Medicine Group. 2024 January 26;5(1):083-088. doi: 10.37871/jbres1874.
Comparative Evaluation of Microorganism Disinfection Methods for N95 Respirators
Satoshi Mitarai1*, Jun Noda2, Satoshi Gondaira3, Ikuo Uchida4 and Rikio Kirisawa4
2Environment Health Sciences, Graduate School of Veterinary Science, Rakuno Gakuen University, Japan
3Laboratory of Animal Health, School of Veterinary Medicine, Rakuno Gakuen University, Japan
4Laboratory of Veterinary Bacteriology, Department of Pathobiology, School of Veterinary Medicine, Rakuno Gakuen University, Japan
- N95 respirator
- Pasteurization
- Sterilization
- Disinfection
- Performance degradation
- COVID-19
Abstract
Background: An excessive demand for N95 respirators occurred during the SARS-CoV-2 pandemic. Therefore, health care workers were obligated to reuse N95 respirators, which were intended to be disposable.
Aim: The primary objective of this study was to establish a standard procedure for safe disinfection or sterilization that does not affect the performance of an N95 respirator.
Methods: As disinfection or sterilization methods, immersion in 70% ethanol, 0.1% hypochlorous acid, 0.3% peracetic acid, 0.2% alkyldiaminoethylglycine hydrochloride aqueous solution, hypochlorous acid water, or plant mineral-activated water, autoclaving, pasteurization and hydrogen peroxide plasma sterilization were used. After sterilization/disinfection, the filtration capacity of each N95 respirator was examined.
Findings: The performance changes in the N95 respirator caused by each sterilization/disinfection method differed for each manufacturer’s product. Seventy percent ethanol, 0.1% sodium hypochlorite aqueous solution, 0.3% peracetic acid aqueous solution, autoclaving, hypochlorous acid water, and plant mineral-activated water significantly deteriorated the performance of N95 respirators. Performance degradation (increased permeability) was observed in 0.2% alkyldiaminoethylglycine hydrochloride aqueous solution and hydrogen peroxide plasma sterilization, and the permeation performance significantly deteriorated by 50–70% in all N95 respirators tested. Only pasteurization resulted in no deterioration in performance, even after five repeated sterilizations.
Conclusion: Verification of sterilization/disinfection methods for the reuse of N95 respirators has shown that the currently recommended hydrogen peroxide plasma sterilization is inadequate as it increases permeability by more than 50% with a single treatment. In this study, pasteurization was found to be the optimal sterilization method.
Introduction
The 2020 SARS-CoV-2 pandemic has caused an explosive demand for personal protective equipment [1]. Given the bioaerosol-infective nature of the pathogen, the N95 respirator is essential for protection against infection. The high demand for N95 respirators caused a critical supply shortage, and many medical professionals were compelled to use one N95 respirator repeatedly, although it was disposable. Therefore, it is necessary to disinfect/inactivate pathogens in N95 respirators.
The N95 filter was invented by Peter Tsai in 1992. It traps 99.97% of 0.3 µm size particles by the corona electrostatic charging method [2]. The technology was utilised for the development of N95 respirators in 1995. They were first used to prevent dust inhalation at manufacturing and construction sites. Later, the pathogen prevention capacity was discovered and utilised in medical practice. In the previous SARS-CoV-1 pandemic, many health care workers reused/rotated N95 respirators with a resting period, expecting a certain survival time of the coronavirus [3]. However, it is uncertain whether all human coronavirus variants have the same survival capacity in natural environments.
Fisher et al. tested four different decontamination methods: UV light (260–285 nm), 70°C dry heat, 70% ethanol and Vaporised Hydrogen Peroxide (VHP). They found that VHP was the best decontamination method without reducing N95 respirator integrity, but ethanol treatment significantly reduced N95 integrity [4]. It is obvious that these decontamination methods are effective against coronavirus; however, other pathogens pose a potential pandemic risk because a large amount of disinfectant has been used in environments for cleansing, sufficient to destroy healthy environmental microbiota [5]. Not only viruses but also bacteria could be emerging pathogens.
