Medicine Group. 2023 November 23;4(11):1601-1606. doi: 10.37871/jbres1836.
Exudative Retinal Detachment as a Prodromal Manifestation of Differentiation Syndrome in Acute Promyelocytic Leukemia: A Case Report and Review of Literature
Saeed Karimi3, Morteza Naderan1*, Salar Bahrami1, Masomeh Sabzevary2 and Mohammad Naderan3
2Department of Medical Genetics, Tehran University of Medical Sciences, Tehran, Iran
3Eye Research Center, Tehran University of Medical Sciences, Tehran, Iran
- Exudative retinal detachment
- Acute myeloblastic leukemia
- Acute promyelocytic leukemia
- multi-modal imaging
- Differentiation syndrome
Abstract
The ocular manifestations of the differentiation syndrome (DS) are rarely reported in the literature with only six cases published until now. Patients with acute promyelocytic leukemia (APL) who undergo treatment with all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) are especially susceptible to developing DS. This is a serious diagnosis with a life-threatening impact on some patients, however, amenable to prophylaxis and treatment by systemic corticosteroids. An exudative retinal detachment (ERD) may either complicate or herald the development of DS. In this article, we present a case of APL who developed ERD in advance of DS symptoms, its multimodal imaging characteristics, and review the published articles concerning this rare disease manifestation.
Introduction
Acute Promyelocytic Leukemia (APL) is a subtype of Acute Myeloid Leukemia (AML) which was formerly known as AML-M3. It comprises 10-15 percent of all AML patients [1]. It is characterized by the inhibition of the myeloid progenitor cells from maturation beyond the promyelocytic stage. The disease results from the production of a fusion oncogene, i.e., Promyelocytic Leukemia/Retinoic Acid Receptor Alpha (PML/RARA), following a balanced translocation between long arms of chromosomes 15 and 17 [2].
Classically, the treatment of AML consists of an induction course of cytarabine and anthracycline followed by consolidation with cytarabine and/or Hematopoietic Stem Cell Transplantation (HSCT) [3]. The latest evidence for the treatment of APL advocates a frontline induction regimen, including the combination of All-Trans Retinoic Acid (ATRA) and Arsenic Trioxide (ATO) compounds, to promote differentiation of the myeloblasts into mature forms. The resultant remission rates of as high as 90% to 100% have been achieved with these agents with promising overall survival rates of 85% to 95%. Some authors have also advocated the administration of these agents as a chemotherapy-free modality in lower-risk patients instead of classic chemotherapy protocols [3].
A well-recognized complication of ATRA and/or ATO regimens is Differentiation Syndrome (DS) [4].
Although it is relatively common (10% to 25%) during induction therapy, there are no strict criteria that define DS. Instead, a combination of respiratory distress, fever, weight gain, pleural effusion, renal failure, pericardial effusion, cardiac failure, and hypotension are used for diagnosis [5].
There are limited data on the ocular manifestations of DS. A few cases have been reported in the literature with an exudative retinal detachment (ERD) after initiation of the induction therapy with ATRA and/or ATO. In this article, we present a case of APL who developed bilateral ERD during induction treatment in advance of the DS episode and review relevant multimodal imaging.
Case History
A 33-year-old male patient was referred to our Ophthalmology Emergency Department for evaluation of acute loss of vision. The patient’s history included two months of easy fatiguability, shortness of breath, and non-healing bruising following an accidental trauma into his office table. After visiting his primary care physician, routine lab tests were obtained which revealed normal leukocyte counts with some premature shapes, mild thrombocytopenia, and anemia. A hematologist referral was scheduled within a week and further evaluation of the bone marrow aspiration demonstrated 25 percent blasts with an abundance of Auer rods. On cytogenetic analysis (the result of which became available after initiation of ATRA/ATO) using fluorescein in-situ hybridization (FISH), t (15,17) translocation was demonstrated corresponding to PML/RARA fusion oncogene, thereby confirming the diagnosis of APL.
The patient was hospitalized immediately after diagnosis and intravenous ATRA was started at 45 mg/m2 per day in two divided doses. ATO was started simultaneously at a dose of 0.15 mg/m2 per day intravenous infusion over 2 hours. The patient did not receive any corticosteroid medications either orally or intravenously as prophylaxis for DS.
