Medicine Group. 2023 November 17;4(11):1557-1569. doi: 10.37871/jbres1830.
Network Pharmacology Study of Huangqi-Huangjing in the Treatment of Diabetic Nephropathy and Diabetic Cardiomyopathy
Bohan Lu3, Lei-Huang1, Xudong Chen1, Jun Hong1, Nake-Jin1, Xuechen Zhao1, Zhongmin Su4 and Jiacheng Rong1,2*
2Graduate School, Shanghai Jiao Tong University School of Medicine, Shanghai, China
3Nephrology Department, Ningbo Hangzhou Bay Hospital, Qianwan New Area, Ningbo, Zhejiang, China
4Cardiac Care Unit, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- Traditional Chinese medicine
- Herbs
- Translational medicine
- Huangqi
- Huangjing
- Network pharmacology
- Molecular docking
- Diabetic nephropathy
- Diabetic cardiomyopathy
Abstract
Objective: To systematically investigate the mechanism of Huangqi-Huangjing in the treatment of Diabetic Nephropathy (DN) and Diabetic Cardiomyopathy (DC) using network pharmacology and molecular docking analysis. To provide a theoretical basis for the development of new drugs for the treatment of DN and DC.
Methods: The active ingredients and therapeutic targets of Huangqi-Huangjing, DN and DC were predicted and screened using TCMSP, GeneCards, DisGeNet and OMIM databases. Networks of active ingredients and targets were mapped using Cytoscape 3.8.2, Protein-Protein Interactions (PPI) were analyzed using the STRING database, and enrichment analysis of key targets was performed using “clusterProfiler” in R. Molecular docking of active ingredients and key targets was performed by Autodock vina.
Results: A total of twenty-six active drug compounds, including diosgenin, formononetin, 7-O-methyl isomucronulatol, and 207 potential targets of Huangqi-Huangjing were obtained. PPI network analysis showed that targets such as AKT1, JUN, TP53, HSP90AA1 and RELA were associated with both huangqi-huangjing and DN-DC. GO and KEGG pathway analysis showed that most of these targets were involved in pathways such as Th17 cell differentiation, IL-17 signaling pathway, and AGE-RAGE signaling pathway in diabetic complications. Docking studies showed that diosgenin has ideal binding activity to TP53, RELA and AKT1.
Conclusion: The active ingredients of Huangqi-Huangjing such as diosgenin may act on DN and DC through different targets such as TP53, RELA and AKT1, which can help to develop innovative drugs for effective treatment of DN and DC.
Introduction
Diabetes Mellitus (DM) is a prevalent clinical disease with hyperglycemia resulting from insulin secretion deficiency or insulin resistance, and it can be classified into type 1 Diabetes Mellitus (T1DM) and Type 2 Diabetes Mellitus (T2DM), with T2DM being more common [1-3]. Diabetic Nephropathy (DN) and Diabetic Cardiomyopathy (DC) are the main complications associated with diabetes, with DN accompanying 20-40% of all diabetes cases [4,5]. DN is characterized by proteinuria and glomerulosclerosis, and its occurrence and development are linked to metabolic disorders, inflammation, oxidative stress, and fibrosis6. While modern medical treatments for DN involve blood glucose and blood pressure control and a reduced-fat, limited-protein diet, renal replacement therapy and organ transplantation are the main treatments for End-Stage Renal Disease (ESRD) [3]. Despite the emerging therapeutic strategies for DN, no single treatment has been discovered to reverse or slow its progression. DC is one of the common causes of death in diabetes mellitus, which can ultimately lead to myocardial fibrosis and other cardiac pathologic changes [5]. Myocardial fibrosis, however, is a typical pathology of many cardiovascular diseases, including diabetic cardiomyopathy, and is basically characterized by the deposition of large amounts of extracellular matrix proteins and disruption of the continuity of the Extracellular Matrix (ECM) as well as the excessive proliferation of activated Cardiac Fibroblasts (CFs) [6]. Myocardial fibrosis is not only one of the major causes of heart failure in patients, but it is also directly related to the risk of cardiovascular mortality [7,8].
Traditional Chinese medicine is renowned for its low toxicity, high efficacy, and multifaceted benefits [7]. Several clinical studies have demonstrated that traditional Chinese medicine can be remarkably effective in alleviating the major symptoms of DN and in slowing its progression [8]. Huangqi (Astragalus membranaceus) has been found to reduce urinary protein, lower urinary albumin, and enhance blood glucose levels [9,10], while Huangqi have hypoglycemic and antioxidant properties, which can help in mitigating the effects of DN [11,12]. Moreover, it is widely used in cardiovascular and renal diseases, suggesting a favorable protective effect [11]. Huangjing (Polygonatum kingianum), as a Chinese medicine with several similar active components to Huangqi, also has clinical benefits for the kidney and cardiovascular system, and has been used in the treatment of diabetic nephropathy as well [12]. However, the molecular targets and mechanisms of the Huangqi-Huangjing mixture in DN and DC are not yet fully known.
The conventional approach to drug research is inadequate for investigating traditional Chinese medicine formulations with complex components and multiple targets [13]. Traditional Chinese medicine network pharmacology is an innovative research method used to study active ingredients and predict effector targets [14]. It is widely employed to analyze the active ingredients and mechanisms of traditional Chinese medicine, predict their safety, and identify their effector targets. In this study, Huangqi and Huangjing were analyzed in terms of composition, and their effective components were screened for and identified. The component target proteins were then validated and subjected to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. Protein-Protein Interaction (PPI) analysis was performed to identify key target proteins, and pharmacological networks were constructed. In the ever-increasingly aware-aroused environment of “synchronous treatment of heart and kidney”, this paper utilizes network pharmacology to investigate the active ingredients of Huangqi-Huangjing mixture, as well as the molecular targets and effector mechanisms associated with treating DN and DC.
Materials and Methods
Identification of Huangqi-Huangjing candidate components
At the very beginning, Traditional Chinese Medicine Systems Pharmacology (TCMSP) database (http://tcmspw.com/tcmsp.php) was used to acquire data on the chemical components of the two Chinese medicinal herbs, including molecule name, drug half-life, oral bioavailability, molecular mass, and drug likeness [15].
Screening of DN and DC related targets and prediction of the DN and DC targets of Huangqi-Huangjing’s active ingredients
DN and DC related targets were obtained from GeneCards (https://www.genecards.org), DisGeNet (https://www.disgenet.org/home/) and OMIM (https://omim.org/) databases, and DN and DC intersecting disease targets were obtained from Venn. The targets of the active ingredients in Huangqi and Huangjing were obtained from the TCMSP database.
Candidate key target screening and Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
We obtained the same targets as DN and DC in the drug prediction and use them as candidate key targets. Venn diagrams were plotted. To understand the candidate key target related functions, the R package “ClusterProfiler” was used for GO and KEGG enrichment analysis of candidate key targets [16].
Construction of the candidate key target-pathway networks and Protein-Protein Interaction (PPI) Network
Cytoscape 3.7.2 maps candidate key target-pathway regulatory networks. The therapeutic targets were imported into the STRING (https://string-db.org) PPI prediction platform, and the species was set as human, to construct the therapeutic target protein PPI network. Cytoscape 3.7.2 was used to build a visual PPI network. The PPI network TSV file obtained was imported into Cytoscape 3.7.2 to construct a PPI network based on “degree” and “combined score”. The “Degree” was used as the limiting condition to screen for hub genes of Huangqi-Huangjing as a treatment for DN and DC.
