprene units [54].
In spite of their important biological functions, the low production of ganoderic acids is a bottleneck for clinical trials and commercial applications [55]. At present, ganoderic acids are mainly obtained from fruiting bodies and mycelia of G. lucidum. Compared to fruiting body cultures, a submerged culture is a promising alternative for the production of triterpenoids, as it is easy to control the product quality and is cost effective. Many efforts, such as by operating fermentation conditions, developing bioprocessing and elicitor strategies, and metabolic engineering, have been conducted to increase the production of gas by fermentation of mycelia. Ganoderma lucidum, common name Ling-Zhi in China and Reishi in Japan, has been used as a traditional medication for prevention and treatment of various human diseases for several thousand years in Asia. Also, according to different functional groups and side-chain types, structural skeleton of ganoderic acids can be classified into three categories. The great extent of oxidative modification (With hydroxyl, oxo, acetoxyl group), especially at C-3, C-7, C-15, and C-22 positions. Until now, GAs is mainly extracted from the solid cultivated fruiting bodies of G. lucidum [56,57].
Ganoderic Acid-DM may be a potential therapeutic candidate to treat an assortment of cancers as well as other diseases. This compound is capable of inducing apoptosis in cancer cells while exhibiting minimal toxicity to healthy cells. Ganoderic Acid-DM is also capable of stimulating an immune response in the tumor environment to potentially provide long-term protection from the malignant tumors [58]. More studies provide great support for utilizing Ganoderic Acid-DM as an alternative or supplemental therapy for various types of cancers, and more research is required to better understand the full scope of molecular targets Ganoderic Acid-DM [58]. Zhang DW, et al. [59], recorded the significant anti-viral activity of Ganoderma lucidum against enterovirus, which is the major cause of hand, foot and mouth diseases. Also Sliva D, et al. [60], and Zhang et al., [61], mentioned that Ganoderic acids from Ganoderma lucidum has Anti-oxidant activity and Anti- Microbial Activity.
Cordycepin
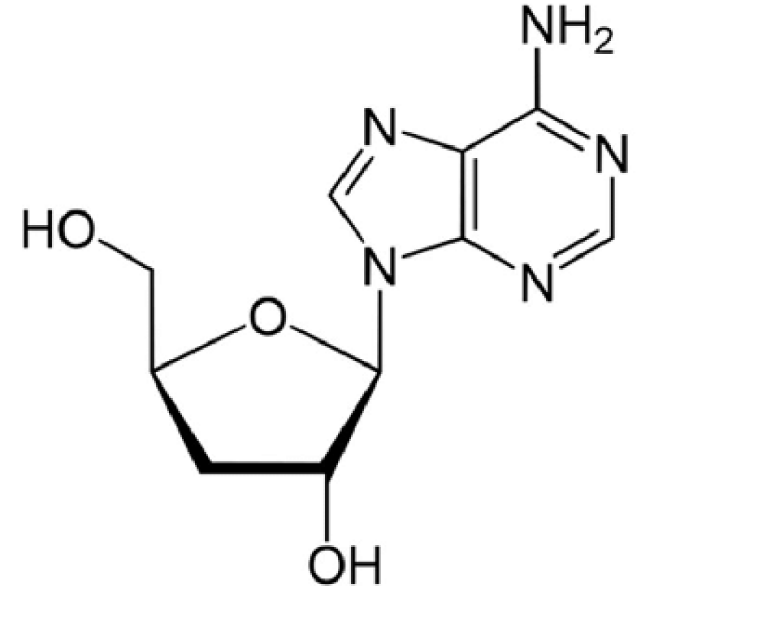
Cordyceps is a type of Ascomycetes which parasitizes insect larvae, grows and gradually changes into a fruit body [62]. In the early 1980s, many scientific institutes began to study the cultivation of Cordyceps sinensis. Previous work, over the course of a decade, was mainly focused on the anamorphic fungi related to Cordyceps sinensis. The Sichuan TCM Institute also achieved the artificial cultivation of Cordyceps sinensis, although commercial production has not been carried out because of the high cost and low stability [63]. Cordyceps militaris and C. sinensis, both have biomedical importance, contains a number of bioactive components. Many of them are biological response modifiers which activate human immune systems for a multitude of defensive functions. The immunomodulating effects are associated with its antitumour and other different biological activities. Cordycepin (Figure 2) was first isolated from C. militaris and its structural formula was confirmed as 3’-deoxyadenosine but it is only found in natural C. militaris with very low content and cannot be detected in the cultured ones [64]. Cordycepin is the most considerable adenosine analogue from some Cordyceps species [65], which is a derivative of the nucleoside adenosine differing from the latter by the absence of oxygen in the 3 ’-position of its ribose entity. Cordycepin is a category of compounds that exhibits significant therapeutic potential and has many intracellular targets, including nucleic acid, apoptosis, and cell cycle. Cordycepin can participate in various molecular processes in cells because of its similarity with adenosine [66]. Cordycepin, a metabolite of C. militaris, cordycepin has been investigated previously for its pharmacological potential, particularly in connection with the use of C. militaris fruiting bodies as a traditional herbal medicine. It has been reported to have anti-inflammatory [67,68] as well as anti-tumour and anti-angiogenic properties.
Up to now, in fact, the artificial cultivation of this valuable fungus has not been successfully achieved; only a product made using a C. sinensis anamorph has been made by fermentation methods. Modern experimental methods in biochemistry have proved that C. sinensis consists of active constituents such as crude fats, proteins, fibre, carbohydrate, cordycepin, cordycepic acid, polysaccharide, a series of vitamins, mannitol, nucleosides, ergosterol, aminophenol and trace elements [69]. It has a broad medical effect, and its function of immunity regulation plays an important role in antitumour effects, organ transplantation and the prevention of kidney, liver and heart disease [70].
Pharmacologically active components of Cordyceps sinensis are still unresolved, at least two chemical constituents, cordycepin and cordycepic acid, have been identified and proposed as important active constituents [3]. It is now believed that cordycepic acid is, in fact, D-mannitol, and that cordycepin is 3’- deoxyadenosine (3 ’- dA), a purine alkaloid, a derivative of the nucleoside adenosine, differing from the latter by the absence of oxygen in the 3 ’- position of its ribose part [3].