Considering the drastic changes in the living environment, it will be necessary to be prepared for a pandemic caused by other pathogens. As a fact, World Health Organization reported during the 28-day period from 11 December 2023 to 7 January 2024, 106 countries reported COVID-19 cases and 51 countries reported COVID-19 deaths. Over 173,000 new hospitalizations and over 1,900 new ICU admissions were reported during this period [6]. Therefore, we tested N95 respirator decontamination against viruses, bacteria, and mycobacteria to provide some useful insights into the rotative use of the N95 respirator.
Materials and Methods
N95 respirators tested
Four different types of N95 respirators were used in this study because other manufacturers were not available as of 2020. 3M (Kita-ku, Tokyo, Japan), KOKEN (Chiyoda-ku, Tokyo, Japan), Shigematsu (Nishigahara, Tokyo, Japan) and Hogi Medical (Minato-ku, Tokyo, Japan) were selected as manufacturers of N95 respirators. The N95 respirators of each manufacturer were 8210 (3M), Hirac 350 (Koken), DD02-N95-2K (Shigematsu) and HPR-R (Hogi).
Preparation of microorganisms
One virus and two bacteria species were used in this study: bovine coronavirus (BCoV), Pasteurella multocida and Mycobacterium tuberculosis H37Ra (ATCC 25177), respectively. BCoV is a member of the family Coronaviridae, genus Betacoronavirus, to which SARS-CoV-2 also belongs.
HRT-18G cells (ATCC CRL-11663) were grown in Dulbecco’s Modified Eagle’s medium (DMEM, Gibco BRL, USA) supplemented with 10% foetal bovine serum (FBS, Gibco BRL, USA), 50 μg/ml gentamicin (Gibco BRL, USA) and 1.5 μg/ml amphotericin B (Bristol-Myers Squibb K.K., Tokyo, Japan). The Kakegawa strain of BCoV was propagated in HRT-18G cells cultured in DMEM supplemented with 4% FBS [7]. BCoV was prepared in approximately 5.5 log10TCID50/25 µl. BCoV was titrated in duplicate using a 10-fold dilution series of specimens from HRT-18G cells.
P. multocida was cultured in Bacto brain heart infusion medium (Becton Dickinson, Fukushima, Japan) and quantified on sheep blood agar medium (Eiken Chemical Ltd., Tokyo, Japan) with a 10-fold dilution series of the bacterial suspension. The concentration was adjusted to 1.41 × 107 CFU/ml.
M. tuberculosis H37Ra was cultured in Middlebrook 7H9 supplemented with OADC enrichment (Becton Dickinson, Sparks, MD, USA) and quantified by a 10-fold dilution series of the original bacterial suspension. The concentration was adjusted in 7H9 medium to 8.3 × 107 CFU/ml.
Disinfectants and decontamination methods
For the decontamination methods, 10 min of immersion in either 70% ethanol (FujiFILM Wako Chemicals, Osaka, Japan), 0.1% hypochlorous acid (FujiFILM Wako Chemicals, Osaka, Japan), 0.3% peracetic acid (Saraya, Tokyo, Japan), 0.2% alkyldiaminoethylglycine hydrochloride aqueous solution (FujiFILM Wako Chemicals, Osaka, Japan), electrolysed-oxidising-water (Aoi Engineering Inc., Shizuoka, Japan), or plant mineral-activated water (Tera Protect, ORIX, Tokyo), autoclaving (121 °C, 30 min), pasteurisation (65 °C, 30 min) and Hydrogen Peroxide Gas Plasma (HPGP) sterilization (Steriace 100, Saraya, Tokyo, Japan) were used. After sterilization/disinfection, the respirators were fully dried and the filtration capacity of each N95 respirator was examined (n = 3) using the N95 integrity test described in the following section.
To evaluate the effect of sterilization/disinfection, 100 µL of BCoV, P. multocida and M. tuberculosis H37Ra were added to each N95 respirator (cut into 2 × 2 cm pieces). They were then dried, immersed in 5 ml of Phosphate Buffer (PB) and vortexed. Each pathogen was recovered by vortex mixing for 2 min (n = 3). For BCoV, TCID50 before and after each sterilization/disinfection treatment was evaluated, and for P. multocida and M. tuberculosis H37Ra, the number of bacteria before and after each sterilization/disinfection was counted using blood agar medium (Becton Dickinson, Fukushima, Japan) and Middlebrook 7H10 medium + OADC supplement (Becton Dickinson, Sparks, MD), respectively.