Construction of “drug-active ingredient-therapeutic target” network
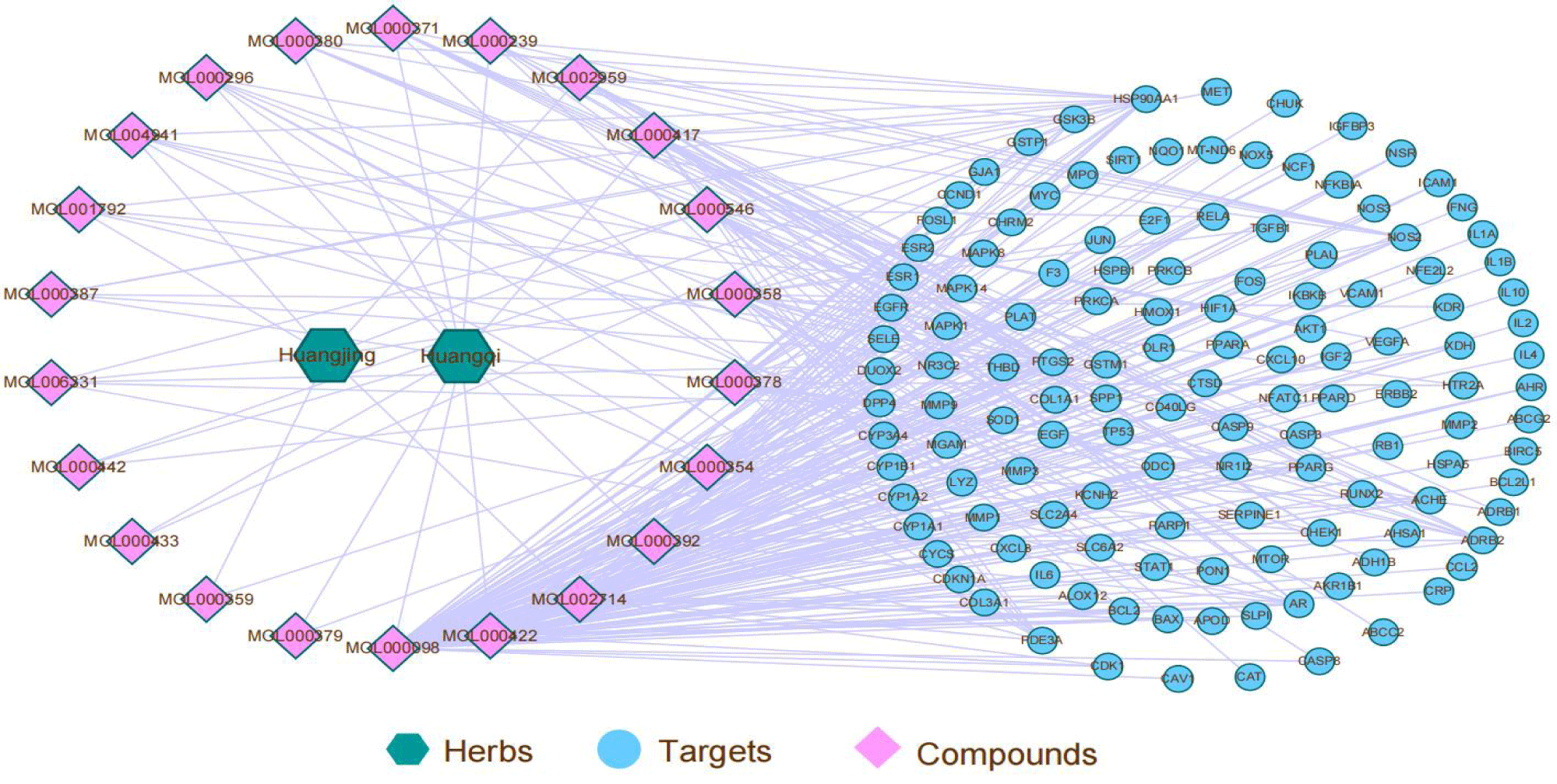
The aforementioned hub genes and active ingredients of Huangqi-Huangjing were mapped. Then the hub genes and active ingredients were mapped and the drug-active ingredient-hub disease network was constructed using Cytoscape 3.7.2.
Molecular Docking
Five key protein target nodes in the pharmacological network (JUN, HSP90AA1, TP53, AKT1, RELA) and the small drug molecules diosgenin, formononetin as well as 7-O-methylisomucronulatol were selected for molecular docking analysis. Information on complexes of the target proteins bound to other ligands was downloaded from the Protein Data Bank (PDB) database (http://www.rcsb.org/) [17]. and used for subsequent studies. The pymol software was used to remove other ligands and water molecules, and target protein was isolated for subsequent molecular docking. The molecular structure files of small molecules were downloaded from the PubChem Compound database (https://pubchem.ncbi.nlm. nih.gov/) in SDF format and converted to PDB format by pymol for subsequent molecular docking. Then, based on Lamarckian GA algorithm, Autodock software (Version 4.2.6) [18] was used to study the possibility of molecular docking between small drug molecule and targets.
Results
Composition and component screening
We searched the TCMSP database using “Huangqi” and “Huangjing” as the keyword to obtain 87 and 38 related compounds, respectively. We filtered these compounds using the criteria OB > 30% and DL ≥ 0.18. Then 26 active ingredients were obtained finally. A Huangqi-Huangjing active compound library containing 26 compounds was established. The basic information of these compounds is shown as follows table 1.
