Medicine Group. 2024 January 05;5(1):008-012. doi: 10.37871/jbres1865.
Auctox: A New Method to Evaluate the Safety of Anticancer Drugs
Florian Scotte1*#, Carole Helissey2, Vincent Launay Vacher3#, Thomas Lloret4, Jerome Stevens4 and Paolo Bossi5
Abstract
The evaluation of anticancer drugs safety is currently based on considering the worst Adverse Event (AE) in a particular patient. The aim of the study was to define a new method which would better describe the impact of treatment on Quality of Life (QoL), combining Patient Reported Outcomes (PROs) and the monitoring of all AEs. A comparative approach to the classic National Cancer Institute-Common Toxicity Criteria for Adverse Events (NCI-CTCAE) classification was performed to assess the interest of monitoring Area Under the Curve (AUC) of toxicities on quality of life.
A negative impact on global health status, assessed by the VAS of EQ-5D-3L, was significantly identified for three AEs based on CTCAE grade alone versus seven when considering AUCtox assessment.
Background
The evaluation of anticancer drugs safety currently relies on the National Cancer Institute-Common Toxicity Criteria for Adverse Events (NCI-CTCAE) classification [1]. This classification defines several grades according to the severity of adverse events, from grade 1, the lowest intensity/severity to 4 or 5, defining the worst. In clinical trials, and in fact in the vast majority if not all of other clinical research in oncology, the methodology used is based on considering the worst AE in a particular patient. For instance, a patient presenting with two episodes of grade 1 nausea and 1 of grade 3 will be recorded as 1 Grade 3 nausea and considered as such in the global evaluation of the safety. Most often in clinical trials and clinical practice, attention is focused on severe AE i.e. grade 3 or more. It is even now usual to list only these grade 3 or more AE in the reports of Phase 2 or 3 clinical trials. However, AEs of lower grades can have a significant deleterious impact on patients’ quality of life (QoL), especially when recurrent or permanent [2].
In addition, the past years showed that the evaluation of anticancer drugs safety by the clinician was somehow only partial, in terms of typology and severity. Most often, AEs are underreported by clinicians as compared to reports from the patients directly or caregivers. A new approach consisting of considering the safety reported by the patients themselves is now more and more often used in studies, and in some clinical trials. Patients Reported Outcomes (PROs) aim at placing the patient at the center of the evaluation of the safety of drugs, considering the impact on QoL, as felt by the patients themselves.
From these two important considerations for a better, more patient-centered, evaluation of drugs’ safety, we aimed at defining a new method which would better describe the impact of treatment on quality of life (QoL), combining PROs and the monitoring of all AEs.
Methods
This approach is first based on the fact that AEs are monitored and recorded by the patients themselves. However, the grading of the severity must be conducted by healthcare professionals, familiar with the classification and the NCI-CTCAE grading system, and specifically trained to translate patients wording into medical wording and subsequent determination and grading of the severity of the AE reported. It was thus necessary to rely on a specific organization which would provide the required trained human resources and validated processes. The oncology patient monitoring program OptiSoins has been developed for metastatic prostate cancer (mPC) in 30 cancer centres in France. OptiSoins provides a centralized web-based platform, with secured extranet access. Oncology nurses, specifically trained for patient interviews, recording of AEs, and grading of severity, regularly call patients during follow-up. Each patient participates in phone calls interviews, before the course is administered, one week after administration (D7), two weeks (D15), and two days before the subsequent course (D-2). For the purpose of this study, a cohort of patients with mPC treated with either docetaxel (DOC) or cabazitaxel (CABA) was isolated in the OptiSoins database. Data consisted of a prospective follow-up of patients, with a focus on safety, through a PRO model, and records of AEs, their severity (NCI-CTCAE grading), frequency and duration together with periodical QoL evaluations.
Secondly, a mathematical approach was chosen to evaluate the safety of the treatment which would take into consideration 1) the severity (grade) of the AE, 2) the frequency of the AE, and 3) the duration of the AE, each episode and cumulative. The model was chosen by analogy with the pharmacokinetic parameter Area Under the concentration-time Curve, which mathematically describes the exposure of the organism to a drug. We thus calculated the Area Under the Curve of Toxicities (AUCtox) for each patient and each AE. All AE episodes recorded during follow-up were chronologically plotted against the severity of each episode. AUCtox was then calculated using the trapezoid rule to estimate the integral of the function plotted.
Third, to evaluate the potential interest of this method, the association of AUCtox with QoL (evaluated by EQ-5D-3L) was statistically tested.
The EQ-5D-3L contains 5 dimensions/domains: Mobility, Autonomy, Activities of Daily Living, Pain/Discomfort, Anxiety/Depression and a VAS scale (Visual Analogic Scale) of general health. The 5 domains of the EQ-5D-3L score were studied as binary variables (0 = no problem vs 1=problem): analysed by logistic regression (OR IC 95%).
