Biology Group . 2023 August 10;4(8):1170-1178. doi: 10.37871/jbres1783.
Unveiling the Role of MX1 and MX2 Gene Expression in Early Pregnancy of Kari Ewes: Insights into INF-tau Signaling and Potential as a Predictive Biomarker for Pregnancy Detection in Domestic Ruminants
Taimur Khan1, Ihtesham-ul-haq2, Muhammad Shahab1, Sohail Ahmad2, Numan Ullah3, Sher Hayat Khan2* and Guojun Zheng1*
2Institute of Biotechnology and Genetic Engineering, University of Agriculture, Peshawar, Pakistan
3Institute of Mobilome and Genome, Yangzhou University, China
- MX genes
- INFt
- PBMC
- Pregnancy
- ewe
Abstract
Myxovirus resistance (MX) genes are relevant to a family of interferon (IFN)-stimulated genes. In domestic ruminants such as ewes and cattle, the MX genes have two isoforms called the Myxovirus Resistance 1 (MX1) gene and the Myxovirus Resistance 2 (MX2) gene. INF-tau (INFt) is released from embryonic trophoblast cells in the early days of pregnancy and induces expression of MX genes in the uterine endometrium. In the present study, we summarized the relative expression of the MX1 and MX2 genes during the early days of pregnancy in Kari ewes. Blood samples were taken from inseminated and non-inseminated (control) ewes on different days. Peripheral Blood Monocytes (PBMC) were isolated, and with the addition of MX1 and MX2 gene primers, complementary deoxyribonucleic acid (cDNA) was synthesized by Reverse Transcriptase Polymerase Chain Reaction (RT-PCR). The cDNA was then amplified by rounds of PCR amplification. The amplified product was run on a 2% Agarose gel. The results showed expression of the MX1 and MX2 genes only in pregnant ewes, while no expression was observed in non-pregnant or control ewes. Expression in pregnant ewes was observed on days 14, 16, 17, and 18 after insemination. When comparing the MX1 and MX2 genes, the expression of the MX2 gene was relatively higher than that of the MX1 gene on all experimental days. The result suggested that INF-tau stimulated MX genes are expressed during the early days of pregnancy and are involved in the establishment of pregnancy. These results justify the development of this biomarker as a predictive tool for the early detection of pregnancy in ewes and other domestic ruminants.
Abbreviations
Myxovirus Resistance (MX); Peripheral Blood Monocytes (PBMC); Interferon tau (IFNt); Oligoadenylate Synthetase 1 (OAS1); Interferon-Stimulated Genes (ISGs); Interferon-stimulated gene 15 ubiquitin-like modifier (ISG15); Signal Transducers And Activators (STAT) 1; IFN regulatory factor 1 (IRF-1); Peripheral Blood Leukocytes (PBLs).
Introduction
The early stages of blastocyst implantation are vital for the maintenance of pregnancy, as they comprise essential events. All through the peri-implantation phase, the developing blastocyst depends on the secretion of histotrophic substances within the uterine environment [1,2]. Comprising a mixture of nutrients, enzymes, hormones, growth factors, and transport proteins, this combination is under the regulation of communication between the embryo and the mother, known as embryo-maternal cross-talk [3,4]. Interferon tau (IFNt) is an important pregnancy-related signal to the maternal system. In the initial maternal recognition mechanism in ruminants, IFNt is one of the first molecules implicated [5]. In cattle, around days 14-15 of pregnancy, the trophectodermal cells of the blastocysts release this protein [6]; and days 16–25 in buffalo [7]; days 13-17 in ewe [8]; and rises with the conceptus elongation [9-11]. This type of interferon controls immunological and luteotropic systems for effective implantation of embryos since it has antiviral, immunomodulatory, and antiproliferative capabilities [12]. Moreover, during the release of IFNt, it induces perceptible temporal changes in local and peripheral tissues [11-14]. In the uterus, IFNt inhibits luteolysis by preventing the release of prostaglandin, resulting in the maintenance of corpus luteum function [15]. In fact, IFNt activates the expression of Interferon-Stimulated Genes (ISGs), including MX1, MX2, interferon-stimulated gene 15 ubiquitin-like modifier (ISG15), and 2/,5/-oligoadenylate synthetase 1 (OAS1), in many cells, for instance luteal, endometrial, and peripheral blood cells [16,17]. In bovines, numerous studies have proved that ISG expression rises during early gestation in peripheral blood leukocytes [18-21]. In the endometrium, IFNs activate the expression of more than 750 other IFN-stimulated genes [22] but it is also possible that these genes are conferred by other type 1 interferons, such as interferon-stimulated gene 15 (ISG 15), and MX 1, MX 2 [22,23]. After infusion of IFN into the uterine vein, expression of ISG-15 was found in ovine corpus luteum cells [24]. The maintenance of a healthy corpus luteum is achieved through this interaction, which abolishes the luteolytic impulses of PGF2. Conceptualized in the endometrium, IFN tau acts locally to regulate many functions, including transcription Signal Transducers and Activators (STAT) 1 and 2, IFN regulatory factor 1 (IRF-1), and IFN regulatory factor 9 (IRF-9). are activated in the endometrium of pigs, dairy cows, ewe, ponies, humans, and mice [13]. MX1 and MX2 expression corresponds to high levels of progesterone 4 (P4) in the midst of the oestrous cycle, suggesting that P4 in uterus may also manage MX1 and MX2 gene expression [25]. The expression of ISGs like MX1 and MX2 gene was seen in peripheral blood monocytes (PBMC) of pregnant ewes, demonstrating contrast in gene expression among pregnant and non-pregnant ewes and cows [26]. In ewe’s endometrium, IFN-tau stimulates MX1 and MX2 genes. The main function of MX1 and MX2 genes is to protect conception from viral diseases. MX1 and MX2 genes have the ability to counteract the luteolytic component to promote early pregnancy [27]. The MX genes is activated by type I IFN, which is a potent inhibitor of viral replication [28]. Actual MX genes expression is activated in all cells possessing type I IFN receptors and has been used as a marker for viral contamination [29].
