Environmental Sciences . 2023 June 30;4(6):1108-1112. doi: 10.37871/jbres1774.
Strategy for Climate Crisis: Introducing Innovative System for CO2 Fixation and Storage
Kenji Sorimachi1*, Toshinori Tsukada2, Wataru Kobayashi3 and Hossam Gabbar4
2KSI, Takasaki, Gunma, Japan
3Environmental Engineering Institute, Inc., Annaka, Gunma, Japan
4Department of Energy and Nuclear Engineering, Faculty of Engineering and Applied Science, Ontario Tech University, Canada
- CO2 fixation
- CO2 storage
- Climate change
- H2 generation
- NaCl electrolysis
- CaCO3
- Limestone
- Direct Air Capture (DAC)
Abstract
The so-called Paris Agreement was reached at the United Nations Climate Change Conference (COP20) in 2015. This agreement was based on the requirement to keep the increase in the mean global temperature below 2°C relative to the temperature prior to the industrial revolution, and preferably less than 1.5°C. At present, this goal is challenging based solely on the development of carbon-neutral energy systems. The concept of the carbon-neutral society by 2050 seems to be far too late. Herein, we propose an innovative system based on simple chemical reactions using NaOH and CaCl2 via the electrolysis of NaCl or seawater. The generated H2 from the electrolysis of NaCl can be used as a clean energy source for fuel batteries, supplying electricity for the operation of the system. When other renewable energy sources power the system, H2 can be generated as a clean energy alternative. Furthermore, this system produces stable and harmless CaCO3 as a final product, along with NaCl, which can be reused as an electrolysis starting material. The proposed system provides a safe and inexpensive approach for simultaneous CO2 fixation and storage.
Introduction
The Intergovernmental Panel on Climate Change (IPCC) concluded on August 9th, 2021, that climate change has been caused by human activities that have produced carbon dioxide (CO2) since the industrial evolution [1]. A few days ago, in early June 2023, Japanese mass media, including television, announced severe mega mountain fires in Canada. The smoke from these fires covered New York City, creating an apocalyptic scene reminiscent of the end of the century. Surprisingly, some smoke even reached Washington D.C., USA, despite the considerable distance from the source. According to reports, the fires in Canada were believed to have been sparked by lightning strikes, with multiple locations being affected owing to climate change. In addition, other natural phenomena have recently increased, such as heavy rains, torrential rains, super hurricanes, super tornadoes, glacier retreats, and the expansion of desert areas.
The planet may be nearing a threshold beyond which unpredictable environmental change may occur, such as increase in the mean global temperature [2]. However, we have not paid a little attention to the effect of increase in atmospheric CO2 concentration for a long time. One of reasons is due to our wide tolerance toward CO2 concentration in daily life. In fact, the atmospheric CO2 concentration at the house room is about 400 ppm, while the concentration easily reaches twice in the presence of several person for certain times in the same room without ventilation. Even under high CO2 concentration around 1,000 ppm for certain time in the limited space, our life doesn’t feel an abnormal symptom. Eventually, many people would be apparently insensitive to a small increase in atmospheric CO2 concentration, although this small change clearly induced climate change crisis on the earth. Our wide tolerance toward CO2 concentration is due to CO2 characteristics that CO2 doesn’t bind with biological compounds such as amines is easily excluded by the lung tissues based on concentration gradient force, like a "pseudo-osmosis" [3]. In order to resist the highly produced CO2, organisms including human beings have acquired the insensitivity to CO2 to adapt to environment during biological evolution. In mitochondrial evolution, active species have more evolved than less active species [4]. This respiratory function based on CO2 characteristics was acquired by organisms during biological evolution [5]. Eventually, the most evolved human beings have induced climate crisis due to consumption of fossil fuel.
Although there are several methods for CO2 capture, such as absorption [6-12] and membrane gas separation [3,13-18], only amines have been currently used [19,20]. However, this organic solvent method for CO2 fixation is limited owing to the decomposition of amines during the heat treatment for CO2 liberation from the amine-CO2 complex. In addition, the amine degradation needs regeneration for further use. In contrast, we developed an innovative method for CO2 fixation and storage using NaOH and CaCl2 [21]. This method converts CO2 from the ambient atmosphere or exhaust gas into CaCO3, a natural, harmless, and stable component in limestone or coral.