Lentinan and Schizophyllan
Lentinan, derived from shiitake (Lentinus edodes), and schizophyllan, derived from the Schizophyllum commune, are the most well-known polysaccharides. Lentinula edodes (Shiitake) because of their rich flavour and meaty texture, shiitake mushrooms are one of the most popular edible “gourmet” mushrooms. However, many people are unaware that shiitake mushrooms are also extremely medicinal, carrying a number of therapeutic compounds. It is made composed of beta-glucans and other polysaccharides like lentinan, emitanin, and KS-2, and it is been intensively researched for its potential pharmacological characteristics [71]. It is also widely used as a nutritional supplement or a hot water extract, which is normally prepared from the whole mushroom, mycelium biomass, or separated bioactive components, including lentinan extracts, for increased therapeutic potency.
On the other hand, Schizophyllum commune is a species of fungus in the genus Schizophyllum. The mushroom resembles undulating waves of tightly packed corals or loose Chinese fan [72]. The species was regarded as non-poisonous by Miller, Orson, and Hope, who considered it to be inedible due to its smallness and toughness [73]. However, some sources indicate that it contains antitumor and antiviral components [74].
Schizophyllan and lentinan are non-ionic polysaccharides, they can be converted into polyelectrolytes when carboxylate groups are introduced onto the β-glucosyl side residues by periodate-chlorite oxidation. The most promising bio pharmacological activities of these biopolymers are their immunomodulation and anti-cancer effects. They are mainly present as glucans with different types of glycosidic linkages such as (1-->3) and (1-->6)-beta-glucans [75,76], while others mostly bind to protein residues as polysaccharide-protein complexes. The two antitumor mushroom polysaccharides, i.e., lentinan and schizophyllan isolated from Lentinus edodes and Schizophyllum commune, have become large market items in Japan. Although the mechanism of their antitumor action is still not completely clear, these polysaccharides are suggested to enhance cell-mediated immune responses in vivo and in vitro and act as biological response modifiers. Among these, Lentinan was found to possess the immunomodulation properties against stomach cancer while schizophyllan was found to possess the anticancer properties against head and neck cancer [77]. In 1986, both of these drugs were licensed to be used in Japan for their chemotherapeutic purpose.
D-fraction or GFP
Grifola frondosa is a Basidiomycetes fungus that belongs to the family of Grifolaceae and the order of Polyporales. In Japan its edible fruiting body is known as maitake. In Japanese, mai means dance and take means mushroom. G. frondosa is known as “hui-shu-hua” (grey tree flower) in Chinese, possibly due to its appearance. G. frondosa grows around the stumps of broadleaf trees or trunks and is edible when young. The environment of the north-eastern part of Japan is suitable for the growth of G. frondosa. The temperate forests in eastern North America, Europe and Asia are also ideal for its growth. Meanwhile, it is a common mushroom in the Unites States and Canada, known as sheep’s head, king of mushrooms, hen-of-the-woods, and cloud mushroom [78].
Grifola frondosa is an edible mushroom is regarded as a healthy food because it is a good source of protein, carbohydrates, dietary fiber [79], vitamin D2 (ergocalciferol) [80] and minerals (K, P, Na, Ca, Mg), with low fat content and caloric value. G. frondosa is delicious, with a sweet and umami taste, which is mainly attributed to its high trehalose, glutamic and aspartic amino acid and 50-nucleotide content [81]. Due to its delicious and special taste, G. frondosa is not only used as a food ingredient, but also as a food-flavouring substance in dried powder form. Apart from its high nutraceutical value, G. frondosa is reported to possess a wide range of pharmacological effects. G. frondosa was first discovered to have antitumor activity in the 1980s from hot water extracts of the G. frondosa fruiting body. The major bioactive components were found to be β-glucans [82].
Several studies have demonstrated its antitumor effect, such as the one conducted by Alonso EN, et al. [83] on MCF-7 human breast cancer cells. Not only did it activate macrophages, T cells, and NK cells, but it also triggered the expression of BCL2-antagonist/killer 1 (BAK-1) and several other genes involved in apoptotic stimulation, the inhibition of cell growth and proliferation and cell cycle arrest, the suppression of tumour cell migration and metastasis, and the down regulation of the PI3K-AKT signalling pathway.
There are in general three methods for the artificial cultivation of the G. frondosa fruiting body, they are bottle culture, bag culture and outdoor bed culture. Bag culture is the most popular cultivation method in Japan [84] because of its advantages such as the low cost of plastic bags, small space requirements and easily-controlled indoor environment. Bag culture can achieve higher yields of mature G. frondosa mushrooms than bottle culture and requires a shorter cultivation time than outdoor bed cultures.
Betulinans A (1) and B (2)
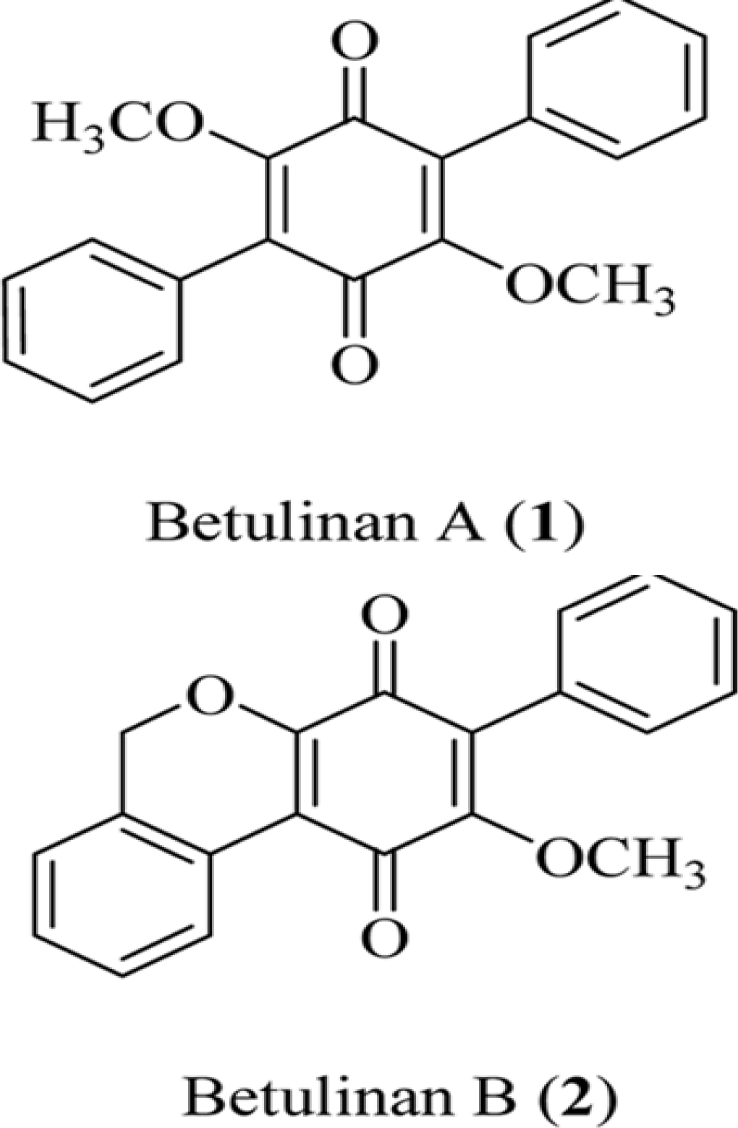
Two lipid peroxidation inhibitors, designated as betulinans A (1) and B (2), were isolated from the MeOH extract of Lenzites betulina [85]. Their structures have been determined to be 2,5-diphenyl-3,6-dimethoxy-p-benzoquinone and 2- phenyl-3-methoxy-[1H-2-enzopyran] [4,3-e] [p]benzoquinone, respectively, on the basis of various spectral data. Betulinans A and B inhibited lipid peroxidation with IC50 values of 0.46 and 2.88 μg/mL, respectively (Figure 3).