| Table 1: TCMSP drug active ingredient. | ||||
| MOL_ID | Molecule Name | OB | MW | DL |
| MOL000098 | Quercetin | 46.43334812 | 302.25 | 0.27525 |
| MOL000422 | Kaempferol | 41.88224954 | 286.25 | 0.24066 |
| MOL000378 | 7-O-Methylisomucronulatol | 74.68613752 | 316.38 | 0.29792 |
| MOL000358 | Beta-sitosterol | 36.91390583 | 414.79 | 0.75123 |
| MOL000380 | (6aR,11aR)-9,10-Dimethoxy-6a,11a-dihydro-6H-benzofurano[3,2-c]chromen-3-ol | 64.25545452 | 300.33 | 0.42486 |
| MOL000371 | 3,9-Di-O-methylnissolin | 53.74152673 | 314.36 | 0.47573 |
| MOL000392 | Formononetin | 69.67388061 | 268.28 | 0.21202 |
| MOL000354 | Isorhamnetin | 49.60437705 | 316.28 | 0.306 |
| MOL000296 | Hederagenin | 36.91390583 | 414.79 | 0.75072 |
| MOL006331 | 4',5-Dihydroxyflavone | 48.55120352 | 254.25 | 0.1859 |
| MOL001792 | DFV | 32.76272375 | 256.27 | 0.18316 |
| MOL004941 | (2R)-7-Hydroxy-2-(4-hydroxyphenyl)chroman-4-one | 71.12298901 | 256.27 | 0.18303 |
| MOL000417 | Calycosin | 47.75182783 | 284.28 | 0.24278 |
| MOL002959 | 3'-Methoxydaidzein | 48.56909374 | 284.28 | 0.24261 |
| MOL002714 | Baicalein | 33.51891869 | 270.25 | 0.20888 |
| MOL000239 | Jaranol | 50.82881677 | 314.31 | 0.29148 |
| MOL000546 | Diosgenin | 80.87792491 | 414.69 | 0.80979 |
| MOL000433 | FA | 68.96043622 | 441.45 | 0.7057 |
| MOL000439 | Isomucronulatol-7,2'-di-O-glucosiole | 49.28105539 | 626.67 | 0.62065 |
| MOL000379 | 9,10-Dimethoxypterocarpan-3-O-β-D-glucoside | 36.73668801 | 462.49 | 0.9243 |
| MOL009763 | (+)-Syringaresinol-O-beta-D-glucoside | 43.35308428 | 580.64 | 0.76682 |
| MOL000387 | Bifendate | 31.09782391 | 418.38 | 0.66553 |
| MOL000442 | 1,7-Dihydroxy-3,9-dimethoxy pterocarpene | 39.04541112 | 314.31 | 0.47943 |
| MOL000359 | Sitosterol | 36.91390583 | 414.79 | 0.7512 |
| MOL000033 | (3S,8S,9S,10R,13R,14S,17R)-10,13-Dimethyl-17-[(2R,5S)-5-propan-2-yloctan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | 36.22847056 | 428.82 | 0.78288 |
| MOL000211 | Mairin | 55.37707338 | 456.78 | 0.7761 |
| Note: DL: Drug-Like; OB: Oral Bioavailability. | ||||
Drug and disease target screening
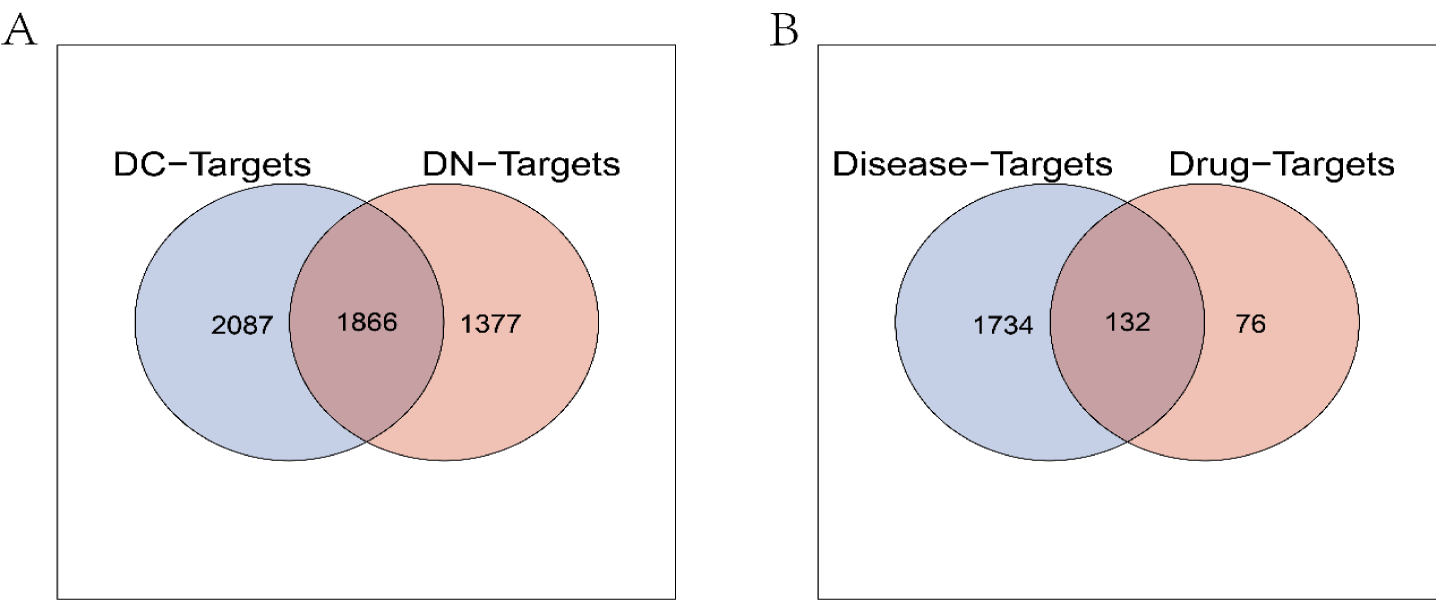
The TCMSP database was used to extract 207 targets corresponding to 26 active compounds. The keywords “Diabetic Cardiomyopathy” and “Diabetic nephropathy” were used to obtain 2819 and 3819, 39 and 189, 1189 and 320 DN and DC-related targets in GeneCards, OMIM and DisGeNET databases, respectively. The disease targets obtained from the three databases were integrated and de-duplicated to obtain 4043 DN and 3953 DC-related targets, respectively. The intersection of DN-Targets and DC-Targets was taken by Venn in R. In total, 1866 overlapping target genes were obtained and visualized (Figure 1A).
Key drug target screening to DN and DC
The 1866 DN and DC-related targets obtained were intersected with 207 drug targets, and a total of 132 common targets were obtained as potential targets of action of Huangqi-Huangjing for DN and DC treatment and visualized by Venn (Figure 1B).
Gene ontology and KEGG enrichment analyses
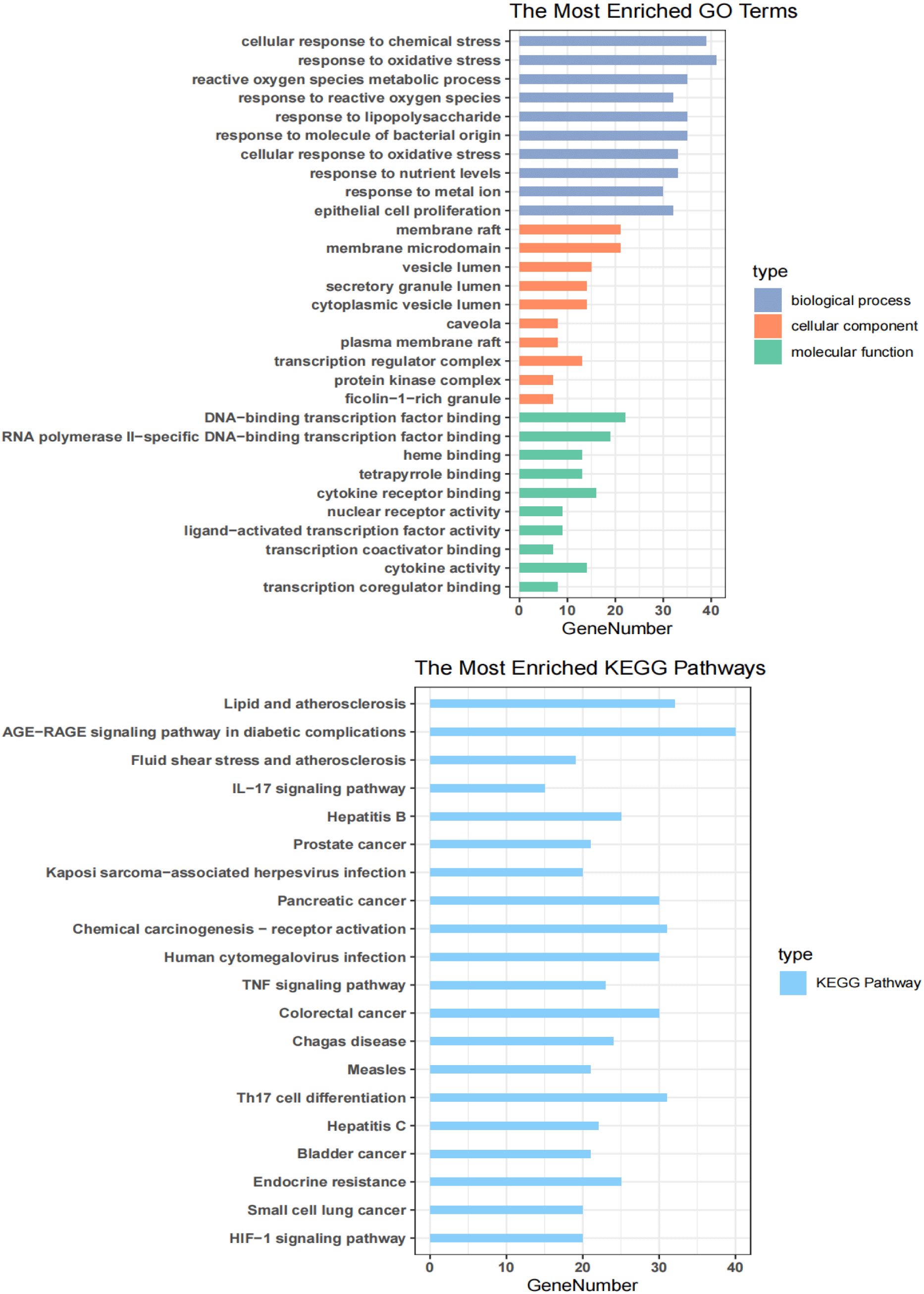
GO enrichment analysis of 132 key targets was performed by the “cluster Profiler” package in R software, and the significantly expressed GO entries were screened with p < 0.05, counts ≥ 1. The current 10 of each entry were selected for visualization (Figure 2A). The obtained entries were mainly focused on BP with 2247 entries, mainly related to some processes like cellular response to oxidative stress, cellular response to chemical stress, response to metal ions, response to lipopolysaccharide and response to reactive oxygen species. And a total of 137 entries were enriched for MF, mainly in tetrapyrrole binding, heme binding and DNA-binding transcription factor binding. 49 entries were enriched for CC, mainly in cytoplasmic vesicle lumen, secretory granule lumen, and vesicle lumen and membrane microregion. The above results suggest that Huangqi-Huangjing improves DN and DC mainly through various BPs.