Currently, there are several methods for detecting pregnancy in ewe, including visual observation, ultrasound, and hormonal assays. However, these methods are often costly, time-consuming. Recent research has shown that the expression of certain genes can be used to detect early pregnancy in ewe with greater accuracy and efficiency. Specifically, the MX1 and MX2 genes have been identified as potential biomarkers for early pregnancy detection in ewe. Thus, the objective of this study was to quantify interferon-stimulated genes (MX1 and MX2) in PBMCs during peri-implantation and early pregnancy in kari ewe. Additionally, the possible relationship between MX1 and MX2 mRNA expression was also investigated. Ultimately, the findings of this study could contribute to the development of a more cost-effective and reliable method for early pregnancy detection in ewe, with potential applications for other livestock industries as well.
Methodology
Animals and experimental design
The proposed study was carried out at the Institute of Biotechnology and Genetic Engineering (IBGE), University of Agriculture, Peshawar, Pakistan. The experiment was carried out according to the guidelines of the ethical committee of the University of Agriculture, Peshawar, Pakistan. In the current research work, a total of 6 ewes were selected including 1 ewe kept as a control. Animals selection and experimental design. Ewe were selected from the Kari breed found in the Chitral region of Pakistan that had been put under synchronization. Regular clinical exams have been carried out, particularly before estrus synchronization, to rule out conditions such as endometritis, mastitis, and metabolic problems. Initial data for all animals were kept, and a protocol was adopted for bringing all the animals into the status of synchronized cyclicity. The following protocol was followed.
- 0.4-0.6 ml of Cylomate®was injected into all screened ewe.
- After 7 days, again, 0.4-0.6 ml of Cyclomate® was injected.
- After 12 hours of cyclomate® injection, 1 ml of GnRH was injected.
- 12 hours after the GnRH injection, rams were introduced to the flock.
Rams were introduced to the flocks for 24 hours and were closely observed to ensure proper mating. After mating, blood samples were collected from the jugular veins in a 10-ml EDTA tube. The blood samples were collected on day 8 of insemination, followed by samples on alternate days until day 13. From day 13 to day 18, blood samples were collected daily. All the EDTA tubes were placed in an ice box while being brought to the laboratory for further processing. The animals were grouped as pregnant, non-pregnant, and control.
Pregnancy diagnosis
Pregnancy was diagnosed through both transrectal ultrasonography and specific gene expression (MX genes). The first pregnancy was detected by gene expression within 18 days of mating. Later, it was confirmed through transrectal ultrasonography on day 30.
Isolation of PBMCs
The collected blood was centrifuged at 12000 rpm for 15 minutes to separate the three layers, and then kept on ice to visualize it clearly. Removed the upper layer and collected the middle, thin layer of PBMCs, which were then aspirated with great care. The collected PBMCs were then subjected to further processing. The isolated PBMCs were stored at -80°C (not 800 oc) until RNA extraction.
Total RNA isolation, reverse transcription, and PCR amplification
After PBMC isolation, total RNA were isolated using Trizol method. The concentration and purity of the extracted RNA were evaluated using the NanoDrop2000 equipment from Thermo Scientific (Waltham, MA, USA). The purity of RNA was measured by calculating the A260/280 ratio, which was recorded to be between 1.8 and 2.0. Furthermore, the RNA concentration in all samples was above 150 ng/μl. A first-strand cDNA synthesis kit (thermos-scientific catalog No. 1622) was used to obtain cDNA. Reverse transcriptase polymerase chain Reaction (RT-PCR) was used for cDNA synthesis. cDNA amplification was done through PCR by using MX1, MX2, and GAPDH gene primers (Table 1). Forward and reverse sequence of primers for all the genes were taken from the literature [14]. The total PCR amplification solution (20 µl) comprised 10 µl of master mix, 1 µl of cDNA, 1µl of primers (forward and reverse) (1+1 = 2 µl), and 7 µl of dH2O.The reaction procedure was as follows: predenaturation at 94°C for 5 min, denaturation at 94°C for 30s, annealing at 53°C for 30s, and extension at 72°C for 30s for 40 cycles. Thereafter, samples were stored at 4°C.