N95 integrity test
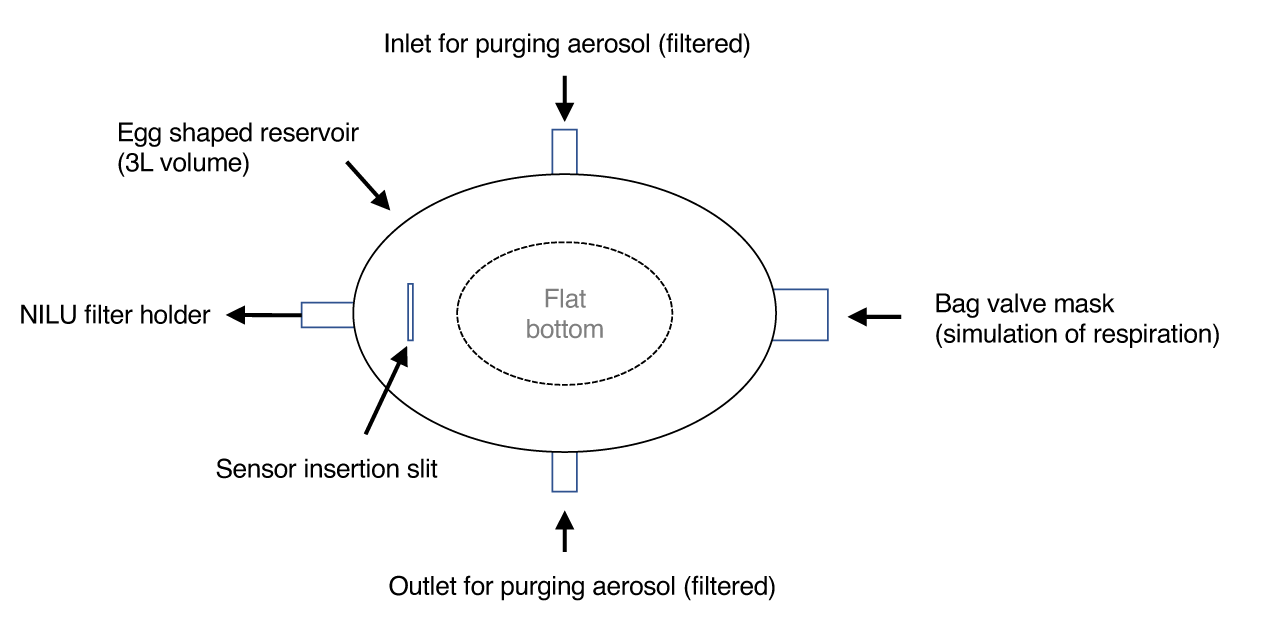
A respiration simulator with a 20 L reservoir was created using a 3D printer (TORAY Precision Co. Ltd., Yokohama, Japan) (Figure 1). The bag valve mask was used to simulate human respiration. The fit tester model MT-05U (Shibata Scientific Technology, Taito, Japan) was used to measure the particle number (outer and inner environments of the reservoir) and leakage rate. The measured particle size was set to ≥0.3 µm. The leakage rate of each N95 respirator was measured in triplicate before and after decontamination.
Statistical Analysis
The N95 permeability was compared before and after each decontamination process. The Wilcoxon signed-rank test was used. A p value of <0.05 was considered significant.
Ethical Considerations
This study used only in-vitro cultured pathogens following national biosafety guidelines. It did not use any clinical samples or patient information; therefore, no ethical approval was required.