To further identify the pathways associated with the 132 key targets, we performed KEGG enrichment analysis on these genes. A total of 169 relevant pathways were identified under the screening condition of p < 0.05, and the top 20 significantly expressed pathways were selected and visualized by enrichplot (Figure 2B). The main pathways included HIF-1 signaling pathway, endocrine resistance, Th17 cell differentiation, tumor necrosis factor signaling pathway, IL-17 signaling pathway, AGE-RAGE signaling pathway in diabetic complications and some diseases. Among them, the tumor-related pathways were significantly enriched, indicating that the active ingredients in Huangqi-Huangjing exerted their ameliorative effects on DC and DN through multiple pathways.
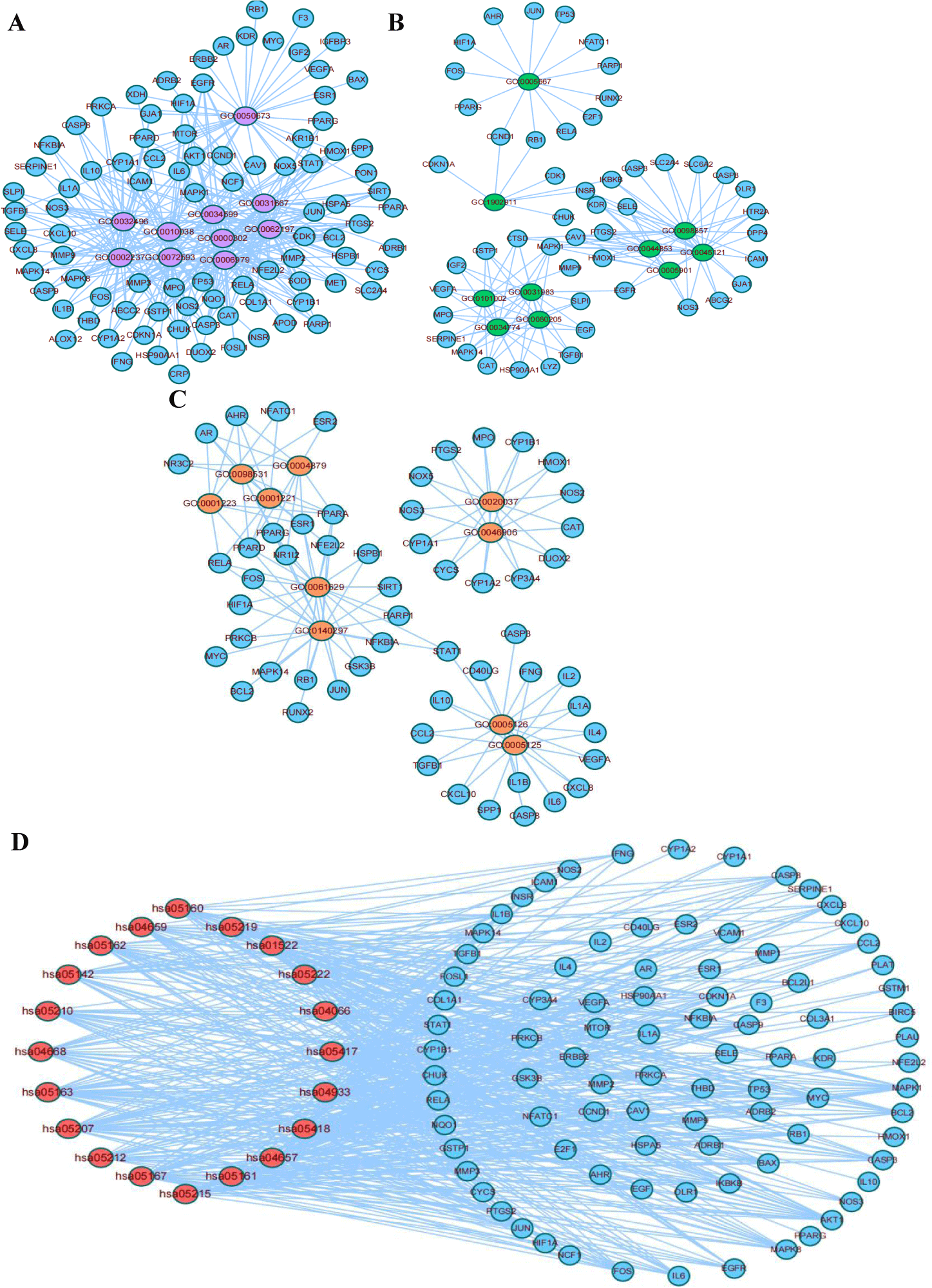
Key target-pathway regulatory network
To explore the correspondence between key targets and pathways, we extracted the top 10 entries in BP, MF and CC in GO enrichment, extracted the target compounds of key targets, constructed the regulatory network map of key target-function, and visualized the results by Cytoscape 3.7.2 (Figures 3A-C). Ninety-two, 50 and 56 key targets were enriched in the key-target-BP, key-target-CC and key-target-MF networks, respectively. The most critical targets were enriched in critical-target-BP with pathway correspondence. The KEGG results were analyzed for key target-pathway regulatory networks, and network maps were drawn and the results were visualized by Cytoscape 3.7.2. A network diagram containing 20 KEGG Pathways, 89 key targets and 500 association pairs was obtained (Figure 3B).