2NaOH + CO2 → Na2CO3 + H2O
Na2CO3 + CaCl2 → CaCO3 + 2NaCl
At high NaOH concentrations, > 0.2 M, NaOH and CaCl2 formed Ca(OH)2 and NaCl in the absence of CO2 [21]. To enhance the reaction efficiency of CO2 with NaOH solution, the NaOH solution was formed as a mist inside the reaction chamber [22], or CO2 was bubbled into the NaOH solution [23].
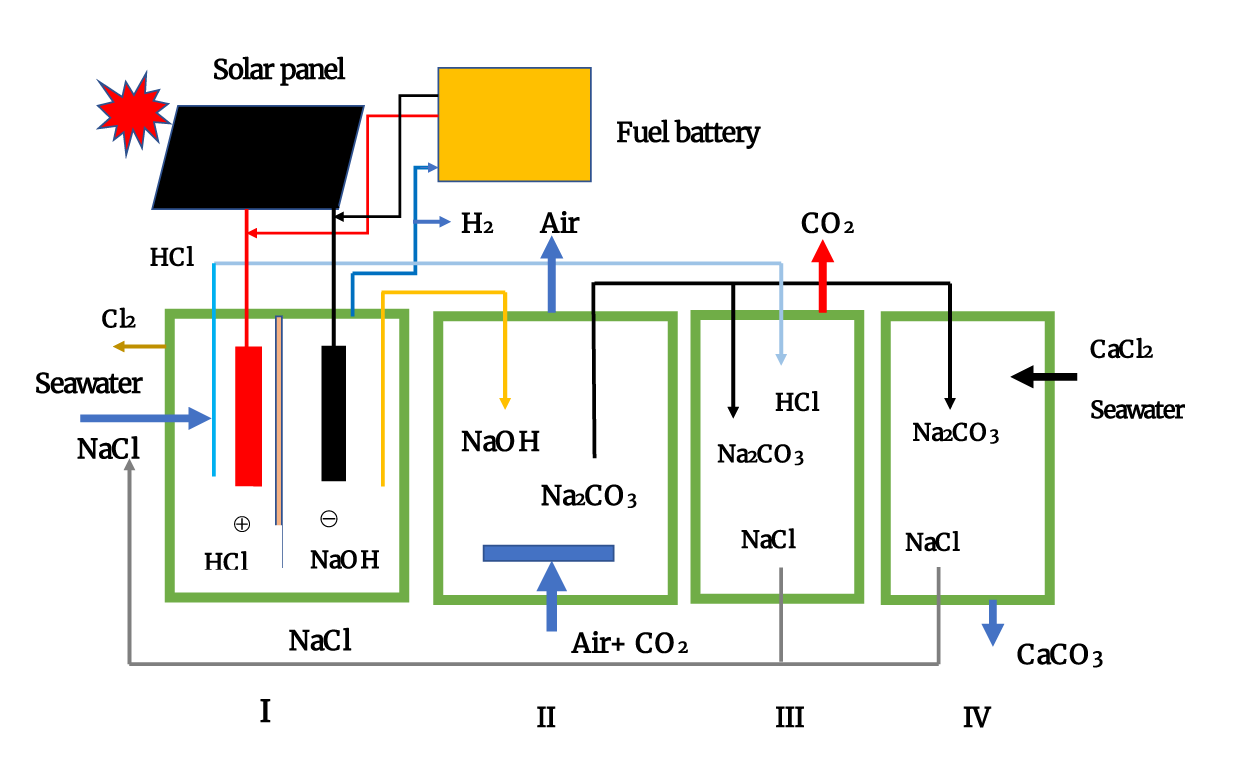
We recently proposed an integrated CO2 fixation system, as shown in figure 1. The system consists of four units. Unit I represent an electrolysis chamber where NaCl or seawater is converted to NaOH in the cathode region and HCl in the anode region. Simultaneously, H2 is generated with NaOH, and some of the generated H2 can be supplied to a fuel battery for electricity generation. Another part of the generated H2 can be used as a clean energy source. If sufficient external electricity is supplied renewable energy sources, such as solar plane and wind power, or from other energy sources, such as thermal and nuclear power, a large amount of H2 can be generated as a clean energy source.