| Table 1: Primers are utilized in PCR reaction. | |||
| Primer name | Sequence | Product size | |
| GAPDH gene | Forward primer | 5-CTCCCAACGTGTCTGTTGTG-3 | 222bp |
| Reverse primer | 5-TGAGCTTGACAAAGTGGTCG-3 | ||
| MX1 gene | Forward primer | 5-ACATGAAACGGAGTCCAAGG-3 | 449bp |
| Reverse primer | 5-TGCCAGGAAGGTCTATCAGG-3 | ||
| MX2 gene | Forward primer | 5-AGGTCATGCAGAACCTCACC-3 | 654 |
| Reverse primer | 5-TAATTTCCATGGCCTTCTGG-3 | ||
Gel electrophoresis
The PCR-amplified product was subjected to agarose gel electrophoresis, which involved preparing a gel in 1xTBE buffer with ethidium bromide as a fluorescent dye. The PCR-amplified product was then run on the 2% agarose gel at 110 volts for 45 minutes. 100-bp DNA ladders were used to identify the size of the PCR products, and the bands were visualized using Gel Doc (Vilber Lourmet). The reference gene used in the analysis was GAPDH, which served as a housekeeping gene. The relative expression of the MX1 and MX2 genes was determined by comparing them to the expression of GAPDH gene.
Data obtained
Image J. software version 1.47 was used to analyze the Gel bands and obtain data. The data was analyzed through SPSS software. A one-way ANOVA and LSD test were applied to the data obtained. The relative expression of MX1, MX2 and GAPDH was demonstrated.
Relative expression = gene data/GAPDH
Results
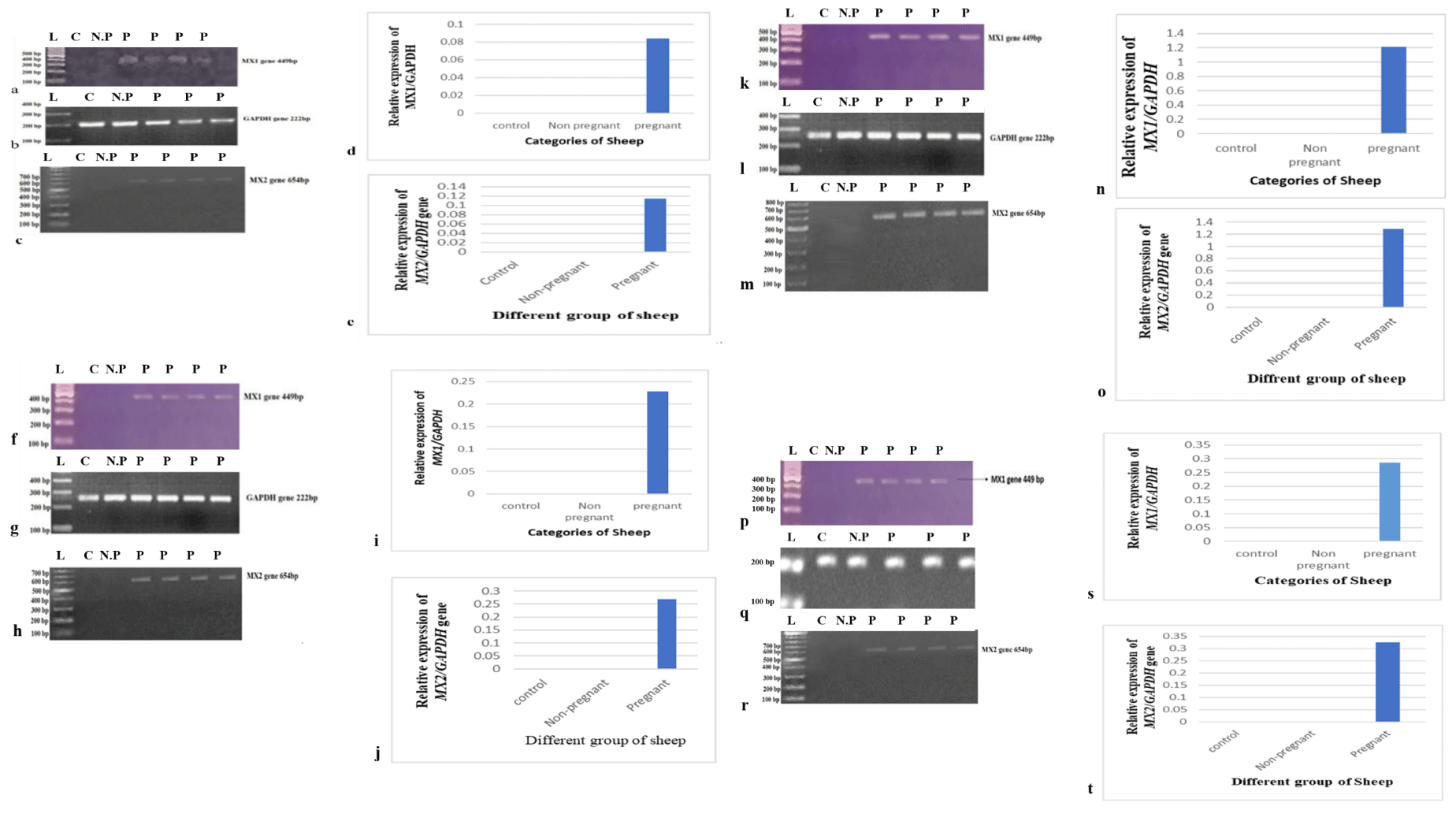
A total of 4 out of 6 ewe enrolled in this study became pregnant, and 1 remained non-pregnant as experimentally proved by MX genes expression, while 1 was kept as a control. The pregnant ewes were confirmed by ultrasound on day 30. MX genes were expressed from days 14 to 17. No expressions were recorded in non-pregnant or control ewe. The housekeeping gene GAPDH, which has 222 bp, was used as a reference gene.