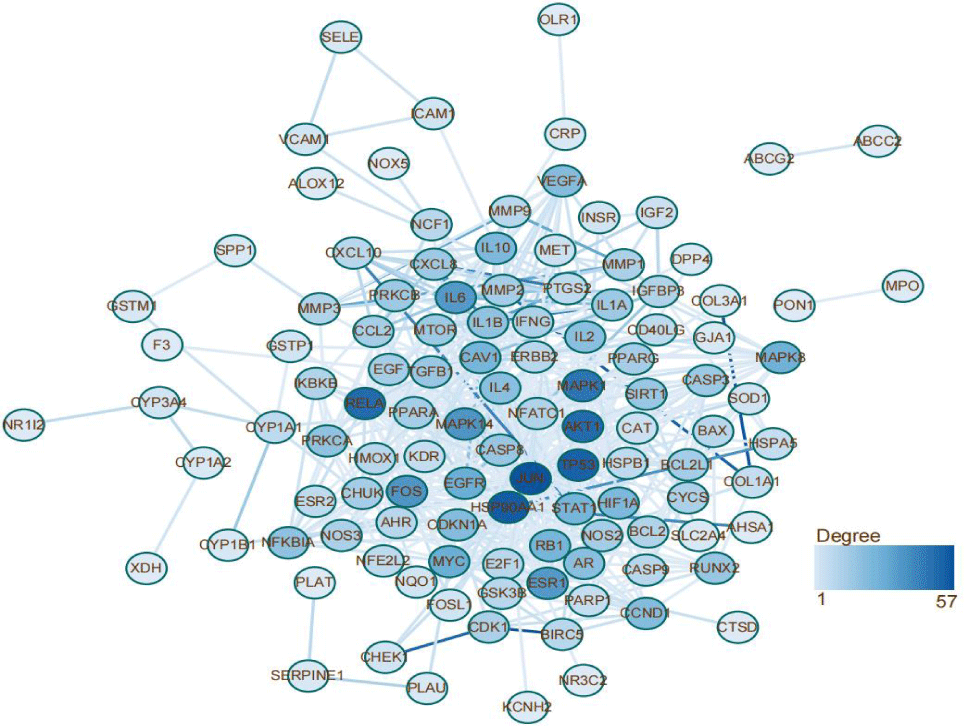
Target proteins PPI network construction and analysis
To determine how the overlapping genes interact, the 132 common targets were imported into the STRING database, and a PPI network diagram was drawn (Figure 4). As stated above, more adjacent genes in the PPI map played more important roles. By calculating the number of nodes connected to each gene, the top 30 genes in terms of centrality were identified as the most important genes of Huangqi-Huangjing with respect to the treatment of DN and DC; these included JUN, HSP90AA1, TP53, AKT1 and RELA.
“Drug-active ingredient-therapeutic target” network diagram construction
A “drug-active ingredient-therapeutic target” network diagram was constructed using Cytoscape 3.7.2 (Figure 5). The network contains 2 herbal nodes, 22 drug active ingredient nodes, 132 key targets and 338 edges. Three ingredients including MOL000546/MOL000378/MOL000392 with the utmost edges connected to other nodes were chosen for molecular docking in the next step.
Molecular Docking
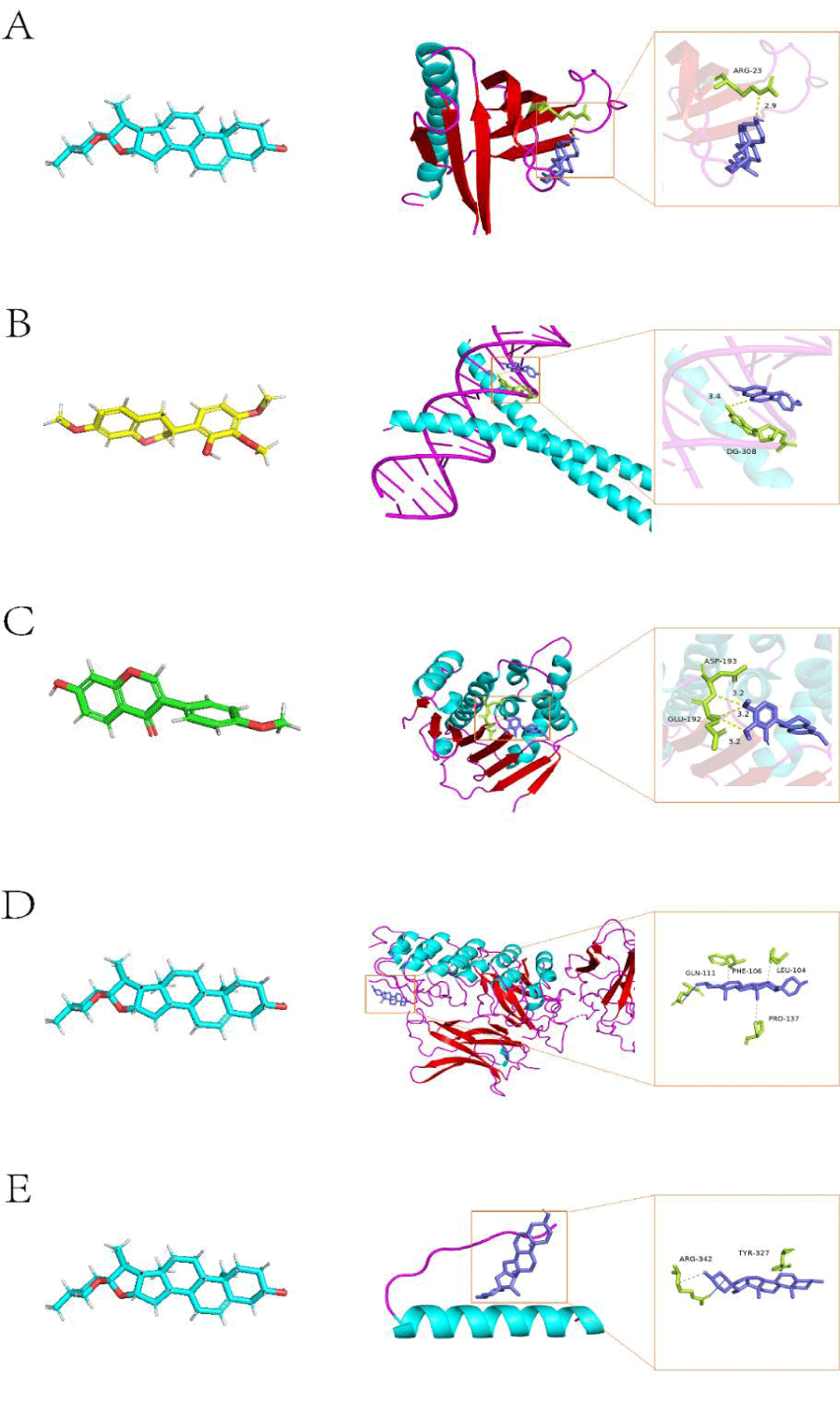
Molecular docking studies were carried out to investigate the binding modes of diosgenin, formononetin and 7-O-methylisomucronulatol with AKT1, JUN, HSP90AA1, RELA and TP53 (Table 2). The binding energy of each target site to small analytical compounds was counted (Table 3). The crystal structure of AKT1 named 1unp was downloaded from the PDB database, molecular docking was performed by AutoDock vina, and then the results were visualized by PyMol software. The residues such as ARG-23 were obtained to have hydrogen bonding interactions with diosgenin molecule with a binding energy of -7.6 kcal/mol (Figure 6A). The residue DG-308 had hydrogen bonding interactions with formononetin molecule with a binding energy of -7.1 kcal/mol for the binding relationship between JUN and formononetin (Figure 6B). The crystal structure of HSP90AA1 named 1uy7 was obtained, and the docking results of 1uy7 with 7_O_methylisomucronulatol showed hydrogen bonding interactions between residues ASP-193, GLU-192 and 7_O_methylisomucronulatol molecules with a binding energy of -5.5 kcal/mol (Figure 6C). The target RELA corresponds to the crystal structure 1ikn, and the molecular docking results of 1ikn with diosgenin indicate that residues GLN-111, PHE-106, LEU-104, PRO-137 have hydrophobic bonding interactions with diosgenin molecules with a binding energy of -9.1 kcal/mol (Figure 6D). TP53 corresponds to the crystal structure 1aie the docking of 1aie with diosgenin molecule showed that residues ARG-342, TYR-327 have hydrophobic bonding mode with diosgenin molecule with binding energy of -8.6 kcal/mol (Figure 6E).