The VAS of the EQ-5D-3L questionnaire was considered as a quantitative variable (from 0 to 100) analysed by linear regression.
The analysis strategy was as follows:
Step 1: Assess the determinants of QoL;
Step 2: Test the relationship between AUC of a toxicity and QoL by adjusting for QoL determinants.
Results
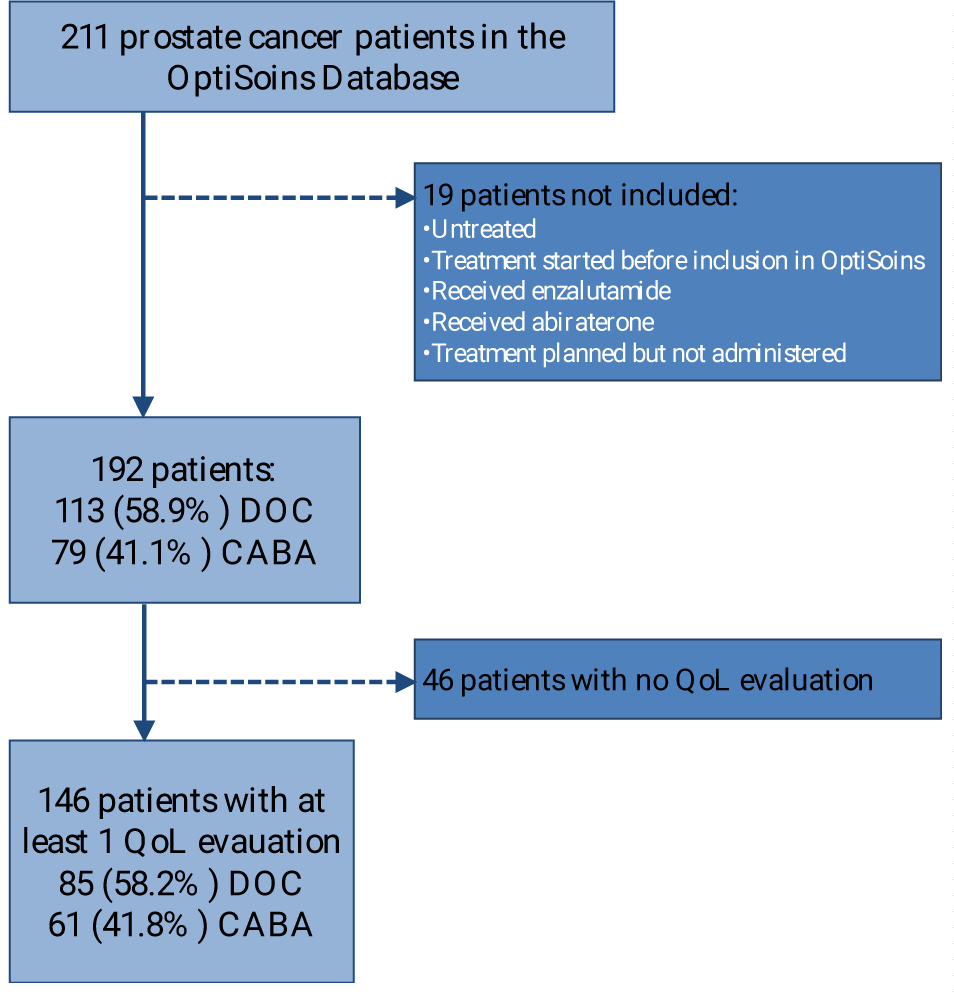
A total of 146 patients of 211 metastatic prostate cancer patients enrolled in the OptiSoins database, were evaluable [DOC 85 (58%), CABA 61 (42%)] (Figure 1). Median age was 70.5 years (Q1-Q3: 64.7; 76.1), median weight was 78.5 kg (Q1-Q3: 70.0-86.5), median body mass index was 24.9 (Q1-Q3: 23.7-28.4). Median follow-up was 4.0 months (Q1-Q3: 3.3-5.4). Patients underwent a median number of 6 cycles (Q1-Q3: 5.0-8.0), with a median number of 15.0 evaluations through monitoring calls (Q1-Q3: 11.0-19.00).