On day 14, the patient noticed a mild blurriness in both of his eyes which gradually increased in the next couple of days. At the time of the ophthalmology visit on day 17, his systemic condition was surprisingly well with no sign of fever, dyspnea, hypotension, renal dysfunction, and weight gain.
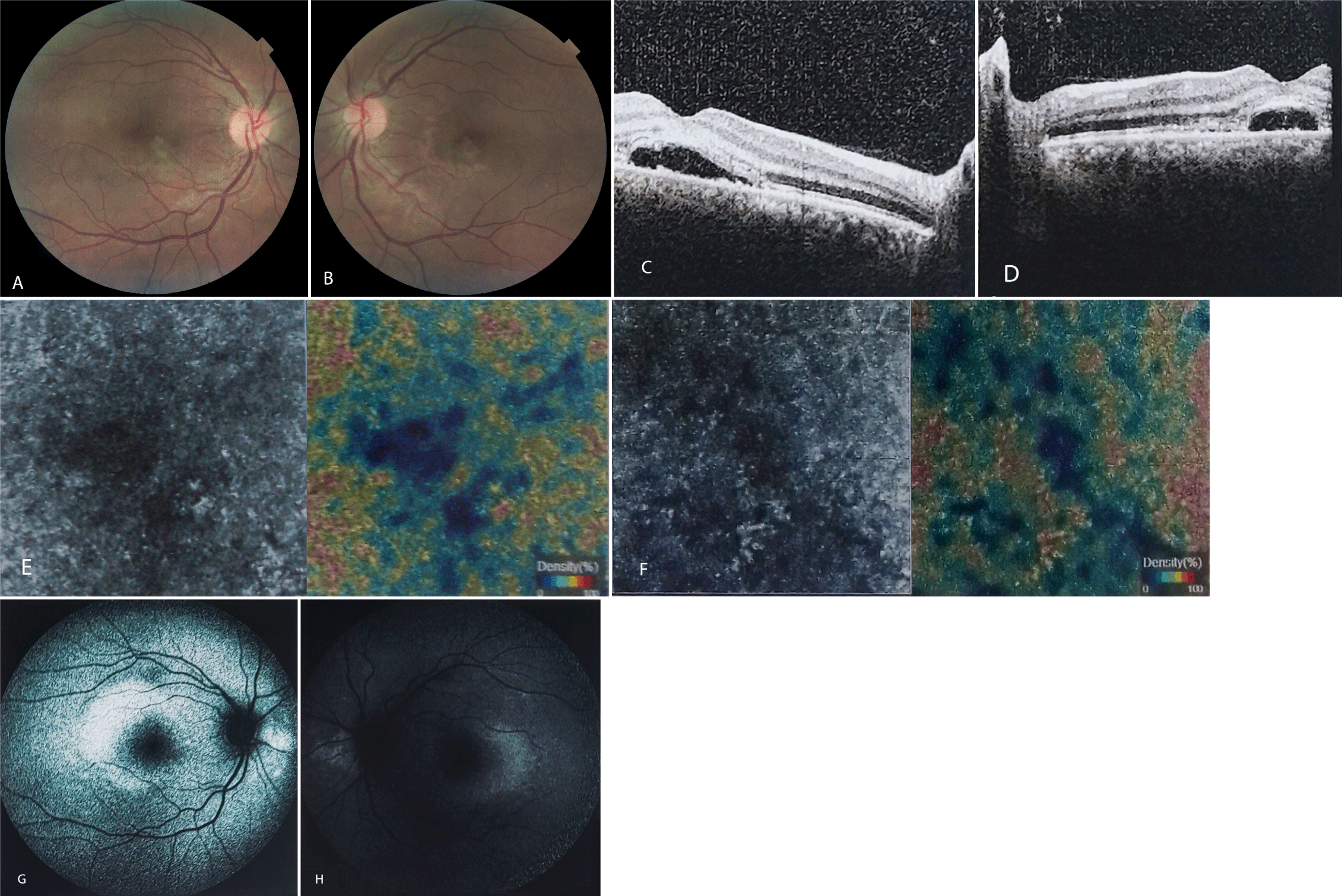
Ophthalmic exam revealed uncorrected Visual Acuity (VA) of Counting Fingers (CF) at 3 meters in the right eye (OD) and CF at 2 meters in the left eye (OS) with a new onset hypermetropic shift. The Best Corrected Visual Acuity (BCVA) was 20/120 (OU). The anterior segment exam was unremarkable with a clear cornea, deep and clear anterior chamber (AC), well-dilating pupil, and normal pupillary responses. The Intraocular Pressure (IOP) was 13 mmHg measured by the Goldman’s applanation tonometer. Fundus exam showed serous retinal detachment in the macula of both eyes with a few yellow small plaques at the level of Retinal Pigment Epithelium (RPE). Optic nerves and retinal vasculature were normal (Figures 1A,B).