| Table 2: Docking molecular parameters and genes. | |||||
| MOL_ID | Molecule_name | OB | MW | DL | Targets |
| MOL000546 | Diosgenin | 80.87792491 | 414.69 | 0.80979 | AKT1 |
| MOL000378 | 7-O-Methylisomucronulatol | 74.68613752 | 316.38 | 0.29792 | HSP90AA1 |
| MOL000392 | Formononetin | 69.67388061 | 268.28 | 0.21202 | JUN |
| MOL000546 | Diosgenin | 80.87792491 | 414.69 | 0.80979 | RELA |
| MOL000546 | Diosgenin | 80.87792491 | 414.69 | 0.80979 | TP53 |
| Table 3: Hub gene and drug docking results. | |||
| Target | Compounds | PDB ID | Total Score (Kcal/mol) |
| AKT1 | Diosgenin | 1 unp | -7.6 |
| JUN | Formononetin | 1 jnm | -7.1 |
| HSP90AA1 | 7-O-Methylisomucronulatol | 1 uy7 | -5.5 |
| RELA | Diosgenin | 1 ikn | -9.1 |
| TP53 | Diosgenin | 1 aie | -8.6 |
Discussion
Diabetes mellitus is a rapidly growing epidemic that has led to diabetic complications, the main cause of morbidity and mortality among patients [19,20]. Cardiovascular disease is the leading cause of death in diabetic patients. Although Coronary Artery Disease (CAD) is the primary Cause of Heart Failure (HF) and cardiovascular death in diabetics, the risk of heart failure remains elevated even after adjusting for CAD and hypertension [21]. Therefore, the term "Diabetic Cardiomyopathy" (DC) was introduced to describe this cardiac entity, characterized by ventricular dysfunction in the absence of CAD and hypertension [22]. Although DC is clinically characterized by cardiac hypertrophy and diastolic dysfunction, which can lead to heart failure with a preserved ejection fraction, it mostly lacks classical features of a cardiomyopathy seen in clinical studies, such as ventricular dilation and significant systolic dysfunction [4]. This has led to controversy regarding the existence of DC. Instead of being a cardiomyopathy in the traditional sense, DC represents a combination of molecular myocardial abnormalities that increases the risk of myocardial dysfunction, especially when additional stressors such as hypertension and CAD are present [19]. Diabetic nephropathy increases morbidity and mortality in both type 1and type 2diabetes mellitus and is the second most-common cause of chronic kidney disease after chronic glomerular disease [23,24]. Clinically, microalbuminuria isused as an important index to evaluate DN progression. Diabetic nephropathy and diabetic cardiomyopathies are both one of the major complications in the pathogenesis of diabetes mellitus [25,26]. Previous studies on diabetic nephropathy are more numerous, but studies on diabetic cardiomyopathies are still not deep enough, and studies on the association of these two diseases are still lacking. In the present study, following network pharmacology analyses based on PPI targets and Drug-active ingredient-therapeutic target network. Three key components (diosgenin, formononetin, 7-O-methylisomucronulatol) were identified from the Huangqi-Huangjing mixture, and these three key components were associated with five key genes (AKT1, JUN, HSP90AA1, RELA, TP53).
The Network pharmacology method analyses indicated that diosgenin, formononetin and 7-O-methylisomucronulatol play key roles in the progression of DN and DC. Previous studies have suggested that diosgenin elements may help maintain functional health by modulating cellular pathways and reducing the risk of diabetes [27]. These elements can affect the pancreatic beta cell renewal pathway, promote insulin secretion, initiate GLUT4, enhance dehydroepiandrosterone, and modify the ER-α-mediated PI3K/AKT pathway [28]. These findings suggest that diosgenin elements could be a promising approach for the development of diabetes treatments. In a study conducted on STZ-induced diabetic rats, diosgenin elements were found to significantly improve the level of oxidative stress, reduce LPO, and increase endogenous antioxidant levels [29]. Diosgenin was also found to have anti-inflammatory effects against myocardial injury in diabetic mice by modulating the RIP140 signaling pathway [30]. Formononetin, a novel isoflavonoid isolated from Astragalus membranaceus, has diverse pharmacological activities [31]. By treating diabetic rats with Formononetin, Huang [32]. Showed that Formononetin attenuated renal tubular epithelial cell apoptosis, mitochondrial fragmentation, and restored the expression of mitochondrial dynamics-related proteins Drp1, Fis1 and Mfn2, as well as Bax, Bcl2 and Caspase-3. Studies have shown that formononetin can upregulate the protein expression of Sirt1 and PGC-1α in the kidneys of diabetic rats [33]. In vitro experiments, we have also demonstrated that it inhibits high glucose-induced apoptosis in HK-2 cells, reducing production of mitochondrial superoxide dismutation products, and attenuates the loss of mitochondrial membrane potential. Overall, formononetin helped alleviate renal tubular injury and mitochondrial damage in rats with diabetic nephropathy by regulating the Sirt1/PGC-1α pathway and may be beneficial in treating this condition [33].
In the pharmacological network, AKT1, JUN, HSP90AA1, RELA, TP53 were important target protein nodes. AKT1 in the renal tubular epithelium and p-Akt1 (Ser(473)) are more prevalent in diabetic patients [34]. The regulation of TP53/AKT1 could be involved in the cell apoptosis and cell autophagy observed in DN [35,36]. TP53 has the same function as TRIAP1. Under low glucose conditions, TRIAP1 can be induced to be expressed by TP53 and reduce the associated apoptosis, and it has been shown that TRIAP1 can interact with heat shock proteins (HSP90AA1) and thus regulate apoptosis [37,38]. HSP90AA1 and TP53 is involved in inflammation and regulates many factors to induce an inflammatory response, thus participating in the pathogenesis of DC.