A total number of 2092 evaluations were performed during the study. The distribution of adverse reactions reported by the enrolled patients based on the different grades of toxicity according to the CTCAE scale is presented in table 1.
| Table 1: Safety analysis of the metastatic prostate cancer Options cohort of patients treated with either Docetaxel or Cabazitaxel. Data present the total number of events (N) and the percentage as compared to the total number of evaluations performed (2092) (%). | |||
| N (%) | |||
| Type of AE | Grade 1 | Grade 2 | Grade 3 or more |
| Alopecia | 415 (19.84) | 165 (7.89) | - |
| Anorexia | 439 (20.98) | 223 (10.66) | 19 (0.91) |
| Constipation | 343 (16.40) | 28 (1.34) | 1 (0.05) |
| Diarrhea | 324 (15.49) | 34 (1.63) | 3 (0.14) |
| Dyspnea | 638 (30.50) | 203 (9.70) | 9 (0.43) |
| Epistaxis | 110 (5.26) | 4 (0.19) | - |
| Fatigue/Asthenia | 949 (45.36) | 415 (19.84) | 63 (3.01) |
| Fever/Hyperthermia | 38 (1.82) | 3 (0.14) | 1 (0.05) |
| Hematuria | 125 (5.98) | 9 (0.43) | 3 (0.14) |
| Tearing, watering eyes | 419 (20.03) | 20 (0.96) | - |
| Nail loss, onycholysis | 360 (17.21) | 38 (1.82) | - |
| Nausea | 260 (12.43) | 52 (2.49) | 2 (0.10) |
| Oral mucositis | 189 (9.03) | 11 (0.53) | 1 (0.05) |
| Pain | 883 (42.21) | 255 (12.19) | 34 (1.63) |
| Peripheral edema | 254 (12.14) | 77 (3.68) | 6 (0.29) |
| Peripheral sensory neuropathy | 696 (33.27) | 161 (7.70) | 3 (0.14) |
| Urinary tract infection | - | 20 (0.96) | 3 (0.14) |
| Weight loss | 213 (10.18) | 31 (1.48) | - |
| Vomiting | 106 (5.07) | 15 (0.72) | 1 (0.05) |
It is useful to note that the patients were receiving either docetaxel or cabazitaxel for metastatic prostate cancer, which may contribute to the adverse effects observed.
Fatigue, pain, peripheral sensory neuropathy, and dyspnea were the most common grade 1 toxicities (45.4%, 42.2%, 33.3% and 30.5% respectively). The most frequent grade 2 toxicities were fatigue (19.9%), pain (12.2%), anorexia (10.7%) and dyspnea (9.7%).
At least one grade ≥3 AE was reported by 45.2% of patients, most frequently fatigue (20.6%) and pain (12.3%). Pain was strongly featured in this cohort, probably in relation to the bone metastatic tropism of prostate cancers.
A negative impact on global health status, assessed by the VAS of EQ-5D-3L, was significantly identified for 3 AEs (anorexia, dyspnea, fatigue/asthenia) based on CTCAE grade alone vs. 7 (alopecia, anorexia, dyspnea, fatigue/asthenia, tearing/watering eyes, nausea, nail loss/onycholysis) when considering AUCtox (Table 2).
| Table 2: Association of adverse events with a negative impact on VAS, univariate analysis. | ||||
| Type of AE | AUCtox | p AUCtox (quanti) |
≥Grade 3 | p Grade 3 |
| Alopecia | ü | 0.0221 | - | - |
| Anorexia | ü | <0.0001 | ü | 0.0427 |
| Dyspnea | ü | 0.0055 | ü | |
| Fatigue/Asthenia | ü | 0.0034 | ü | |
| Tearing, watering eyes | - | 0.1131 | - | |
| Nausea | - | 0.1389 | - | |
| Peripheral sensory neuropathy | ü | 0.0299 | - | |
| Nail loss, onycholysis | ü | 0.0210 | - | |
| ü: p ≤ 0.05 | ||||
| Type of AE | AUCtox | p AUCtox (>median) |
≥Grade 3 | p Grade 3 (>median) |
| Alopecia | ü | 0.0324 | - | |
| Anorexia | ü | <0.001 | ü | |
| Dyspnea | ü | 0.0405 | ü | |
| Fatigue/Asthenia | ü | 0.0002 | ü | |
| Tearing, watering eyes | ü | 0.0120 | - | |
| Nausea | ü | 0.0500 | - | |
| Peripheral sensory neuropathy | - | 0.1478 | - | |
| Nail loss, onycholysis | ü | 0.0216 | - | |
| ü: p ≤ 0.05 | ||||
Discussion
Assessing the safety of anti-cancer treatments is part of the daily work of cancerology teams, and new digital technologies are providing tools to ensure continuous, high-quality monitoring [3,4]. The involvement of teams of nurse navigators ensures direct contact with patients and optimized referral according to the severity of toxicities encountered [5]. This assessment also enables the appropriate decisions to be taken, particularly in terms of cancer treatment adaptations.