Feedback was provided to the patient’s hematologist. Due to favorable systemic condition, the therapeutic plan was continued for another week. On day 22, the patient developed a mild fever and a mild shortness of breath. Broad-spectrum intravenous antibiotics were started and appropriate infectious workup was done to evaluate pulmonary, cardiac, hepatobiliary, and genitourinary systems, which yielded negative. The diagnosis of mild DS was considered and oral prednisolone 1 mg/kg/day was initiated, together with the cessation of ATRA and ATO. A week later (day 29) the patient’s systemic manifestations had resolved. Accordingly, the ATRA/ATO regimen was resumed and the prednisolone was stopped at day 37. On ophthalmic examination, the Sub-Retinal Fluid (SRF) had been absorbed, the retina was flat, and a mild RPE mottling was evident in the macula. The retinal imaging studies returned to normal by this time. The VA returned to 20/20 OU. Repeat bone marrow analysis revealed both molecular and clinical complete remission at day 40 post-treatment.
Imaging studies
The Spectral Domain Optical Coherence Tomography (SD-OCT) revealed neurosensory retinal detachment with few drusenoid deposits overlying RPE and a shagginess of the outer segments of the photoreceptor layer. The subretinal space appeared to contain a hyperreflective material in the outer aspect of the photoreceptor layer. Also, a hyperreflective band was found in the outer nuclear layer overlying the detached retina. Despite thickened inner retinal layers, cystoid macular edema was absent (Figures 1C,D).
OCT Angiography (OCTA) demonstrated normal vascular densities in the Superficial Capillary Plexus (SCP) and Deep Capillary Plexus (DCP). The choriocapillaris was intact with no sign of neovascularization, however, hyporeflective areas were discernible beneath the placoid lesions (Figures 1E,F).
Fundus Autofluorescence (FAF) imaging showed mild hyperautofluorescence in the macula of both eyes (Figures 1G,H).
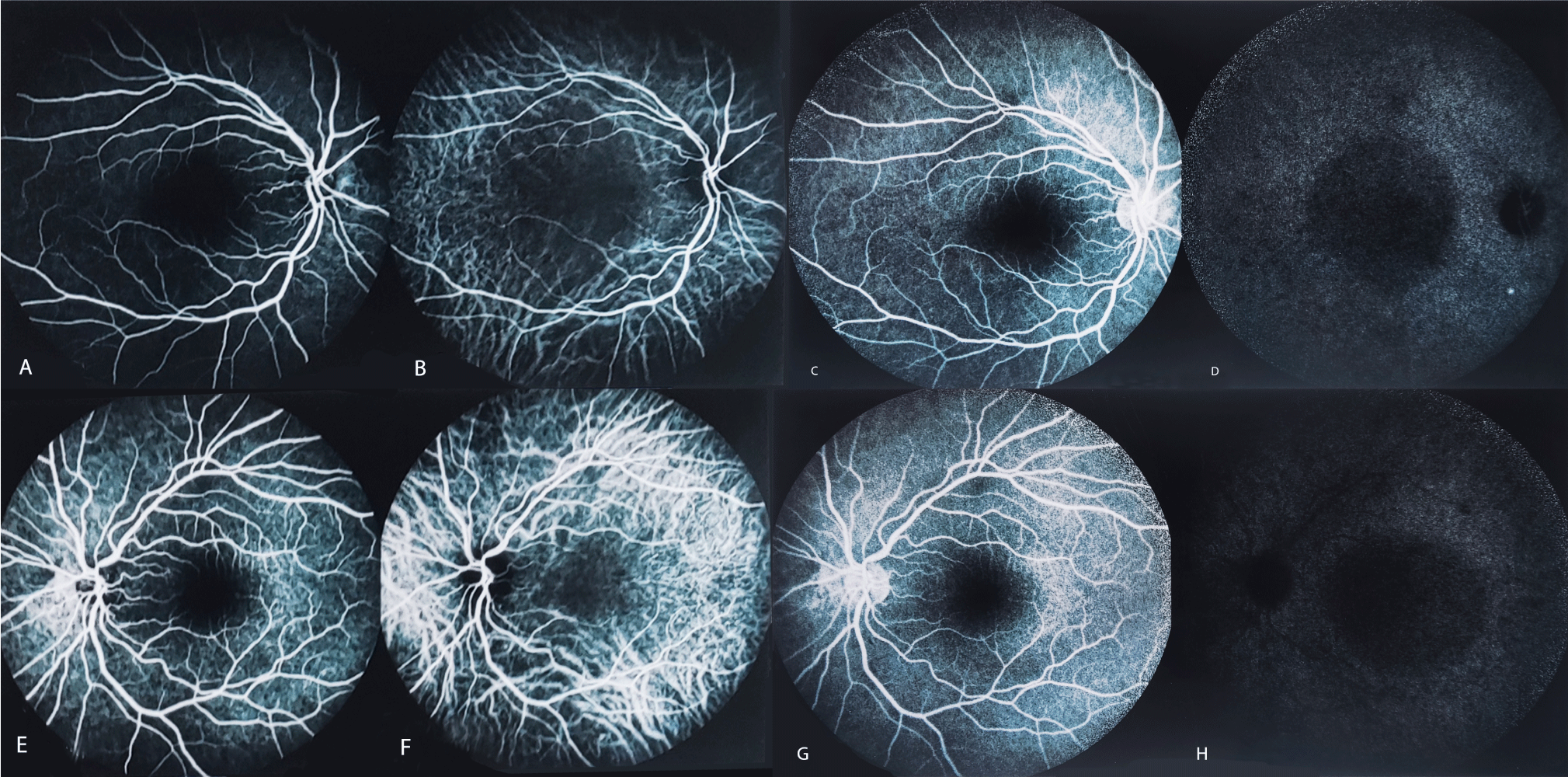