There were 132 predicted DN and DC-related targets of Huangqi-Huangjing treatment were identified. The 20 pathways of KEGG analysis with the highest degree of enrichment were selected for analysis. Many of the top 20 pathways in the KEGG enrichment analysis were related to DN, the pathway with the highest degree of enrichment and a strong correlation with DN was the AGE-RAGE signaling pathway in diabetic complications. One study showed that AGE can bind to its receptor (RAGE) to induce oxidative stress and promote inflammation and thrombosis, thereby resulting in diabetes-related vascular complications [39,40]. Furthermore, the formation and accumulation of AGEs can promote mitochondrial peroxide synthesis, resulting in further cytotoxicity and causing further kidney injury [39]. Th17 is a new type of effector CD4+ T-cell in the IL-17 signaling pathway that secretes the IL-17A, which can further promote the secretion of proinflammatory factors and macrophage infiltration, thereby exacerbating kidney damage in DN [41,42]. Huangqi-Huangjing effector targets in this pathway shows that its therapeutic effects on DN and DC may result from its inhibition of the effects of IL17A to alleviate kidney injury in DN and DC. Network correlation analysis of 132 key targets with GO and KEGG enrichment results revealed a high degree of overlap with the results of GO and KEGG enrichment analysis for 132 targets, such as the AGE-RAGE signaling pathway and IL-17 signaling pathway in diabetic complications. It indicates the feasibility of using the network pharmacology procedure in this study to identify core therapeutic targets and pathways. The results of this study suggest that Huangqi-Huangjing may exhibit therapeutic effects on DN and DC by affecting metabolism, controlling apoptosis and inhibiting inflammation.
Our study has some limitations. First of all, all results were output based on the present online stored big data of TCM and the virtual analysis, of which the real in-vivo situation could not be accurately simulated. Secondly, the referred genes in this process might not be completely enrolled due to the collection of databases. Ultimately, some non-functional genes would possibly be included on account of the poor selection and simple intersection for genes in common. Therefore, more formal databases could be adopted for more in-depth analyses, while further experiments were essentially needed in the future.
Conclusion
In conclusion, the diosgenin, formononetin, 7-O-methylisomucronulatol were identified as key components of Huangqi-Huangjing for the treatment of DN and DC. AKT1, JUN, HSP90AA1, RELA and TP53 may be key targets for Huangqi-Huangjing to exert its therapeutic effects on DN and DC. The AGE-RAGE and IL-17 signaling pathways are important in DN and DC and may be the key pathways for Huangqi-Huangjing to exert its therapeutic effects. However, the conclusion still needs to be validated by further experiments.
Author Contributions
Conceptualization, Xuechen Zhao, Lei huang and Jiacheng Rong; Data curation, Nake Jin, Zhongmin Su and Jiacheng Rong; Formal analysis, Jiacheng Rong; Funding acquisition, Xudong Chen and Jiacheng Rong; Investigation, Jun Hong; Methodology, Xudong Chen and Jiacheng Rong; Resources, Xuechen Zhao; Software, Jiacheng Rong; Supervision, Xudong Chen and Jiacheng Rong; Validation, Jun Hong; Visualization, Xuechen Zhao and Jiacheng Rong; Writing – original draft, Nake Jin; Writing – review & editing, Zhongmin Su and Jiacheng Rong.
Funding
There is no funding of this study.
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
Data Availability
The datasets applied in this study are readily accessible from the online repositories. This article contains the names of the repositories.
Ethical Statement
Ethical approval was not applicable in this study.
References
- Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, Rossing P, Groop PH, Cooper ME. Diabetic kidney disease. Nat Rev Dis Primers. 2015 Jul 30;1:15018. doi: 10.1038/nrdp.2015.18. PMID: 27188921; PMCID: PMC7724636.
- Blair M. Diabetes Mellitus Review. Urol Nurs. 2016 Jan-Feb;36(1):27-36. PMID: 27093761.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017 Dec 7;12(12):2032-2045. doi: 10.2215/CJN.11491116. Epub 2017 May 18. PMID: 28522654; PMCID: PMC5718284.
- Hölscher ME, Bode C, Bugger H. Diabetic Cardiomyopathy: Does the Type of Diabetes Matter? Int J Mol Sci. 2016 Dec 18;17(12):2136. doi: 10.3390/ijms17122136. PMID: 27999359; PMCID: PMC5187936.
- Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH. Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988-2014. JAMA. 2016 Aug 9;316(6):602-10. doi: 10.1001/jama.2016.10924. PMID: 27532915; PMCID: PMC5444809.
- Boucek P. Diabetická nefropatie/diabetické onemocnení ledvin [Diabetic nephropathy/diabetic kidney disease]. Vnitr Lek. 2013 Mar;59(3):201-3. Czech. PMID: 23713188.
- Wang Y, Liu T, Ma F, Lu X, Mao H, Zhou W, Yang L, Li P, Zhan Y. A Network Pharmacology-Based Strategy for Unveiling the Mechanisms of Tripterygium Wilfordii Hook F against Diabetic Kidney Disease. J Diabetes Res. 2020 Nov 20;2020:2421631. doi: 10.1155/2020/2421631. PMID: 33274236; PMCID: PMC7695487.
- Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013 Mar;11(2):110-20. doi: 10.1016/S1875-5364(13)60037-0. PMID: 23787177.
- Sang Z, Zhou L, Fan X, McCrimmon RJ. Radix astragali (huangqi) as a treatment for defective hypoglycemia counterregulation in diabetes. Am J Chin Med. 2010;38(6):1027-1038.
- Kim J, Moon E, Kwon S. Effect of Astragalus membranaceus extract on diabetic nephropathy. Endocrinol Diabetes Metab Case Rep. 2014;2014:140063. doi: 10.1530/EDM-14-0063. Epub 2014 Sep 1. PMID: 25298884; PMCID: PMC4176648.
- Liu S, Jia QJ, Peng YQ, Feng TH, Hu ST, Dong JE, Liang ZS. Advances in Mechanism Research on Polygonatum in Prevention and Treatment of Diabetes. Front Pharmacol. 2022 Feb 8;13:758501. doi: 10.3389/fphar.2022.758501. PMID: 35211009; PMCID: PMC8861320.
- Shi Y, Si D, Chen D, Zhang X, Han Z, Yu Q, Liu J, Si J. Bioactive compounds from Polygonatum genus as anti-diabetic agents with future perspectives. Food Chem. 2023 May 15;408:135183. doi: 10.1016/j.foodchem.2022.135183. Epub 2022 Dec 12. PMID: 36566543.
- Wang ZY, Wang X, Zhang DY, Hu YJ, Li S. [Traditional Chinese medicine network pharmacology: development in new era under guidance of network pharmacology evaluation method guidance]. Zhongguo Zhong Yao Za Zhi. 2022 Jan;47(1):7-17. Chinese. doi: 10.19540/j.cnki.cjcmm.20210914.702. PMID: 35178906.
- Lai X, Xin W, Hu Y, Su SB, Li W, Lu A. Network Pharmacology and Traditional Medicine. Front Pharmacol. 2020;11:1194.
- Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014 Apr 16;6:13. doi: 10.1186/1758-2946-6-13. PMID: 24735618; PMCID: PMC4001360.
- Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L, Fu X, Liu S, Bo X, Yu G. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). 2021 Jul 1;2(3):100141. doi: 10.1016/j.xinn.2021.100141. PMID: 34557778; PMCID: PMC8454663.
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. doi: 10.1093/nar/28.1.235. PMID: 10592235; PMCID: PMC102472.
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009 Dec;30(16):2785-91. doi: 10.1002/jcc.21256. PMID: 19399780; PMCID: PMC2760638.
- Lee MMY, McMurray JJV, Lorenzo-Almorós A, Kristensen SL, Sattar N, Jhund PS, Petrie MC. Diabetic cardiomyopathy. Heart. 2019 Feb;105(4):337-345. doi: 10.1136/heartjnl-2016-310342. Epub 2018 Oct 18. PMID: 30337334.
- Mou X, Zhou DY, Zhou D, Liu K, Chen LJ, Liu WH. A bioinformatics and network pharmacology approach to the mechanisms of action of Shenxiao decoction for the treatment of diabetic nephropathy. Phytomedicine. 2020 Apr;69:153192. doi: 10.1016/j.phymed.2020.153192. Epub 2020 Feb 22. PMID: 32200292.