MX1 and MX2 gene Expression of Pregnant and Non-Pregnant ewe on specific days.
The gel bands show amplification results of the MX1 and MX2 genes and GAPDH on day 14 post-insemination (Figure 1(a-e)). In the given figure, C indicates control, N.P indicates non-pregnant, and P indicates pregnant ewes. The results showed the expression of the MX1 and MX2 genes on day 14 only in pregnant ewes, as shown in lines 3, 4, 5, and 6 of the gel electrophoresis picture. The GAPDH gene was also successfully amplified in all samples (Figure 1b), which showed expression in all ewes on day 14. The bar graph shows the relative expression of the MX1 and MX2 genes on day 14 (Figure 1(d,e)). The samples were taken on day 16, and subsequent PCR amplification showed the expression of the MX1 and MX2 genes in all pregnant ewes, as shown in figure 1(f-j), but no expression was detected in the control and non-pregnant ewes on day 16. GAPDH gene were expressed in all samples (Figure 1g) and (Figure 1 (i,j)) represent the relative expression of MX1 and MX2 gene in day 16 Same as before. On day 17 (Figure 1(k-o) and 18 (Figure 1 (p-t)), the MX1 and MX2 genes expression was detected in pregnant ewes, but in non-pregnant ewes, the expression was not detected. The GAPDH gene showed full expression in all experimental ewe (Figure 1 (i,q)).
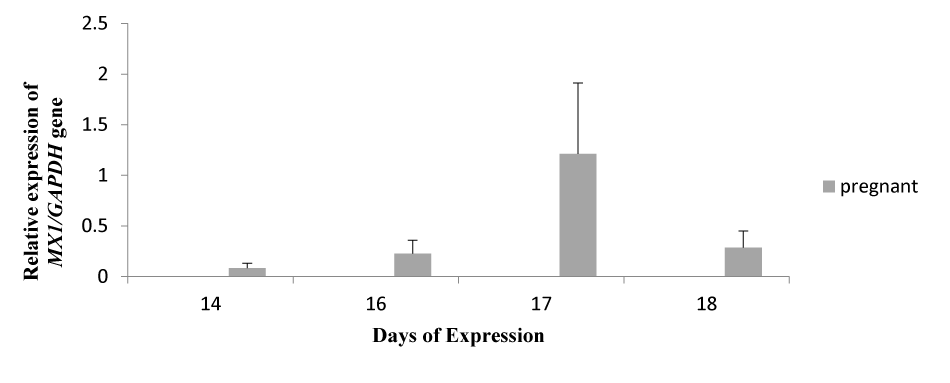
MX1 Expression on successive days
The bar graph shows the relative expression pattern of the MX1 gene on days 14, 16, 17, and 18 post-inseminations (Figure 2). The expression was only detected in pregnant ewes, which was confirmed by ultrasonic examination of all ewes on day 30 post-insemination. But in the non-pregnant and control, no expression was observed. On day 17, the expression was detected higher as compared to other expression days. The Y-axis shows the relative expression of the MX1/GAPDH gene, while the x-axis shows the days of expression.