This application to the clinical evaluation of tolerance to anti-cancer treatments is based on many similar approaches used in biological and toxicological research, in particular to identify the cytotoxic or antioxidant activities of some compounds [6]. The synthesis and evaluation in the development of new pharmacological molecules, for example, follow the concentration that inhibits 50% of growth (IC50%). Recently, in the context of therapeutic research against COVID-19, a similar method following ProTox-II (Prediction of Toxicity of Chemicals) Virtual Laboratory disclosed the predicted relative biosafety of the compound cordycepin in order to demonstrate that the cordycepin is able to potently inhibit the multiplication of the new resistant strains of SARS-CoV-2 [7]. More specifically in the field of cancerology, a new pyrazine derivative, 1-(5-bromopyrazin-2-yl)-1-[3-(trifluoromethyl)benzyl]urea (BPU), was designed via structural optimization and synthesized to investigate its anticancer potential with less side effects and higher antitumor activities [8].
The impact of anticancer drugs toxicities on patients' daily lives is often considered in the case of severe toxicities assessed at a specific time and often present over a short period [2]. Consideration of an area under the curve, highlighting not only the severity but also the duration of exposure to toxicity, could provide a better clinical assessment of the impact of treatment tolerance on quality of life. The results presented in this study of patients treated for metastatic prostate cancer corroborate this hypothesis.
There are several limitations to this study, first and foremost the questionnaire used to analyze patients' quality of life. The exclusively male gender, the type of pathology and the treatment (chemotherapy) are also limitations in the current context of developing personalized medicine.
Further studies, carried out on other diseases and on a wider range of cancer treatments, are needed to confirm this new approach, which could nevertheless help us to understand the experiences of patients undergoing cancer treatment.
Conclusion
Current evaluation of toxicity based on severity grade alone underestimates the impact of anticancer drugs on QoL. Using the area under the curve of toxicities (AUCtox) allows to identify AEs that significantly impact QoL and which are not reported when considering only Grade 3 or more toxicities or when limiting the analysis on a single, punctual evaluation and not on a time-dependent one. AUCtox can better detect the impact of all toxicities, allowing a more patient-oriented evaluation of drug safety.
References
- Common terminology criteria for adverse events (CTCAE). 2017.
- de Mol M, Visser S, den Oudsten BL, Lodder P, van Walree N, Belderbos H, Aerts JG. Frequency of low-grade adverse events and quality of life during chemotherapy determine patients' judgement about treatment in advanced-stage thoracic cancer. Support Care Cancer. 2019 Sep;27(9):3563-3572. doi: 10.1007/s00520-019-4659-x. Epub 2019 Jan 28. PMID: 30690684; PMCID: PMC6660482.
- Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. 2017 Jul 11;318(2):197-198. doi: 10.1001/jama.2017.7156. PMID: 28586821; PMCID: PMC5817466.
- Mooney K, Berry DL, Whisenant M, Sjoberg D. Improving Cancer Care Through the Patient Experience: How to Use Patient-Reported Outcomes in Clinical Practice. Am Soc Clin Oncol Educ Book. 2017;37:695-704. doi: 10.1200/EDBK_175418. PMID: 28561689.
- Mir O, Ferrua M, Fourcade A, Mathivon D, Duflot-Boukobza A, Dumont S, Baudin E, Delaloge S, Malka D, Albiges L, Pautier P, Robert C, Planchard D, de Botton S, Scotté F, Lemare F, Abbas M, Guillet M, Puglisi V, Di Palma M, Minvielle E. Digital remote monitoring plus usual care versus usual care in patients treated with oral anticancer agents: the randomized phase 3 CAPRI trial. Nat Med. 2022 Jun;28(6):1224-1231. doi: 10.1038/s41591-022-01788-1. Epub 2022 Apr 25. PMID: 35469070.
- Nashaan F, Al Rawi S, Alhammer M, Rabie A, Tomma AHJ. Synthesis, characterization, and cytotoxic activity of some imides from galloyl hydrazide', Eurasian Chemical Communications. 2022;4(10):966-975. doi: 10.22034/ecc.2022.340135.1453.
- Rabie AM. Potent Inhibitory Activities of the Adenosine Analogue Cordycepin on SARS-CoV-2 Replication. ACS Omega. 2022 Jan 11;7(3):2960-2969. doi: 10.1021/acsomega.1c05998. PMID: 35071937; PMCID: PMC8767658.
- Mohammed YHI, Shamkh IM, Alharthi NS, Shanawaz MA, Alzahrani HA, Jabbar B, Beigh S, Alghamdi S, Alsakhen N, Khidir EB, Alhuthali HM, Karamalla THE, Rabie AM. Discovery of 1-(5-bromopyrazin-2-yl)-1-[3-(trifluoromethyl)benzyl]urea as a promising anticancer drug via synthesis, characterization, biological screening, and computational studies. Sci Rep. 2023 Dec 20;13(1):22824. doi: 10.1038/s41598-023-44662-x. PMID: 38129413; PMCID: PMC10739849.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.