Unit II represents the CO2 reaction chamber, where CO2 reacts with the OH− formed from NaOH. In this unit, mist formation [22] or CO2 bubbling [23] in the NaOH solution increases the reaction efficiency by expanding the surface area. In addition, increasing the volume of the reaction chamber enhances CO2 fixation efficiency. The mist formation technique of NaOH solution is suitable for direct air capture, which treats an extremely large volume of air [22], while the method involving CO2 bubbling is well-suited for fixing high concentrations of CO2 [23].
Unit III represents the CO2 liberation chamber, where Na2CO3 is converted into NaCl and CO2 by adding HCl, produced in unit I [20]. The acidification of Na2CO3 releases CO2 and NaCl, while the CO2–ethanol amine complex releases CO2 and ethanol amine [20]. This unit can produce concentrated pure CO2 from the ambient air, which can be used as a starting material for methanation [24]. For CO2 storage, geo-sequestration by injecting CO2 into underground geological formations, such as oil fields, gas fields, and saline formations, has been suggested [25,26], although these systems are still projects for future. Na2CO3 is produced via the Solvay method and used as a material in the glass industry. Furthermore, this compound mimics insulin in glucose consumption in cultured cells [27,28]. Additionally, amines also mimic insulin in glucose consumption not only in vitro but also in vivo [29].
Unit IV represents the CaCO3 formation chamber in the presence of CaCl2 [21]. Seawater can be used instead of CaCl2, although it is advisable to concentrate it before use because of its low concentration. A reverse osmotic membrane is beneficial to concentrate seawater salts, and simple seawater evaporation applies to concentrate. As the precipitate of CaCO3 is heavy, the decantation is applied to separate CaCO3 from the solution. This method is a more inexpensive alternative to filtration. Each unit can be integrated into one unit, while each can be divided into multiple units for different purposes.
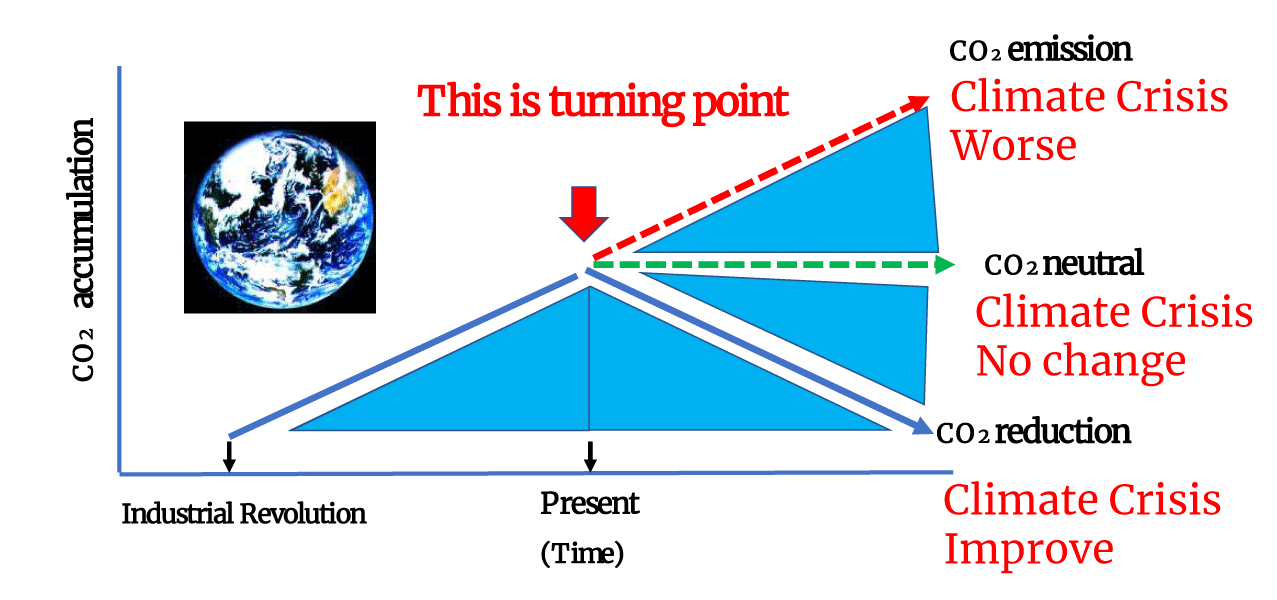
As mentioned earlier, the accumulated CO2 in the Earth’s atmosphere has induced the present climate change (Figure 2). Gradual reduction of atmospheric CO2 levels would considerably improve the present climate change situation. However, even if a carbon-neutral society with zero CO2 emission would be achieved at present, the present climate change could not be improved permanently because of the persistent CO2 concentration. In contrast, if carbon emission continues in future, the CO2 concentration in the atmosphere will increase, exacerbating the climate crisis. This scenario would have detrimental effects on both humans and other organisms on the Earth. We comprehend this fundamental theory and select a course of action that effectively reduces the accumulated CO2 in the atmosphere for future generations and a beautiful blue planet.