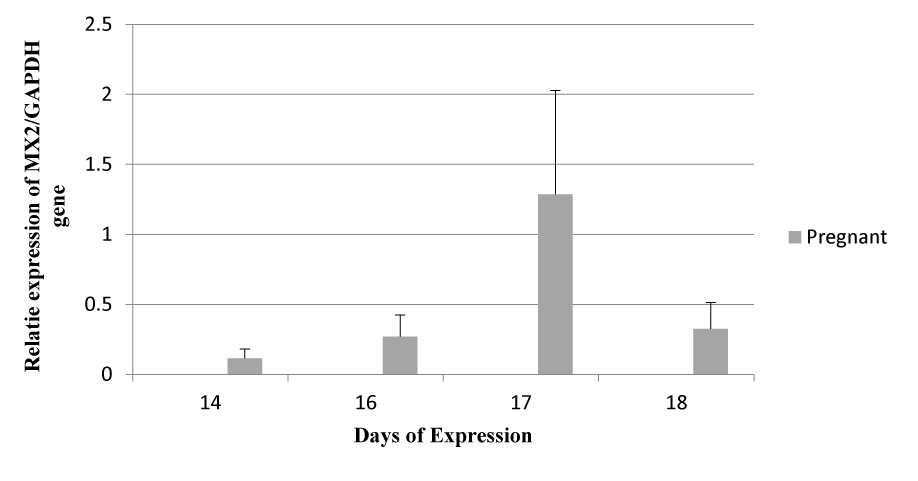
MX2 Expression on successive days
The graph shows the expression pattern of the MX2 gene on days 14, 16, 17, and 18. Expression was detected only in pregnant ewes, but in non-pregnant and control ewes, no expression was observed (Figure 3). Like MX1, on day 17, the expression of the MX2 gene was detected higher as compared to other expression days, but MX2 gene expression on successive days was detected higher as compared to the MX1 gene on successive days.
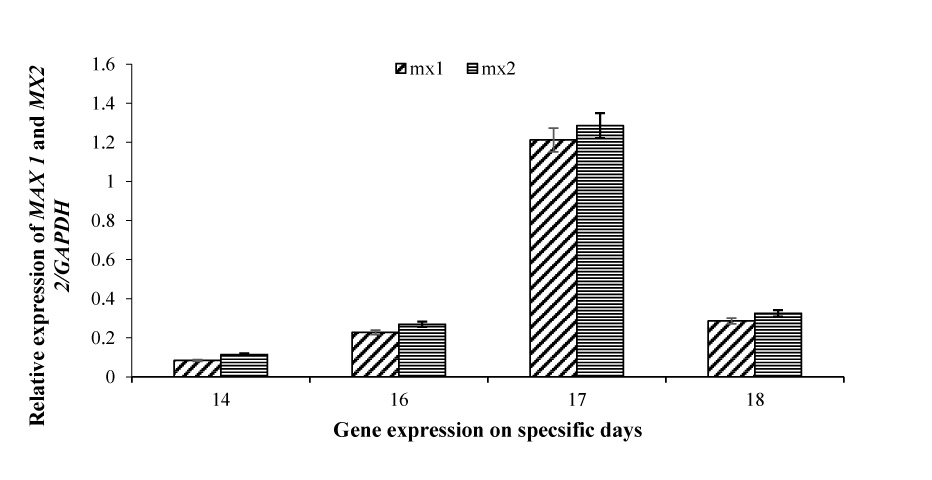
Comparison of relative expression of MX1 and MX2 between pregnant and non-pregnant ewes on different days
The relative expression pattern of the MX1 and MX2 genes on days 14, 16, 17, and 18, as I mentioned before, the expression was detected only in pregnant ewes, but in non-pregnant and control animals, no expression was observed. As shown in figure 4, on day 14, the expression of MX1 is low as compared to MX2. Similarly, on the other days, MX2 gene expression was relatively higher as compared to MX1 gene expression. Both genes showed significantly higher expression on day 17 as compared to other days. The expression pattern of both the genes was almost similar, starting from day 14 and then gradually increasing, reaching its peak on day 17 and then decreasing on day 18. The highest expression was detected by the MX2 gene on day 17 (Figure 4).
Discussion
The present research is intended to investigate the expression pattern of MX1 and MX2 genes during the early pregnancy in ewes. The Kari breed (a local breed) of ewes was used for the study. The selection of the Kari breed was due to its short gestation period and the unavailability of early studies on this breed [30]. A total of six sheep were selected for the experiment, including one sheep kept as a control. Due to the institutional ethical committee guidelines, which accentuate on limiting the number of experimental animals, we only use one ewe as control. Blood was obtained from both experimental and control ewes. However, expression of MX genes was only found in pregnant ewes at days 14, 16, 17, and 18. While no expression was observed in non-pregnant ewes. The reason for the expression of the selected genes in pregnant ewes may be due to the fact that MX genes are interferon-stimulating genes, and interferon tau is released from trophoblast cells of the embryo only in pregnant ewes and stimulates the expression of many endometrial genes responsible for it. Maternal acknowledgment of pregnancy and progression is required. The expression of MX-1 is linked to the progression of pregnancy. [31]. In similarity to our results [32], we found that the expression of MX genes is upregulated in pregnant ewes. Illustrated that the expression of MX2 and OAS1 was upregulated in pregnant cows compared to cycling cows [31]. Our results showed that expression of the MX1 and MX2 genes started on the 14th day of mating (Figure 1). Similarly, [33] found that expression of interferon-stimulating genes in ewes begins as early as day 12 of mating. Similarly, [34] reviewed MX1 RNA levels in LE stroma and myometrium. The maximum RNA level was found on day 15, and RNA was detected as early as day 11. However, no expression of MX1 mRNA was found in non-pregnant ewes. Another study by [35] examining the relative expression of MX RNA found that the expression of MX genes starts at day 14 of mating and peaks at day 17 after mating. Some of these studies reported differences in the day of first expression of MX genes from what we have reported. A possible explanation for this is that the breed of ewe we used in our study (Kari ewe) is a non-seasonal breed. It is known that Kari ewe have a relatively shorter gestation period compared to other ewe breeds [30]. Therefore, non-seasonality and fluctuations in their gestation periods can be a possible reason for the different expression on certain days. Regarding the expression pattern of the MX genes, our results showed a change in the expression pattern in pregnant and non-pregnant ewes. Expression of the MX1 and MX2 genes in pregnant ewes begins on day 14 and peaks on day 17, decreasing slightly from day 18 until completely disappearing on day 20. On the other hand, expression was not observed on any of the test days in non-pregnant ewes. In the study [21]], synthetic interferon-tau was administered intramuscularly to examine its effect on the gene