Usnic Acid
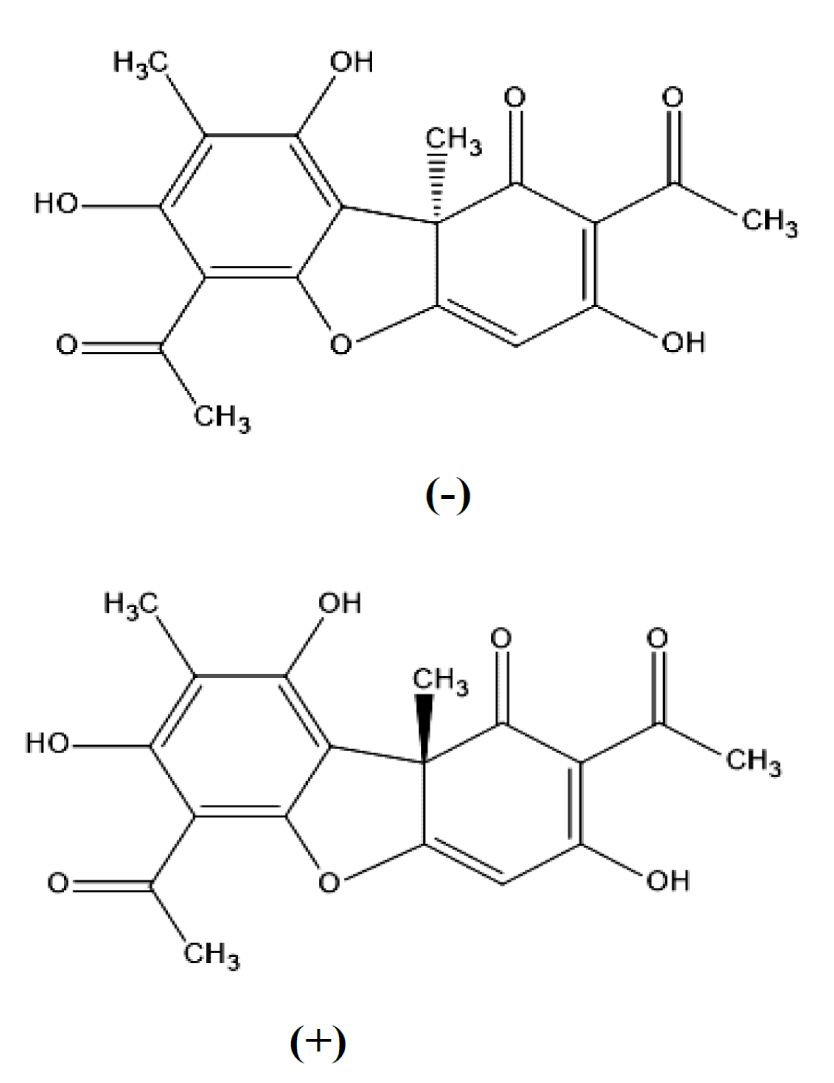
Lichen substances are derived for defense against most of the pathogens in nature. There are several interesting examples found reported in literature. Atranorin isolated from Physcia aipolia, fumarprotocetraric acid isolated from Cladonia furcata, gyrophoric acid isolated from Umbilicaria polyphylla, lecanoric acid isolated from Ochrolechia androgyna, physodic acid isolated from Hypogymnia physodes), protocetraric acid isolated from Parmelia caperata, stictic acid isolated from Parmelia conspersa and the most important lichen substances is usnic acid isolated from Flavoparmelia caperata, Pseudocyphellaria glabra, P. homoeophylla, Usnea campestris, U. diffracta, U. longissima, U. misaminensis and other exhibited potential antimicrobial effects against six types of bacteria and 10 types of fungi, including human, animal, plant pathogens, mycotoxin producers and food-spoilage organisms [86]. Usnic acid (Figure 4), is a yellowish pigment produced by several lichen species. It is a product of the secondary metabolism of the fungal partner and it exists in two enantiomers which differ in the orientation of the methyl group located in position 9b [87].
Usnic acid seems to play many biological roles such as: antibiotic, antimycotic, antifeedant, phytotoxic, photobiont regulator, UV filter. The difficulty of culturing whole lichen thalli in controlled conditions for an appropriate length of time clearly represents a limitation for research. Usnic acid probably does not have a single main biological role, but can play different, species-specific roles, also depending on habitat factors. Further investigations are necessary in order to test these hypotheses [87]. Usnic acid seems to be an exclusive lichen product. No synthetic derivatives more effective than the natural form are known. On the other hand, both the (+) and (–) enantiomers of usnic acid are effective against a large variety of Gram-positive (G+) bacterial strains. The (+) usnic acid enantiomer appears to be selective against Streptococcus mutans without inducing perturbing side effects on the oral saprophyte flora. Furthermore, the (–) usnic acid enantiomer is a selective natural herbicide because of its blocking action against a specific key plant enzyme [87].
Usnic acid has significant biological profile against pathogenic gram +ve organisms and anaerobic bacteria [88]. In addition, usnic acid has also been used in topical preparations, toothpastes and mouth washes. Lichen compounds significantly inhibit bacterial growth at lower concentration, while comparing with other antibiotics [50]. Usnic acid is class of dibenzofuran functional group and it has multiple biological activities. It shows antitumor activity against Lewis Lung carcinoma and P388 leukaemia through the mechanism of mitosis inhibition and apoptotic induction [89]. Usnic acid was found to be a good antimicrobial agent when compared to streptomycin [90]. The (+)-usnic acid produced by Cladonia arbuscula (have antimycobacterial activity) [91], and the (–) usnic acid produced by Cladonia leptoclada (have anti-tumour activity) [92]. Odimegwu DC, et al. [93] and Bhattacharjya R, et al. [94], also reported that, usnic acid produced by Pseudocyphellaria glabra and Pseudocyphellaria homoeophylla have antiviral, antimicrobial and cytotoxic activities. Usnea campestris, U. diffracta, U. longissima and U. misaminensis can produced usnic acid which have different biological activities (Antimicrobial and anti-inflammatory) [94].