- Schmidt AM. Diabetes Mellitus and Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2019 Apr;39(4):558-568. doi: 10.1161/ATVBAHA.119.310961. PMID: 30786741; PMCID: PMC6532416.
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972 Nov 8;30(6):595-602. doi: 10.1016/0002-9149(72)90595-4. PMID: 4263660.
- Liu ZH. Nephrology in china. Nat Rev Nephrol. 2013 Sep;9(9):523-8. doi: 10.1038/nrneph.2013.146. Epub 2013 Jul 23. PMID: 23877587.
- Zhou XF, Zhou WE, Liu WJ, Luo MJ, Wu XQ, Wang Y, Liu P, Wen YM, Li JL, Zhao TT, Zhang HJ, Zhao HL, Li P. A Network Pharmacology Approach to Explore the Mechanism of HuangZhi YiShen Capsule for Treatment of Diabetic Kidney Disease. J Transl Int Med. 2021 Jun 18;9(2):98-113. doi: 10.2478/jtim-2021-0020. PMID: 34497749; PMCID: PMC8386324.
- Qi C, Mao X, Zhang Z, Wu H. Classification and Differential Diagnosis of Diabetic Nephropathy. J Diabetes Res. 2017;2017:8637138. doi: 10.1155/2017/8637138. Epub 2017 Feb 20. PMID: 28316995; PMCID: PMC5337846.
- Chen Y, Song X, Luo Y, Li G, Luo Y, Wang Z, He R, Lu J, Xiong G, Cheng H, Li H, Yang S. Network Pharmacology Approach to Investigate the Mechanism of Danggui-Shaoyao-San against Diabetic Kidney Disease. Evid Based Complement Alternat Med. 2023 Jan 3;2023:9208017. doi: 10.1155/2023/9208017. PMID: 36636607; PMCID: PMC9831705.
- Gan Q, Wang J, Hu J, Lou G, Xiong H, Peng C, Zheng S, Huang Q. The role of diosgenin in diabetes and diabetic complications. J Steroid Biochem Mol Biol. 2020 Apr;198:105575. doi: 10.1016/j.jsbmb.2019.105575. Epub 2019 Dec 30. PMID: 31899316.
- Arya P, Kumar P. Diosgenin: An ingress towards solving puzzle for diabetes treatment. J Food Biochem. 2022 Dec;46(12):e14390. doi: 10.1111/jfbc.14390. Epub 2022 Sep 15. PMID: 36106684.
- Kanchan DM, Somani GS, Peshattiwar VV, Kaikini AA, Sathaye S. Renoprotective effect of diosgenin in streptozotocin induced diabetic rats. Pharmacol Rep. 2016 Apr;68(2):370-7. doi: 10.1016/j.pharep.2015.10.011. Epub 2015 Nov 14. PMID: 26922541.
- Wu Y, Ye F, Lu Y, Yong H, Yin R, Chen B, Yong Y. Diosgenin glucoside protects against myocardial injury in diabetic mice by inhibiting RIP140 signaling. Am J Transl Res. 2018 Nov 15;10(11):3742-3749. PMID: 30662624; PMCID: PMC6291728.
- Oza MJ, Kulkarni YA. Formononetin Treatment in Type 2 Diabetic Rats Reduces Insulin Resistance and Hyperglycemia. Front Pharmacol. 2018 Jul 18;9:739. doi: 10.3389/fphar.2018.00739. PMID: 30072892; PMCID: PMC6058024.
- Huang Q, Chen H, Yin K, Shen Y, Lin K, Guo X, Zhang X, Wang N, Xin W, Xu Y, Gui D. Formononetin Attenuates Renal Tubular Injury and Mitochondrial Damage in Diabetic Nephropathy Partly via Regulating Sirt1/PGC-1α Pathway. Front Pharmacol. 2022 May 12;13:901234. doi: 10.3389/fphar.2022.901234. PMID: 35645821; PMCID: PMC9133725.
- Zhuang K, Jiang X, Liu R, Ye C, Wang Y, Wang Y, Quan S, Huang H. Formononetin Activates the Nrf2/ARE Signaling Pathway Via Sirt1 to Improve Diabetic Renal Fibrosis. Front Pharmacol. 2021 Jan 13;11:616378. doi: 10.3389/fphar.2020.616378. PMID: 33519483; PMCID: PMC7845558.
- Wang YY, Liu RX, Guo B, Xiao Y, Shi MJ, Pi MJ, Wen QY, Zhang GZ. [Down-regulation of PTEN expression in kidney and its role in development of diabetic nephropathy in rats]. Sheng Li Xue Bao. 2011 Aug 25;63(4):325-32. Chinese. PMID: 21861051.
- Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IJcken WF, Houtsmuller AB, Pothof J, de Bruin RWF, Madl T, Hoeijmakers JHJ, Campisi J, de Keizer PLJ. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017 Mar 23;169(1):132-147.e16. doi: 10.1016/j.cell.2017.02.031. PMID: 28340339; PMCID: PMC5556182.
- Zhang SZ, Qiu XJ, Dong SS, Zhou LN, Zhu Y, Wang MD, Jin LW. MicroRNA-770-5p is involved in the development of diabetic nephropathy through regulating podocyte apoptosis by targeting TP53 regulated inhibitor of apoptosis 1. Eur Rev Med Pharmacol Sci. 2019 Feb;23(3):1248-1256. doi: 10.26355/eurrev_201902_17018. PMID: 30779094.
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008 May;9(5):402-12. doi: 10.1038/nrm2395. PMID: 18431400.
- Ohiro Y, Garkavtsev I, Kobayashi S, Sreekumar KR, Nantz R, Higashikubo BT, Duffy SL, Higashikubo R, Usheva A, Gius D, Kley N, Horikoshi N. A novel p53-inducible apoptogenic gene, PRG3, encodes a homologue of the apoptosis-inducing factor (AIF). FEBS Lett. 2002 Jul 31;524(1-3):163-71. doi: 10.1016/s0014-5793(02)03049-1. PMID: 12135761.
- Yamagishi S. Advanced glycation end products and receptor-oxidative stress system in diabetic vascular complications. Ther Apher Dial. 2009 Dec;13(6):534-9. doi: 10.1111/j.1744-9987.2009.00775.x. PMID: 19954478.
- Vlassara H, Striker GE. AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol. 2011 May 24;7(9):526-39. doi: 10.1038/nrendo.2011.74. PMID: 21610689; PMCID: PMC3708644.
- Wang B, Yao K, Wise AF, Lau R, Shen HH, Tesch GH, Ricardo SD. miR-378 reduces mesangial hypertrophy and kidney tubular fibrosis via MAPK signalling. Clin Sci (Lond). 2017 Mar 1;131(5):411-423. doi: 10.1042/CS20160571. Epub 2017 Jan 4. PMID: 28053239.
- Ururahy MA, de Souza KS, Oliveira YM, Loureiro MB, da Silva HP, Freire-Neto FP, Bezerra JF, Luchessi AD, Doi SQ, Hirata RD, Almeida Md, Arrais RF, Hirata MH, de Rezende AA. Association of polymorphisms in IL6 gene promoter region with type 1 diabetes and increased albumin-to-creatinine ratio. Diabetes Metab Res Rev. 2015 Jul;31(5):500-6. doi: 10.1002/dmrr.2621. Epub 2014 Dec 8. PMID: 25384728.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.