expression of MX2 and ISG15 in bovine peripheral blood mononuclear cells. The results of the study showed that there is a correlation between the pattern of gene expression and the interferon administered. The study also linked embryonic deaths to the expression pattern of genes. The study concluded that INF-tau amounts can be estimated from ISG15 and MX gene expression in PBMCs. The differences in the expression pattern of MX genes in these studies and in our studies may be due to the experimental ewe used in this study having shorter gestation periods. Since MX genes are interferon-stimulating genes and interferon tau is released by the dividing term, these differences in pattern may be due to the use of different breeds of ewe. In the case of non-pregnant ewes, since there was no release of interferon, MX genes were not expressed, and therefore their expression was not recorded in non-pregnant ewes. From this study, it is concluded that in pregnancy, MX1 and MX2 are expressed due to the release of interferon tau from the newborn. Because these genes play an antiviral role, they are actually targeted against negative-strand RNA viruses. Their expression is observed in those cells that have INT receptors and are normally prognostic for viral attacks. Once the ewes are conceived, these genes begin to be expressed by interferon tau. The study also suggests that the MX genes could be used indirectly for early detection of pregnancy in ewes.
Conclusion and Recommendation
The MX1 and MX2 genes were particularly expressed in pregnant ewes. No expression was observed in non-pregnant and control ewes. Expression of the MX genes was at its maximum on day 17. Expression of the MX genes started their expression from day 14, while expression down regulated after day 17. Moreover, MX2 gene expression was recorded higher as compared to MX1 gene. In addition, a larger research sample size is required to fully explore the role of the MX genes. A comparative expression analysis of the gene should be carried out in different ewe breeds. It is necessary to find other ways to detect early pregnancy in order to reduce the loss during the open period.
Acknowledgment
This work was supported by the state-key laboratories of chemical Resources engineering at the Beijing University of Chemical Technology, Beijing 100029, and China.
Author’s Contributions
TK conceptualized the study, designed the experiment, and performed manuscript writing. MS performed the statistical analyses and drew the graphs. IH and SH's overall guidance, read the study. GZ approved and submitted the final manuscript.
Funding
This research did not receive any specific grants from funding agencies.
Declarations
The research article submitting "Expression analysis of MXI and MX2 genes for maternal recognition of concept us in ewes" for publication in your journal of repute is a unique article, and nobody did it earlier.
Consent for Publication
Consent was obtained from all participants for this publication.
Competing Interests
The authors declare that they have no competing interests.
References
- Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction. 2002 Aug;124(2):289-300. PMID: 12141942.
- Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction. 2009 Aug;138(2):195-209. doi: 10.1530/REP-09-0158. Epub 2009 Jun 5. PMID: 19502456.
- Groebner AE, Rubio-Aliaga I, Schulke K, Reichenbach HD, Daniel H, Wolf E, Meyer HH, Ulbrich SE. Increase of essential amino acids in the bovine uterine lumen during preimplantation development. Reproduction. 2011 May;141(5):685-95. doi: 10.1530/REP-10-0533. Epub 2011 Mar 7. PMID: 21383026.
- Forde N, Simintiras CA, Sturmey R, Mamo S, Kelly AK, Spencer TE, Bazer FW, Lonergan P. Amino acids in the uterine luminal fluid reflects the temporal changes in transporter expression in the endometrium and conceptus during early pregnancy in cattle. PLoS One. 2014 Jun 24;9(6):e100010. doi: 10.1371/journal.pone.0100010. PMID: 24960174; PMCID: PMC4069017.
- Thatcher W, Meyer M, Danet-Desnoyers G. Maternal recognition of pregnancy. Journal of Reproduction and Fertility-Supplements only. 1995;49:15-28.
- Hansen PJ, Tríbulo P. Regulation of present and future development by maternal regulatory signals acting on the embryo during the morula to blastocyst transition - insights from the cow. Biol Reprod. 2019 Sep 1;101(3):526-537. doi: 10.1093/biolre/ioz030. PMID: 31220231; PMCID: PMC8127039.