Moura JB, et al. [91], and Gylfason AE [95], mentioned that, usnic acid produced by Cladonia sp. and Alectoria ochroleuca, have antibiotic activity and antifungal. Protousnea poeppigii produced usnic acid, isodivaricatic acid, 5-propylresorcinol and divaricaticacid have different biological activities like Antiprotozoal, antifungal [96]. D-Usnic acid, evernic acid and atranorin produced by Pseudoevernia furfuracea have significant anti-Allergy activity [97]. Parmotrema dilatatum, Parmotrema tinctorum and Pseudoparmelia sphaerospora all can produce usnic acid, orsellinic acid esters and salazinic acid, all have significant anti-Mycobacterium tuberculosis [98]. Usnic acid isolated from Ramalina terebrata and Parmelia caperata inhibits the growth of Bacillus subtilis, Staphyrococcus aurens, Escherichia coliand Pseudomonas aeruginosa [99]. The (-)-usnic acid isolated from Cladonia stellaris and Alectoria sarmentosa, active against Staphylococcus aureus [100,101].
More researches are needed to make possible intensive lichen culture, in order to produce large quantities of lichen usnic acid and other substances for pharmaceutical, cosmetic and agricultural purposes. Some biological aspects, i.e., the possible biological roles of usnic acid, need to be investigated and discussed.
Future Prospects
Different members of mushrooms and Lichens have encouraged researchers to investigate further applications of these macrofungi and lichens in fields of food and pharmaceutical industries and also other than food and pharmaceutical industries. Shortage in global materials and resources, besides the increase in world population, has encouraged research in eco-friendly alternatives, which are also relatively cheap. Capabilities of macrofungi in general, and mushrooms in particular, have supported their use in further applications in fields other than food and pharmaceutical industries. Some mushrooms are able to produce highly stable dyes which can be used in textiles and other dye-related products; some mushrooms can be used as cathodes or anodes in Microbial Fuel Cell (MFC). Mushroom mycelial growth contributes to production of bioplastics and as an alternative insulation material in building, infrastructure construction, packaging, foams and green building materials, like bricks. Moreover, mushrooms have potent enzymatic machinery that allows them to contribute in bioremediation processes. Also lichens have been used by humans for centuries as food and as a source of dye, and for their therapeutic properties in traditional medicine. More researches are needed to make possible intensive mushrooms and lichens culturing, in order to produce large quantities of their important natural products and their important applications.
Conclusion
Studies with mushrooms and lichens have been developed recently and it is figured out those potent properties of secondary metabolites from different wild mushroom and lichens species showed great biological activities. The aim of this review is to discuss mechanism of action involved in antioxidant, anti-inflammatory, cytotoxic/anticancer and other bioactivities attributed to the most common important bioactive compounds from mushrooms and lichens like Lentinan, derived from shiitake (Lentinus edodes), and schizophyllan, derived from the Schizophyllum commune, betulinans A and B, were isolated from Lenzites betulina and ganoderic acid and cordycepin, from the genera Ganoderma and Cordyceps belonging to the phylum Basidiomycota and Ascomycota, respectively and secondary metabolites, usnic acid in common lichen. There is a growing interest in active metabolites that are obtained from these natural sources as an alternative to synthetic drugs like ganoderic acid and cordycepin from wild mushrooms and usnic acid from lichens. Several compounds are responsible for the therapeutic activities of many medicinal mushrooms and lichens genera, the main groups of these compounds are polysaccharides, terpenes, phenolic compounds, and essential amino acids, as well as minerals. Ganoderic acid and cordycepin from wild mushrooms and Usnic acid from lichens are showing the most potential therapeutic activities. Further studies are required to explore the most common important bioactive compounds from different mushrooms and lichens.
Funding
This research does not receive any external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elkhateeb WA, Daba GM, Thomas PW, Wen TC. Medicinal mushrooms as a new source of natural therapeutic bioactive compounds. Egypt Pharmaceu J. 2019;18(2):88-101. doi: 10.4103/epj.epj_17_19.
- Elkhateeb WA, Daba GM, Elnahas M, Thomas P, Emam M. Metabolic profile and skin-related bioactivities of Cerioporus squamosus hydromethanolic extract. Biodiversitas J Biological Div. 2020;21(10). doi: 10.13057/biodiv/d211037.
- Elkhateeb WA, Daba, G. The endless nutritional and pharmaceutical benefits of the Himalayan gold, Cordyceps; Current knowledge and prospective potentials. Biofarmasi Journal of Natural Product Biochemistry. 2020;18(2):70-77. doi: 10.13057/biofar/f180204.
- Elkhateeb WA, Daba GM. Termitomyces marvel medicinal mushroom having a unique life cycle. Open Access Journal of Pharmaceutical Research. 2020;4(1):1-4. doi: 10.23880/oajpr-16000196.
- Daba GM, Elkhateeb W, ELDien AN, Fadl E, Elhagrasi A, Fayad W, Wen TC. Therapeutic potentials of n-hexane extracts of the three medicinal mushrooms regarding their anti-colon cancer, antioxidant, and hypocholesterolemic capabilities. Biodiversitas Journal of Biological Diversity. 2020;21(6):1-10. doi: 10.13057/biodiv/d210615.
- Elkhateeb WA. What medicinal mushroom can do? Chem Res J. 2020;5(1):106-118.
- Elkhateeb WA, Daba GM, Elmahdy EM, Thomas PW, Wen TC, Mohamed N. Antiviral potential of mushrooms in the light of their biological active compounds. ARC J Pharmac Sci. 2019;5:8-12. doi: 10.20431/2455-1538.0502003.
- El-Hagrassi A, Daba G, Elkhateeb W, Ahmed E, El-Dein AN, Fayad W, Shaheen M, Shehata R, El-Manawaty M, Wen T. In vitro bioactive potential and chemical analysis of the n-hexane extract of the medicinal mushroom, Cordyceps militaris. Malays J Microbiol. 2020;16(1):40-48. doi: 10.21161/mjm.190346.