Conclusion
In order to improve the present climate changes, there is no method other than CO2 reduction from the ambient atmosphere immediately. In addition, the introduced system based on NaCl electrolysis and chemical reaction of NaOH and CaCl2 provides a safe, inexpensive approach to simultaneous CO2 fixation and storage. Thus, the introduced system demonstrated a simple approach to fit worldwide usage without environmental concerns.
Acknowledgment
The author thanks Enago (https://www.enago.jp) for editing a draft of this manuscript.
References
- Allan RP, et al. Climate change 2021. The physical science basis, Intergovernmental panel on climate change (IPCC). 2021;1-41.
- Rockström J, Steffen W, Noone K, Persson A, Chapin FS 3rd, Lambin EF, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ, Nykvist B, de Wit CA, Hughes T, van der Leeuw S, Rodhe H, Sörlin S, Snyder PK, Costanza R, Svedin U, Falkenmark M, Karlberg L, Corell RW, Fabry VJ, Hansen J, Walker B, Liverman D, Richardson K, Crutzen P, Foley JA. A safe operating space for humanity. Nature. 2009 Sep 24;461(7263):472-5. doi: 10.1038/461472a. PMID: 19779433.
- Sorimachi K. Epoch-making discovery for CO2 characteristics: “Pseudo osmosis” in the gas phase. Sci J Health Sci Res. 2022;1(1):49-57.
- Sorimachi K. Study on ultimate human evolution: Cooperation of cerebral and five-fingernail development. New Visions in Biological Science. 2021;3:50-64. doi: 10.9734/bpi/nvbs/v3/12202D.
- Sorimachi K. Human and earth evolution through CO2: Perspective for climate crisis. J Biomed Res. 2022;3(1):11-17.
- Lv B, Guo B, Zhou Z, Jing G. Mechanisms of CO2 Capture into Monoethanolamine Solution with Different CO2 Loading during the Absorption/Desorption Processes. Environ Sci Technol. 2015;49:10728-35.
- Choi S, Drese JH, Jones CW. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem. 2009;2(9):796-854. doi: 10.1002/cssc.200900036. PMID: 19731282.
- Jones CW. CO(2) capture from dilute gases as a component of modern global carbon management. Annu Rev Chem Biomol Eng. 2011;2:31-52. doi: 10.1146/annurev-chembioeng-061010-114252. PMID: 22432609.
- Nandi M, et al. Unprecedented CO2 uptake over highly porous N-doped activated carbon monoliths prepared by physical activation. Chem Commun. 2012;48:10283-10285.
- Hajra S, Biswas A. Efficient chemical fixation and defixation cycle of carbon dioxide under ambient conditions. Sci Rep. 2022;10:15825.
- Hiraide S. et al. High-throughput gas separation by flexible metal-organic framework with fast gating and thermal management capabilities. Nat Commun. 2020;11:3867.
- Modak A, Nandi M, Mondal J. Bhaumik A. Porphyrin based porous organic polymers: novel synthetic strategy and exceptionally high CO2 adsorption capacity. Chem Commun. 2021;48:248-250.