- Saugandhika S, Sharma V, Malik H, Saini S, Bag S, Kumar S, Singh NK, Mohanty AK, Malakar D. Expression and purification of buffalo interferon-tau and efficacy of recombinant buffalo interferon-tau for in vitro embryo development. Cytokine. 2015 Sep;75(1):186-96. doi: 10.1016/j.cyto.2015.03.012. Epub 2015 Apr 15. PMID: 25890875.
- Zhu D, Ott TL, Bazer FW. Enzyme-linked immunosorbent assay for ovine interferon-tau. J Interferon Cytokine Res. 1996 Feb;16(2):147-50. doi: 10.1089/jir.1996.16.147. PMID: 8742367.
- Ealy AD, Yang QE. Control of interferon-tau expression during early pregnancy in ruminants. Am J Reprod Immunol. 2009 Feb;61(2):95-106. doi: 10.1111/j.1600-0897.2008.00673.x. PMID: 19143673.
- Roberts RM. Interferon-tau, a Type 1 interferon involved in maternal recognition of pregnancy. Cytokine Growth Factor Rev. 2007 Oct-Dec;18(5-6):403-8. doi: 10.1016/j.cytogfr.2007.06.010. Epub 2007 Jul 27. PMID: 17662642; PMCID: PMC2000448.
- Bazer FW. Pregnancy recognition signaling mechanisms in ruminants and pigs. J Anim Sci Biotechnol. 2013 Jun 26;4(1):23. doi: 10.1186/2049-1891-4-23. PMID: 23800120; PMCID: PMC3710217.
- Kowalczyk A, Czerniawska-Piątkowska E, Wrzecińska M. The Importance of Interferon-Tau in the Diagnosis of Pregnancy. Biomed Res Int. 2021 Sep 2;2021:9915814. doi: 10.1155/2021/9915814. PMID: 34513997; PMCID: PMC8429012.
- Pugliesi G, Miagawa BT, Paiva YN, França MR, Silva LA, Binelli M. Conceptus-induced changes in the gene expression of blood immune cells and the ultrasound-accessed luteal function in beef cattle: how early can we detect pregnancy? Biol Reprod. 2014 Oct;91(4):95. doi: 10.1095/biolreprod.114.121525. Epub 2014 Sep 10. PMID: 25210129.
- Ruhmann B, Giller K, Hankele AK, Ulbrich SE, Schmicke M. Interferon-τ induced gene expression in bovine hepatocytes during early pregnancy. Theriogenology. 2017 Dec;104:198-204. doi: 10.1016/j.theriogenology.2017.07.051. Epub 2017 Jul 31. PMID: 28888122.
- Spencer TE, Bazer FW. Conceptus signals for establishment and maintenance of pregnancy. Reprod Biol Endocrinol. 2004 Jul 5;2:49. doi: 10.1186/1477-7827-2-49. PMID: 15236653; PMCID: PMC471568.
- Shirasuna K, Matsumoto H, Kobayashi E, Nitta A, Haneda S, Matsui M, Kawashima C, Kida K, Shimizu T, Miyamoto A. Upregulation of interferon-stimulated genes and interleukin-10 in peripheral blood immune cells during early pregnancy in dairy cows. J Reprod Dev. 2012;58(1):84-90. doi: 10.1262/jrd.11-094k. Epub 2011 Nov 4. PMID: 22052007.
- Toji N, Koshi K, Furusawa T, Takahashi T, Ishiguro-Oonuma T, Kizaki K, Hashizume K. A cell-based interferon-tau assay with an interferon-stimulated gene 15 promoter. Biomed Res. 2018;39(1):13-20. doi: 10.2220/biomedres.39.13. PMID: 29467347.
- Gifford CA, Racicot K, Clark DS, Austin KJ, Hansen TR, Lucy MC, Davies CJ, Ott TL. Regulation of interferon-stimulated genes in peripheral blood leukocytes in pregnant and bred, nonpregnant dairy cows. J Dairy Sci. 2007 Jan;90(1):274-80. doi: 10.3168/jds.S0022-0302(07)72628-0. PMID: 17183095.
- Green JC, Okamura CS, Poock SE, Lucy MC. Measurement of interferon-tau (IFN-tau) stimulated gene expression in blood leukocytes for pregnancy diagnosis within 18-20d after insemination in dairy cattle. Anim Reprod Sci. 2010 Aug;121(1-2):24-33. doi: 10.1016/j.anireprosci.2010.05.010. Epub 2010 May 20. PMID: 20554404.
- Kizaki K, Shichijo-Kizaki A, Furusawa T, Takahashi T, Hosoe M, Hashizume K. Differential neutrophil gene expression in early bovine pregnancy. Reprod Biol Endocrinol. 2013 Feb 5;11:6. doi: 10.1186/1477-7827-11-6. PMID: 23384108; PMCID: PMC3570308.