- Elkhateeb WA, Daba GM, El-Dein AN, Sheir DH, Fayad W, Shaheen MN, Wen TC. Insights into the in-vitro hypocholesterolemic, antioxidant, antirotavirus, and anticolon cancer activities of the methanolic extracts of a Japanese lichen, Candelariella vitellina, and a Japanese mushroom, Ganoderma applanatum. Egyptian Pharmaceutical Journal. 2020;19(1):67. doi: 10.4103/epj.epj_56_19.
- Elkhateeb WA, Zaghlol GM, El-Garawani IM, Ahmed EF, Rateb ME, Abdel Moneim AE. Ganoderma applanatum secondary metabolites induced apoptosis through different pathways: In vivo and in vitro anticancer studies. Biomed Pharmacother. 2018 May;101:264-277. doi: 10.1016/j.biopha.2018.02.058. Epub 2018 Feb 27. PMID: 29494964.
- Elkhateeb WA, Elnahas MO, Thomas PW, Daba GM. To heal or not to heal? Medicinal mushrooms wound healing capacities. ARC Journal of Pharmaceutical Sciences. 2019;5(4):28-35. doi: 10.20431/2455-1538.0504004.
- Elkhateeb WA, Daba GM, Elnahas MO, Thomas PW. Anticoagulant capacities of some medicinal mushrooms. ARC J Pharma Sci. 2019;5:12-16. doi: 10.20431/2455-1538.0504001.
- Elkhateeb W, Elnahas MO, Paul W, Daba GM. Fomes fomentarius and Polyporus squamosus models of marvel medicinal mushrooms. Biomed Res Rev. 2020;3:119. doi: 10.31021/brr.20203119.
- Elkhateeb WA, Daba GM. Mycotherapy of the good and the tasty medicinal mushrooms Lentinus, Pleurotus, and Tremella. Journal of Pharmaceutics and Pharmacology Research. 2021;4(3):1-6. doi: 10.31579/2693-7247/029.
- Elkhateeb WA, Daba GM. The fascinating bird’s nest mushroom, secondary metabolites and biological activities. International Journal of Pharma Research and Health Sciences, 2021;9(1):3265-3269. doi: 10.21276/ijprhs.2021.01.01.
- Elkhateeb WA, Daba GM, Gaziea SM. The anti-nemic potential of mushroom against plant-parasitic nematodes. Open Access Journal of Microbiology & Biotechnology. 2021;6(1):1-6. doi: 10.23880/oajmb-16000186.
- Elkhateeb WA, Elnahas MO, Thomas PW, Daba GM. Trametes Versicolor and Dictyophora Indusiata Champions of Medicinal Mushrooms. Open Access Journal of Pharmaceutical Research. 2020;4(1):1-7. doi: 10.23880/oajpr-16000192.
- Elkhateeb WA, Daba G. The endless nutritional and pharmaceutical benefits of the Himalayan gold, Cordyceps; Current knowledge and prospective potentials. Biofarmasi Journal of Natural Product Biochemistry. 2020;18(2):1-10. doi: 10.13057/biofar/f180204.
- Daba GM, Elkhateeb W, ELDien AN, Fadl E, Elhagrasi A, Fayad W, Wen TC. (2020). Therapeutic potentials of n-hexane extracts of the three medicinal mushrooms regarding their anti-colon cancer, antioxidant, and hypocholesterolemic capabilities. Biodiversitas Journal of Biological Diversity. 2020;21(6):1-10. doi: 10.13057/biodiv/d210615.
- Thomas PW, Elkhateeb WA, Daba GM. Chaga (Inonotus obliquus): A medical marvel but a conservation dilemma? Sydowia. 2020;72:123-130. doi: 10.12905/0380.sydowia72-2020-0123.
- Thomas P, Elkhateeb WA, Daba GM. Industrial applications of truffles and truffle-like fungi. In: Advances in Macrofungi. CRC Press; 2021. p.82-88. doi: 10.1201/9781003096818-8.
- Elkhateeb W, Thomas P, Elnahas M, Daba G. Hypogeous and epigeous mushrooms in human health. In: Advances in Macrofungi. CRC Press; 2021. p.7-19. doi: 10.1201/9781003191278-2.
- Elkhateeb W, Elnahas M, Daba G. Infrequent current and potential applications of mushrooms. CRC Press; 2021. p.70-81. doi: 10.1201/9781003096818-7.
- Elkhateeb WA, El Ghwas DE, Gundoju NR, Somasekhar T, Akram M, Daba GM. Chicken of the woods Laetiporus sulphureus and Schizophyllum commune treasure of medicinal mushrooms. Open Access Journal of Microbiology & Biotechnology. 2021;6(3):1-7. doi: 10.23880/oajmb-16000201.
- Elkhateeb WA, Daba GM. Highlights on unique orange pore cap mushroom favolaschia sp. and beech orange mushroom Cyttaria sp. and their biological activities. Open Access Journal of Pharmaceutical Research. 2021;5(3):1-6. doi: 10.23880/oajpr-16000246.
- Elkhateeb WA, Daba GM. Highlights on the wood blue-leg mushroom Clitocybe nuda and blue-milk mushroom Lactarius indigo ecology and biological activities. Open Access Journal of Pharmaceutical Research. 2021;5(3):1-6. doi: 10.23880/oajpr-16000249.
- Elkhateeb WA, Daba GM. Highlights on the golden mushroom Cantharellus cibarius and unique shaggy ink cap mushroom Coprinus comatus and smoky bracket mushroom Bjerkandera adusta ecology and biological activities. Open Access Journal of Mycology & Mycological Sciences. 2021;4(2):1-8. doi: 10.23880/oajmms-16000143.
- Thomas PW, Elkhateeb WA, Daba G. Truffle and truffle-like fungi from continental Africa. Acta Mycological. 2019;54(2):1-15.
- ALKolaibe AG, Elkhateeb WA, Elnahas MO, El-Manawaty M, Deng CY, Wen, TC, Daba GM. Wound healing, anti-pancreatic cancer, and α-amylase inhibitory potentials of the edible mushroom, Metacordyceps neogunnii. Research Journal of Pharmacy and Technology. 2021;14(10):5249-5253. doi: 10.52711/0974-360X.2021.00914.
- Elkhateeb WA, Daba GM. The coral mushrooms Ramaria and Clavaria. Studies in Fungi. 2021;6(1):495-506. doi: 10.5943/sif/6/1/39.
- Elkhateeb WA, Daba GM. Medicinal mushroom: What should we know? International Journal of Pharmaceutical Chemistry and Analysis. 2021;9(1):1-19.