- Qiao Z, Zhao S, Wang J, Wang S, Wang Z, Guiver MD. A Highly Permeable Aligned Montmorillonite Mixed-Matrix Membrane for CO2 Separation. Angew Chem Int Ed Engl. 2016 Aug 1;55(32):9321-5. doi: 10.1002/anie.201603211. Epub 2016 Jun 17. PMID: 27312314.
- Takeda B, Yamaguchi B. Gas penetrability of polymer membranes. (In Japanese). J Ind Chem. 1959;62(12):1897-1904.
- Kenbishi H. Gas penetrability of gum. J Japan Gum Assoc. (In Japanese) 1980;53 (12):719-727.
- Pulyalina A, Polotskaya G, Rostovtseva V, Pientka Z, Toikka A. Improved Hydrogen Separation Using Hybrid Membrane Composed of Nanodiamonds and P84 Copolyimide. Polymers (Basel). 2018 Jul 27;10(8):828. doi: 10.3390/polym10080828. PMID: 30960753; PMCID: PMC6404051.
- Elhenawy S, Khraisheh M, AlMomani F, Hassan M. Key Applications and Potential Limitations of Ionic Liquid Membranes in the Gas Separation Process of CO2, CH4, N2, H2 or Mixtures of These Gases from Various Gas Streams. Molecules. 2020 Sep 18;25(18):4274. doi: 10.3390/molecules25184274. PMID: 32961921; PMCID: PMC7570638.
- Park HB, Kamcev J, Robeson LM, Elimelech M, Freeman BD. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science. 2017 Jun 16;356(6343):eaab0530. doi: 10.1126/science.aab0530. PMID: 28619885.
- Dutcher B, Fan M, Russell AG. Amine-based CO2 capture technology development from the beginning of 2013-a review. ACS Appl Mater Interfaces. 2015 Feb 4;7(4):2137-48. doi: 10.1021/am507465f. Epub 2015 Jan 21. PMID: 25607244.
- Sorimachi K, Gabbar H. Sustainable system for CO2 capturing with multiple products. Petro Chem Indus Intern. 2023;6(3):182-192.
- Sorimachi K. Innovative method for CO2 fixation and storage. Sci Rep. 2022 Feb 1;12(1):1694. doi: 10.1038/s41598-022-05151-9. PMID: 35105896; PMCID: PMC8807835.
- Sorimachi K. Novel method for CO2 fixation and storage preventing climate crisis: an “artificial forest” model. Petro Chem Indus Intern. 2022;5(1):1-4.
- Sorimachi K. Pilot plant model for sequential CO2 fixation and storage. Adv Environ Stu. 2022;6(1):479-483. doi: 10.36959/742/241.
- Guil-López R, Mota N, Llorente J, Millán E, Pawelec B, Fierro JLG, Navarro RM. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials (Basel). 2019 Nov 26;12(23):3902. doi: 10.3390/ma12233902. PMID: 31779127; PMCID: PMC6926878.
- Eccles J, Pratson LF, Chandel MK. Effects of well spacing on geological storage site distribution costs and surface footprint. Environ Sci Technol. 2012 Apr 17;46(8):4649-56. doi: 10.1021/es203553e. Epub 2012 Apr 2. PMID: 22433004.
- Carroll SA, Iyer J, Walsh SDC. Influence of Chemical, Mechanical, and Transport Processes on Wellbore Leakage from Geologic CO2 Storage Reservoirs. Acc Chem Res. 2017 Aug 15;50(8):1829-1837. doi: 10.1021/acs.accounts.7b00094. Epub 2017 Jul 25. PMID: 28741360.
- Sorimachi K. Direct evidence for glucose consumption acceleration by carbonates in cultured cells. Int J Pharma Phytopharm Res. 2019;9(3):1-5.
- Sorimachi K. Direct evidence for intracellular homeostasis in mammalian cells: Insulin-independent glucose metabolisms. Ann Res Rev Biol. 2022;37(3):10-19. doi: 10.9734/ARRB/2022/v37i330493.
- Sorimachi K. Amines mimic insulin in vito and in vivo: Acceleration of cellular glucose consumption. New Advances in Medicine and Medical Sciences. 2023;2:47-63. doi: 10.9734/bpi/namms/v2/19254D.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.