- Matsuyama S, Kojima T, Kato S, Kimura K. Relationship between quantity of IFNT estimated by IFN-stimulated gene expression in peripheral blood mononuclear cells and bovine embryonic mortality after AI or ET. Reprod Biol Endocrinol. 2012 Mar 22;10:21. doi: 10.1186/1477-7827-10-21. PMID: 22439976; PMCID: PMC3364858.
- Forde N, Carter F, Spencer TE, Bazer FW, Sandra O, Mansouri-Attia N, Okumu LA, McGettigan PA, Mehta JP, McBride R, O'Gaora P, Roche JF, Lonergan P. Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biol Reprod. 2011 Jul;85(1):144-56. doi: 10.1095/biolreprod.110.090019. Epub 2011 Feb 23. PMID: 21349821.
- Geisert RD, Bazer FW, Regulation of implantation and establishment of pregnancy in mammals. Tribute to 45 Year Anniversary of Roger V. Short’s ‘Maternal Recognition of Pregnancy. Springer. 2015.
- Bott RC, Ashley RL, Henkes LE, Antoniazzi AQ, Bruemmer JE, Niswender GD, Bazer FW, Spencer TE, Smirnova NP, Anthony RV, Hansen TR. Uterine vein infusion of interferon tau (IFNT) extends luteal life span in ewes. Biol Reprod. 2010 Apr;82(4):725-35. doi: 10.1095/biolreprod.109.079467. Epub 2009 Dec 30. PMID: 20042537.
- Oliveira LJ, Hansen PJ. Deviations in populations of peripheral blood mononuclear cells and endometrial macrophages in the cow during pregnancy. Reproduction. 2008 Oct;136(4):481-90. doi: 10.1530/REP-08-0218. Epub 2008 Jul 17. PMID: 18635742.
- Köse M, Görgülü M, Kaya MS, Aydilek N, Bozkaya F, Bayril T, Kurar E, Kiyma Z, Güzeloğlu A, Atli MO. Expression profiles of Interferon-tau Stimulated Genes (ISGs) in Peripheral Blood Leucocytes (PBLs) and milk cells in pregnant dairy cows. 2014;20(2). doi:10.9775/kvfd.2013.9776.
- Hicks BA, Etter SJ, Carnahan KG, Joyce MM, Assiri AA, Carling SJ, Kodali K, Johnson GA, Hansen TR, Mirando MA, Woods GL, Vanderwall DK, Ott TL. Expression of the uterine Mx protein in cyclic and pregnant cows, gilts, and mares. J Anim Sci. 2003 Jun;81(6):1552-61. doi: 10.2527/2003.8161552x. PMID: 12817504.
- Horisberger MA, Gunst MC. Interferon-induced proteins: identification of Mx proteins in various mammalian species. Virology. 1991 Jan;180(1):185-90. doi: 10.1016/0042-6822(91)90022-4. PMID: 1984648.
- Haller O, Kochs G. Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity. Traffic. 2002 Oct;3(10):710-7. doi: 10.1034/j.1600-0854.2002.31003.x. PMID: 12230469.
- Ahmad S. Gestation length of Kari sheep. Nature Precedings. 2008. doi: 10.1038/npre.2008.1620.1.
- Johnson GA, Joyce MM, Yankey SJ, Hansen TR, Ott TL. The Interferon Stimulated Genes (ISG) 17 and Mx have different temporal and spatial expression in the ovine uterus suggesting more complex regulation of the Mx gene. J Endocrinol. 2002 Aug;174(2):R7-R11. doi: 10.1677/joe.0.174r007. PMID: 12176677.
- Yankey SJ, Hicks BA, Carnahan KG, Assiri AM, Sinor SJ, Kodali K, Stellflug JN, Stellflug JN, Ott TL. Expression of the antiviral protein Mx in peripheral blood mononuclear cells of pregnant and bred, non-pregnant ewes. J Endocrinol. 2001 Aug;170(2):R7-11. doi: 10.1677/joe.0.170r007. PMID: 11479146.
- Bazer FW, Spencer TE, Ott TL, Ing NH. Regulation of endometrial responsiveness to estrogen and progesterone by pregnancy recognition signals during the periimplantation period. Springer. 1995;27-47.
- Ott TL, Yin J, Wiley AA, Kim HT, Gerami-Naini B, Spencer TE, Bartol FF, Burghardt RC, Bazer FW. Effects of the estrous cycle and early pregnancy on uterine expression of Mx protein in sheep (Ovis aries). Biol Reprod. 1998 Oct;59(4):784-94. doi: 10.1095/biolreprod59.4.784. PMID: 9746726.
- Shirozu T, Sasaki K, Kawahara M, Yanagawa Y, Nagano M, Yamauchi N, Takahashi M. Expression dynamics of bovine MX genes in the endometrium and placenta during early to mid pregnancy. J Reprod Dev. 2016;62(1):29-35. doi: 10.1262/jrd.2015-086. Epub 2015 Oct 26. PMID: 26498202; PMCID: PMC4768776.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.