Fluorescein Angiography (FA) yielded mild enlargement of the Foveal Avascular Zone (FAZ), a few early hyperfluorescent dots, and diffuse patchy faint hyperfluorescence consistent with RPE dysfunction (Figure 2). Retinal vasculature appeared normal without any staining of the vascular wall.
On Indocyanine Green Angiography (ICGA) Choroidal Vascular Hyper-Permeability (CVH) and a pachyvessel-like appearance were evident, in addition to areas of faint hypercyanescence due to leakage of the dye (Figure 2).
Discussion
APL is a fatal condition. Population-based studies have shown that one-third of patients die within the first month after diagnosis, of whom one-third occur before treatment with ATRA is initiated [6]. Hence, the need for urgent treatment following suspicion of APL cannot be overemphasized [1].
ATRA and ATO have long been used in clinical practice [7]. Their safety profile and differentiating capacity have made them favorable agents in frontline management of APL patients. Their effect results from targeted degradation of the PML/RARA oncoprotein, thereby, inducing terminal differentiation of immature promyelocytes into mature granulocytes [4]. Combined ATRA/ATO is now recommended as chemotherapy-free regimens in low-risk patients with reported complete remission and survival rates of 90 percent in clinical studies [1]. Both of these agents may cause DS, however, their combination has been associated with less incidence of complications [8,9].
DS is a serious treatment-related complication in APL with a mortality rate as high as 20 percent [10]. Despite its prevalence (10-25%), the diagnosis of DS requires a high index of suspicion and prompt treatment with intravenous corticosteroids, due to a lack of diagnostic criteria. The underlying mechanism of DS is not well known but it is probably related to the release of inflammatory cytokines from differentiating cells with resultant over-flux of cells in various organs, capillary leakage, and organ failure [11]. The nature of the hyperreflective deposits in the outer retina and thickened retinal layers in our study remains elusive due to the lack of biopsy specimens.