- Elkhateeb WA, Daba GM. The wild non edible mushrooms, what should we know so far? International Journal of Advanced Biochemistry Research. 2022;6(1):43-50. doi: 10.33545/26174693.2022.v6.i1a.83.
- Elkhateeb WA and Daba GM. Bioactive potential of some fascinating edible mushrooms Flammulina, lyophyllum, Agaricus, Boletus, Letinula, and Pleurotus as a treasure of multipurpose therapeutic natural product. Pharm Res. 2022;6(1):1-10. doi: 10.23880/oajpr-16000263.
- Ahmed E, Elkhateeb W, Taie H, Rateb M, Fayad W. Biological capacity and chemical composition of secondary metabolites from representatives Japanese lichens. Journal of Applied Pharmaceutical Science. 2017;7(01):098-103. doi: 10.7324/JAPS.2017.70113.
- Elkhateeb WA, Daba, GM. Lichens, an alternative drugs for modern diseases. International Journal of Research in Pharmacy and Biosciences. 2019;6(10):5-9.
- El-Garawani IM, Elkhateeb WA, Zaghlol GM, Almeer RS, Ahmed EF, Rateb ME, Abdel Moneim AE. Candelariella vitellina extract triggers in vitro and in vivo cell death through induction of apoptosis: A novel anticancer agent. Food Chem Toxicol. 2019 May;127:110-119. doi: 10.1016/j.fct.2019.03.003. Epub 2019 Mar 7. PMID: 30853555.
- El-Garawani I, Emam M, Elkhateeb W, El-Seedi H, Khalifa S, Oshiba S, Abou-Ghanima S, Daba G. In Vitro Antigenotoxic, Antihelminthic and Antioxidant Potentials Based on the Extracted Metabolites from Lichen, Candelariella vitellina. Pharmaceutics. 2020 May 24;12(5):477. doi: 10.3390/pharmaceutics12050477. PMID: 32456266; PMCID: PMC7285106.
- Elkhateeb WA, Daba, GM. Occurrence of terpenes, polyketides, and tannins in some Japanese lichens and green mosses. Egyptian Pharmaceutical Journal. 2020;19(3):216.
- Elkhateeb WA, Daba, GM, El-Dein AN, Sheir DH, Fayad W, Shaheen MN, Wen TC. Insights into the in-vitro hypocholesterolemic, antioxidant, antirotavirus, and anticolon cancer activities of the methanolic extracts of a Japanese lichen, Candelariella vitellina, and a Japanese mushroom, Ganoderma applanatum. Egyptian Pharmaceutical Journal. 2020;19(1):67.
- Elkhateeb WA, Daba GM, Sheir D, Hapuarachchi KK, Thomas PW. Mysterious world of lichens: Highlights on their history, applications, and pharmaceutical potentials. The Natural Products Journal 2021;11(3):275-287. doi: 10.2174/2210315510666200128123237.
- Elkhateeb WA, Daba GM. Fungi over fungi, endophytic fungi associated with mushroom fruiting bodies and lichens. Journal of Pharmaceutics and Pharmacology Research. 2021;4(2):1-4. doi: 10.31579/2693-7247/028.
- Elkhateeb W, Somasekhar T, Thomas P, Wen TC, Daba G. Mycorrhiza and lichens as two models of fungal symbiosis. Journal of Microbiology, Biotechnology and Food Sciences. 2021;11(3):e4644-e4644. doi: 10.15414/jmbfs.4644.
- Elkhateeb WA, Elnahas MO, Daba GM. Lichentherapy: Highlights on the pharmaceutical potentials of lichens. Open Access Journal of Microbiology & Biotechnology. 2021;6(1):1-10. doi: 10.23880/oajmb-16000190.
- Elkhateeb WA, El-Ghwas DE, Daba GM. Lichens uses surprising uses of lichens that improve human life. J Biomed Res Environ Sci. 2022;3:189-194. doi: 10.37871/jbres1420.
- Sharma GN, Gupta G, Sharma P. A Comprehensive Review of Free Radicals, Antioxidants, and Their Relationship with Human Ailments. Crit Rev Eukaryot Gene Expr. 2018;28(2):139-154. doi: 10.1615/CritRevEukaryotGeneExpr.2018022258. PMID: 30055541.
- Shukla V, Joshi GP, Rawat MS. Lichens as a potential natural source of bioactive compounds: A review. Phytochemistry Reviews. 2010;9:303-314. doi: 10.1007/s11101-010-9189-6.
- Seymour FA, Crittenden PD, Dickinson MJ, Paoletti M, Montiel D, Cho L, Dyer PS. Breeding systems in the lichen-forming fungal genus Cladonia. Fungal Genet Biol. 2005 Jun;42(6):554-63. doi: 10.1016/j.fgb.2005.03.006. PMID: 15893256.
- Nayaka S. Methods, techniques in collection, preservation and identification of lichens. Plant Taxonomy and Biosystematics. In: Classical and modern methods. Rana TS, Nair KN, Upreti DK, editors. New India Publishing Agency; 2014. p.101-105.
- Monge-Najera J. Relative humidity, temperature, substrate type, and height of terrestrial lichens in a tropical paramo. Revista de Biologia Tropical. 2019;67(1):1-10. doi: 10.15517/rbt.v67i1.33948.
- Bhattacharyya S, Deep PR, Singh S, Nayak B. Lichen secondary metabolites and its biological activity. American Journal of PharmTech Research. 2016;6(6):29-44.
- Chaturvedi VK, Agarwal S, Gupta KK, Ramteke PW, Singh MP. Medicinal mushroom: boon for therapeutic applications. 3 Biotech. 2018 Aug;8(8):334. doi: 10.1007/s13205-018-1358-0. Epub 2018 Jul 23. PMID: 30073119; PMCID: PMC6056353.
- Paterson RR. Ganoderma - a therapeutic fungal biofactory. Phytochemistry. 2006 Sep;67(18):1985-2001. doi: 10.1016/j.phytochem.2006.07.004. Epub 2006 Aug 14. PMID: 16905165.
- Hapuarachchi KK, Elkhateeb WA, Karunarathna SC, Cheng CR, Bandara AR, Kakumyan P, Wen TC. Current status of global Ganoderma cultivation, products, industry and market. Mycosphere. 2018;9(5):1025-1052. doi 10.5943/mycosphere/9/5/6.
- Bryant JM, Bouchard M, Haque A. Anticancer Activity of Ganoderic Acid DM: Current Status and Future Perspective. J Clin Cell Immunol. 2017;8(6):535. doi: 10.4172/2155-9899.1000535. Epub 2017 Dec 12. PMID: 29399381; PMCID: PMC5795599.
- Feng J, Zhang JS, Jia W, Yang Y, Liu F, Lin CC. An unstructured kinetic model for the improvement of triterpenes production by Ganoderma lucidum G0119 based on nitrogen source effect. Biotechnology and Bioprocess Engineering. 2014;19:727-32. doi: 10.1007/s12257-014-0049-x.
- Xu P, Ding ZY, Qian Z, Zhao CX, Zhang KC. Improved production of mycelial biomass and ganoderic acid by submerged culture of Ganoderma lucidum SB97 using complex media. Enzyme and Microbial Technology. 2008;42:325-331. doi: 10.1016/j.enzmictec.2007.10.016.
- Shiao MS. Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chem Rec. 2003;3(3):172-80. doi: 10.1002/tcr.10058. PMID: 12900937.
- Mitchell DA, Sassaki GL, De Almeida Amazonas MAL, Berovic M. Current techniques for the cultivation of Ganoderma lucidum for the production of biomass, ganoderic acid and polysaccharides. Food Technology and Biotechnology. 2003;41:371-382.
- Zhang DW, Wang ZL, Qi W, Lei W, Zhao GY. Cordycepin (3'-deoxyadenosine) down-regulates the proinflammatory cytokines in inflammation-induced osteoporosis model. Inflammation. 2014 Aug;37(4):1044-9. doi: 10.1007/s10753-014-9827-z. PMID: 24493324.
- Sliva D, Sedlak M, Slivova V, Valachovicova T, Lloyd FP Jr, Ho NW. Biologic activity of spores and dried powder from Ganoderma lucidum for the inhibition of highly invasive human breast and prostate cancer cells. J Altern Complement Med. 2003 Aug;9(4):491-7. doi: 10.1089/107555303322284776. PMID: 14499024.
- Zhang XQ, Ip FC, Zhang DM, Chen LX, Zhang W, Li YL, Ip NY, Ye WC. Triterpenoids with neurotrophic activity from Ganoderma lucidum. Nat Prod Res. 2011 Oct;25(17):1607-13. doi: 10.1080/14786419.2010.496367. Epub 2011 Jun 13. PMID: 21671206.
- Jianghai GDZ, Xin L. A review and prospect on the studies of Cordyceps sinensis (Berk) Sacc [J]. Journal of Chinese Institute of Food Science and Technology. 2006.
- Hongchun G, Jiquan G, Qianyun X, Xiaohong, L. Research and development for Cordyceps sinensis. 2003;23(1):50-55.
- Yang FQ, Li DQ, Feng K, Hu DJ, Li SP. Determination of nucleotides, nucleosides and their transformation products in Cordyceps by ion-pairing reversed-phase liquid chromatography-mass spectrometry. J Chromatogr A. 2010 Aug 20;1217(34):5501-10. doi: 10.1016/j.chroma.2010.06.062. Epub 2010 Jun 30. PMID: 20637470.
- Paterson RR. Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008 May;69(7):1469-95. doi: 10.1016/j.phytochem.2008.01.027. Epub 2008 Mar 17. PMID: 18343466; PMCID: PMC7111646.
- Yue K, Ye M, Zhou Z, Sun W, Lin X. The genus Cordyceps: a chemical and pharmacological review. J Pharm Pharmacol. 2013 Apr;65(4):474-93. doi: 10.1111/j.2042-7158.2012.01601.x. Epub 2012 Oct 23. PMID: 23488776.
- Kondrashov A, Meijer HA, Barthet-Barateig A, Parker HN, Khurshid A, Tessier S, Sicard M, Knox AJ, Pang L, De Moor CH. Inhibition of polyadenylation reduces inflammatory gene induction. RNA. 2012 Dec;18(12):2236-50. doi: 10.1261/rna.032391.112. Epub 2012 Nov 1. PMID: 23118416; PMCID: PMC3504674.
- Ashraf S, Radhi M, Gowler P, Burston JJ, Gandhi RD, Thorn GJ, Piccinini AM, Walsh DA, Chapman V, de Moor CH. The polyadenylation inhibitor cordycepin reduces pain, inflammation and joint pathology in rodent models of osteoarthritis. Sci Rep. 2019 Mar 18;9(1):4696. doi: 10.1038/s41598-019-41140-1. PMID: 30886197; PMCID: PMC6423048.
- Wei L. Research advanced in functional composition content of Cordyceps. West Chin Journal of Pharmaceutical Sciences. 2003;18:359-360.
- Shi YX. Update on researches of pharmacological effects of Cordyceps. Chin Pharm. 2005;14:72-74.
- He X, Wang X, Fang J, Chang Y, Ning N, Guo H, Huang L, Huang X, Zhao Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion's Mane) mushroom: A review. Int J Biol Macromol. 2017 Apr;97:228-237. doi: 10.1016/j.ijbiomac.2017.01.040. Epub 2017 Jan 10. PMID: 28087447.
- Kuo M. Schizophyllum Commune. 2003.
- Miller H, Miller OK. North American Mushrooms: A field guide to edible and inedible fungi. Guilford, CN: FalconGuide; 2003. p.139.
- Davis RM, Sommer R, Menge JA. Field guide to mushrooms of Western North America. Berkeley: University of California Press; 2012. p.131-132.
- Sindhu RK, Najda A, Kaur P, Shah M, Singh H, Kaur P, Cavalu S, Jaroszuk-Sierocińska M, Rahman MH. Potentiality of Nanoenzymes for Cancer Treatment and Other Diseases: Current Status and Future Challenges. Materials (Basel). 2021 Oct 11;14(20):5965. doi: 10.3390/ma14205965. PMID: 34683560; PMCID: PMC8539628.
- Rahman MM, Islam MR, Shohag S, Hossain ME, Rahaman MS, Islam F, Ahmed M, Mitra S, Khandaker MU, Idris AM, Chidambaram K, Emran TB, Cavalu S. The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules. 2022 Mar 6;27(5):1713. doi: 10.3390/molecules27051713. PMID: 35268815; PMCID: PMC8911649.
- Hosseini M, Salam A, and Makhlouf H. Industrial Applications for Intelligent Polymers and Coatings. Berlin, Germany. Spinger; 2016.
- Mayell M. Maitake extracts and their therapeutic potential. Altern Med Rev. 2001 Feb;6(1):48-60. PMID: 11207456.
- Cohen N, Cohen J, Asatiani MD, Varshney VK, Yu HT, Yang YC, Li YH, Mau JL, Wasser SP. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int J Med Mushrooms. 2014;16(3):273-91. doi: 10.1615/intjmedmushr.v16.i3.80. PMID: 24941169.