The literature is scant regarding the ocular manifestations of DS. One reason may be the real scarcity of such manifestations. Another reason is that the severity of DS symptoms and the priority of their management may postpone the appreciation of ocular symptoms by patients and timely ophthalmology consultation [12]. Furthermore, it seems that treatment of DS with systemic corticosteroids can effectively and rapidly relieve ophthalmic signs with no residual disease which obscures the retrospective diagnosis of ocular involvement.
As far as we know, only six cases of ocular involvement in the DS syndrome have been reported (Table 1). All cases were bilateral and involved the chorioretinal structures with no indication of vitritis or vasculitis. The onset of ocular disease was during the first 14 days after initiation of ATRA and/or ATO. Although most cases occurred during an episode of DS, Kim and Gim reported a 66-year-old woman with APL who developed serous retinal detachment before the onset of DS symptoms [16]. Likewise, our patient developed symptoms of ERD before the onset of other DS symptoms. Interestingly, our patient was different from other cases in that his fundus exam revealed any retinal hemorrhage, which was universally present in other cases.
| Table 1: Published case reports on the ocular manifestations of differentiation syndrome. | |||||||||
| Author | Year | Cases (No.) |
Primary disease | Signs | Onset (day) |
Age (year) | Sex | Ocular complication | |
| 1 | [12] | 2013 | 1 | APL | Multifocal serous retinal detachment, Intraretinal hemorrhage | 10 | 39 | M | None |
| 2 | [13] | 2018 | 2 | APL | Multifocal yellow choroidal lesions, retinal hemorrhage, Roth’s spot, SRF | 14 10 |
35 30 |
F F |
None |
| 3 | [14] | 2020 | 1 | APL | Intraretinal hemorrhage, choroidal effusion, SRF | 14 | 51 | M | None |
| 4 | [15] | 2021 | 1 | APL | Intraretinal hemorrhage, optic disc swelling, intraretinal edema, SRF | 10 | 25 | M | None |
| 5 | [16] | 2021 | 1 | APL | Intraretinal hemorrhage, Roth’s spot, SRF | 5 | 66 | F | None |
| 6 | Our study | - | 1 | APL | Yellow subretinal plaque, SRF, no hemorrhage | 14 | 33 | M | None |
| APL: Acute Promyelocytic Leukemia; SRF: Sub Retinal Fluid; M: Male; F: Female | |||||||||
The treatment for ERD in the setting of DS is straightforward and consists of withholding ATRA/ATO and starting systemic corticosteroids irrespective of previous prophylactic steroid therapy. The prognosis is good and the visual acuity recovers completely with treatment. It should be emphasized that although the differential diagnoses of ERD include a variety of infectious and non-infectious etiologies, corticosteroid therapy should not be postponed while awaiting the workup results. We propose that, until the characteristics, risk factors, and pathophysiologic mechanisms for the development of ERD during DS are not well-defined, it is best considered a mild or incomplete form of DS which may evolve into full-blown systemic syndrome and necessitates urgent treatment.
Conflict of Interest
The authors declare no conflict of interests.
References
- Yilmaz M, Kantarjian H, Ravandi F. Acute promyelocytic leukemia current treatment algorithms. Blood Cancer J. 2021;11(6):123. doi: 10.1038/s41408-021-00514-3.
- Grimwade D, Lo Coco F. Acute promyelocytic leukemia: a model for the role of molecular diagnosis and residual disease monitoring in directing treatment approach in acute myeloid leukemia. Leukemia. 2002 Oct;16(10):1959-73. doi: 10.1038/sj.leu.2402721. PMID: 12357347.
- Zhu HH, Hu J, Lo-Coco F, Jin J. The simpler, the better: oral arsenic for acute promyelocytic leukemia. Blood. 2019 Aug 15;134(7):597-605. doi: 10.1182/blood.2019000760. Epub 2019 May 21. PMID: 31113776.
- Rego EM, De Santis GC. Differentiation syndrome in promyelocytic leukemia: clinical presentation, pathogenesis and treatment. Mediterr J Hematol Infect Dis. 2011;3(1):e2011048. doi: 10.4084/MJHID.2011.048. Epub 2011 Oct 24. PMID: 22110898; PMCID: PMC3219650.