- Tabata T, Yamasaki Y, Ogura T. Comparison of chemical compositions of Maitake (Grifola frondosa (Fr.) SF Gray) cultivated on logs and sawdust substrate. Food Sci Technol Res. 2004;10:21-24.
- Huang SJ, Tsai SY, Lin SY, Liang CH, Mau JL. Nonvolatile taste components of culinary-medicinal maitake mushroom, Grifola frondosa (Dicks.:Fr.) S.F. Gray. Int J Med Mushrooms. 2011;13(3):265-72. doi: 10.1615/intjmedmushr.v13.i3.60. PMID: 22135878.
- Nanba H, Hamaguchi A, Kuroda H. The chemical structure of an antitumor polysaccharide in fruit bodies of Grifola frondosa (maitake). Chem Pharm Bull (Tokyo). 1987 Mar;35(3):1162-8. doi: 10.1248/cpb.35.1162. PMID: 3607939.
- Alonso EN, Orozco M, Eloy Nieto A, Balogh GA. Genes related to suppression of malignant phenotype induced by Maitake D-Fraction in breast cancer cells. J Med Food. 2013 Jul;16(7):602-17. doi: 10.1089/jmf.2012.0222. PMID: 23875900; PMCID: PMC3719462.
- Mayuzumi Y, Mizuno T. III. Cultivation methods of maitake (Grifola frondosa). Food Rev Int. 1997;13:357-364. doi: 10.1080/87559129709541117.
- Lee IK, Yun BS, Cho SM, Kim WG, Kim JP, Ryoo IJ, Koshino H, Yoo ID. Betulinans A and B, two benzoquinone compounds from Lenzites betulina. J Nat Prod. 1996 Nov;59(11):1090-2. doi: 10.1021/np960253z. PMID: 8946751.
- Branislav R, Marijana M. The antimicrobial activity of substances derived from the lichens Physcia aipolia, Umbilicaria polyphylla, Parmelia caperata and Hypogymnia physodes. World Journal of Microbiology and Biotechnology. 2008;24:1239-1242. doi: 10.1007/s11274-007-9580-7.
- Cocchietto M, Skert N, Nimis PL, Sava G. A review on usnic acid, an interesting natural compound. Naturwissenschaften. 2002 Apr;89(4):137-46. doi: 10.1007/s00114-002-0305-3. PMID: 12061397.
- Galanty A, Paśko P, Podolak I. Enantioselective activity of usnic acid: A comprehensive review and future perspectives. Phytochem Rev. 2019;18(2):527-548. doi: 10.1007/s11101-019-09605-3.
- Türk H, Yilmaz M, Tay T, Türk AO, Kivanç M. Antimicrobial activity of extracts of chemical races of the lichen Pseudevernia furfuracea and their physodic acid, chloroatranorin, atranorin, and olivetoric acid constituents. Z Naturforsch C J Biosci. 2006 Jul-Aug;61(7-8):499-507. doi: 10.1515/znc-2006-7-806. PMID: 16989308.
- Macedo DC, Almeida FJ, Wanderley MS, Ferraz MS, Santos NP, López AM, Santos-Magalhães NS, Lira-Nogueira MC. Usnic acid: from an ancient lichen derivative to promising biological and nanotechnology applications. Photochemistry Reviews. 2020;20(4):1-22. doi: 10.1007/s11101-020-09717-1.
- Moura JB, Vargas AC, Gouveia GV, Gouveia JJ, Ramos-Júnior JC, Botton SD, Pereira EC, Costa M. In vitro antimicrobial activity of the organic extract of Cladonia substellata Vainio and usnic acid against Staphylococcus spp. obtained from cats and dogs. Pesquisa Veterinária Brasileira. 2017;37(4):368-378. doi: 10.1590/S0100-736X2017000400011.
- Noël A, Garnier A, Clément M, Rouaud I, Sauvager A, Bousarghin L, Vásquez-Ocmín P, Maciuk A, Tomasi S. Lichen-associated bacteria transform antibacterial usnic acid to products of lower antibiotic activity. Phytochemistry. 2021 Jan;181:112535. doi: 10.1016/j.phytochem.2020.112535. Epub 2020 Oct 21. PMID: 33099225.
- Odimegwu DC, Ngwoke K, Ejikeugwu C, Esimone CO. Lichen secondary metabolites as possible antiviral agents. In: Lichen secondary metabolites. Springer Cham; 2019. p.199-214.
- Bhattacharjya R, Begum A, Tiwari A. Role of Algae–fungi relationship in sustainable agriculture. In: Agriculturally important fungi for sustainable agriculture. Springer Cham; 2020. p.227-254.
- Gylfason AE. Content and distribution of usnic acid enantiomers in three Icelandic lichen taxa (Doctoral dissertation); 2019.
- Yousuf S, Choudhary M, Rahman A. Lichens: Chemistry and biological activities. Studies in Natural Products Chemistry. 2014;43:223-259. doi: 10.1016/B978-0-444-63430-6.00007-2.
- Schmeda-Hirschmann G, Tapia A, Lima B, Pertino M, Sortino M, Zacchino S, Arias AR, Feresin GE. A new antifungal and antiprotozoal depside from the Andean lichen Protousnea poeppigii. Phytother Res. 2008 Mar;22(3):349-55. doi: 10.1002/ptr.2321. PMID: 18058986.
- Sarikurkcu C, Kocak MS, Calapoglu M, Ocal C, Tepe B. Biological and phytochemical evaluation: Pseudevernia furfuracea as an alternative multifunctional agent. Journal of Functional Foods. 2016;24:11-17. doi: 10.1016/j.jff.2016.03.022.
- Honda NK, Pavan FR, Coelho RG, de Andrade Leite SR, Micheletti AC, Lopes TI, Misutsu MY, Beatriz A, Brum RL, Leite CQ. Antimycobacterial activity of lichen substances. Phytomedicine. 2010 Apr;17(5):328-32. doi: 10.1016/j.phymed.2009.07.018. Epub 2009 Aug 14. PMID: 19683421.
- Paudel B, Bhattarai HD, Lee HK, Oh H, Shin HW, Yim JH. Antibacterial activities of Ramalin, usnic acid and its three derivatives isolated from the Antarctic lichen Ramalina terebrata. Z Naturforsch C J Biosci. 2010 Jan-Feb;65(1-2):34-8. doi: 10.1515/znc-2010-1-206. PMID: 20355318.
- Ranković B. Lichen Secondary Metabolites. Cham: Springer International Publishing; 2015. p.202.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.