- Stahl M, Tallman MS. Acute promyelocytic leukemia (APL): remaining challenges towards a cure for all. Leuk Lymphoma. 2019 Dec;60(13): 3107-3115. doi: 10.1080/10428194.2019.1613540.
- Lehmann S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Möllgård L, Derolf AR, Stockelberg D, Tidefelt U, Wahlin A, Wennström L, Höglund M, Juliusson G. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011 Jul;25(7):1128-34. doi: 10.1038/leu.2011.78. Epub 2011 Apr 19. PMID: 21502956.
- Nowak D, Stewart D, Koeffler HP. Differentiation therapy of leukemia: 3 decades of development. Blood. 2009 Apr 16;113(16):3655-65. doi: 10.1182/blood-2009-01-198911. Epub 2009 Feb 12. PMID: 19221035; PMCID: PMC2943835.
- Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutré S, Dahlberg S, Ellison R, Warrell RP Jr. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001 Sep 15;19(18):3852-60. doi: 10.1200/JCO.2001.19.18.3852. PMID: 11559723.
- Wang G, Li W, Cui J, Gao S, Yao C, Jiang Z, Song Y, Yuan CJ, Yang Y, Liu Z, Cai L. An efficient therapeutic approach to patients with acute promyelocytic leukemia using a combination of arsenic trioxide with low-dose all-trans retinoic acid. Hematol Oncol. 2004 Jun;22(2):63-71. doi: 10.1002/hon.728. PMID: 15468344.
- de la Serna J, Montesinos P, Vellenga E, Rayón C, Parody R, León A, Esteve J, Bergua JM, Milone G, Debén G, Rivas C, González M, Tormo M, Díaz-Mediavilla J, González JD, Negri S, Amutio E, Brunet S, Lowenberg B, Sanz MA. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008 Apr 1;111(7):3395-402. doi: 10.1182/blood-2007-07-100669. Epub 2008 Jan 14. PMID: 18195095.
- Luesink M, Pennings JL, Wissink WM, Linssen PC, Muus P, Pfundt R, de Witte TJ, van der Reijden BA, Jansen JH. Chemokine induction by all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia: triggering the differentiation syndrome. Blood. 2009 Dec 24;114(27):5512-21. doi: 10.1182/blood-2009-02-204834. Epub 2009 Oct 14. PMID: 19828696.
- Levasseur SD, Tantiworawik A, Lambert Maberley DA. All-trans retinoic Acid differentiation syndrome chorioretinopathy: a case of multifocal serous neurosensory detachments in a patient with acute promyelocytic leukemia treated with all-trans retinoic Acid. Retin Cases Brief Rep. 2013 Winter;7(1):46-9. doi: 10.1097/ICB.0b013e3182618d6c. PMID: 25390520.
- Newman AR, Leung B, Richards A, Campbell TG, Wellwood J, Imrie FR. Two cases of differentiation syndrome with ocular manifestations in patients with acute promyelocytic leukaemia treated with all-trans retinoic acid and arsenic trioxide. Am J Ophthalmol Case Rep. 2018 Jan 17;9:106-111. doi: 10.1016/j.ajoc.2018.01.026. PMID: 29468228; PMCID: PMC5790809.
- Hua HU, Rayess N, Moshfeghi AA. Acute Promyelocytic Leukemia With Sudden Vision Loss. JAMA Ophthalmol. 2020 Feb 1;138(2):206-207. doi: 10.1001/jamaophthalmol.2019.4838. PMID: 31804661.
- Tam EK, Ness S, Peeler CE. Exudative hemorrhagic retinopathy related to all-trans retinoic acid differentiation syndrome in a patient with acute promyelocytic leukemia. Int J Ophthalmol. 2021 Feb 18;14(2):323-325. doi: 10.18240/ijo.2021.02.22. PMID: 33614465; PMCID: PMC7840356.
- Gim Y, Kim HJ. Ocular Symptom Can Be the First Presentation of Differentiation Syndrome in Acute Promyelocytic Leukemia. Korean J Ophthalmol. 2021 Feb;35(1):94-96. doi: 10.3341/kjo.2020.0101. Epub 2020 Dec 11. PMID: 33307628; PMCID: PMC7904416.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.