Results
Performance changes of N95 respirator due to decontamination
The performance changes of the N95 respirator owing to each decontamination method differed for each manufacturer’s product. Seventy percent ethanol, 0.1% sodium hypochlorite aqueous solution, 0.3% peracetic acid aqueous solution, autoclaving, electrolysed-oxidising water, and plant mineral-activated water significantly deteriorated the performance of all tested N95 respirators. Performance degradation (increased permeability) was observed in 0.2% alkyldiaminoethylglycine hydrochloride aqueous solution and HPGP sterilization, and the permeation performance significantly deteriorated by 50 - 70% in all N95 respirators tested. Only pasteurisation resulted in no deterioration in performance, even after five repeated sterilizations (data not shown). Changes in permeability before and after disinfection are shown in table 1.
| Table 1: Permeability changes (%) of N95 respirator before and after disinfection treatment. | ||||||||||||
| 3M | Hogy | Koken | Shigematsu | |||||||||
| Methods | Before | After | p value | Before | After | p value | Before | After | p value | Before | After | p value |
| 70% EtOH | 3.017% | 11.923% | 0.0585 | 1.743% | 4.593% | 0.0449* | 0.337% | 14.323% | 0.0007* | 0.573% | 14.613% | 0.1977 |
| 0.1% Hypochlorous acid | 3.017% | 2.113% | 0.3762 | 1.743% | 1.237% | 0.6601 | 0.337% | 1.14% | 0.1401 | 0.573% | 21.667% | 0.1493 |
| 0.3% Peracetic acid | 3.017% | 39.607% | 0.0251* | 1.743% | 2.997% | 0.3823 | 0.337% | 0.89% | 0.0647 | 0.573% | 36.11% | 0.1293 |
| Autoclave (121 °C, 30 min) | 3.017% | 8.08% | 0.0532 | 1.743% | 11.443% | 0.0079* | 0.337% | 2.853% | 0.0645 | 0.573% | 33.3% | 0.0598 |
| 0.2% Alkyldiaminoethylglycine hydrochloride aqueous solution | 3.017% | 62.207% | 0.0027* | 1.743% | 43.257% | 0.0212* | 0.337% | 75.593% | 0.0005* | 0.573% | 50.02% | 0.001* |
| Electrolysed-oxidising water | 3.017% | 5.347% | 0.0592 | 1.743% | 1.46% | 0.7496 | 0.337% | 1.133% | 0.1071 | 0.573% | 4.12% | 0.04510* |
| Plant mineral-activated water | 3.017% | 32.677% | 0.0478* | 1.743% | 2.213% | 0.7065 | 0.337% | 3.653% | 0.185 | 0.573% | 19.943% | 0.1357 |
| Pasteurisation (65 °C, 30 min) | 3.017% | 1.3% | 0.1363 | 1.743% | 1.007% | 0.4994 | 0.337% | 1.023% | 0.2593 | 0.573% | 3.117% | 0.0514 |
| Hydrogen peroxide plasma sterilisation | 3.017% | 83.04% | 0.0107* | 1.743% | 60.58% | 0.0421* | 0.337% | 82.447% | 0.0012* | 0.573% | 59.453% | 0.0394* |
| Before and after: mean value of triplicated test results, *p < 0.05 | ||||||||||||
Microbiological effect of decontamination
All decontamination methods showed a decrease in the virus titre below the detection limit. The limit of detection of virus titre was ≤-0.5 (log10TCID50/25ul), and that of bacteria was 10 CFU/ml theoretically. With P. multocida, the inoculum volume was 1.41 × 106 CFU and the recovery rate was 0.85 - 1.02%. No live P. multocida were recovered from the decontaminated samples. At least 99.99% of the solution was sterilised or remained trapped in the filter. Furthermore, with M. tuberculosis H37Ra, the inoculum amount was 0.83 × 105 CFU, and the recovery rate was 0.23 – 0.96%. At least 99.95% was considered to have been sterilised or remained trapped in the filter.
Discussion
Verification of various decontamination methods for the reuse of N95 respirators has shown that immersive methods generally damage N95 integrity at different levels. In particular, 0.2% alkyldiaminoethylglycine hydrochloride aqueous solution and hydrogen peroxide plasma sterilization showed severe damage to all four N95 respirators tested in this study. Hypochlorous acid (0.1%) and conventional pasteurisation were found to be the optimal decontamination methods that did not damage N95 integrity.
Visucusi et al. reported that HPGP did not significantly affect filter aerosol penetration or filter airflow resistance [8]. However, Bergman et al. reported that three cycles of the HPGP decontamination process reduced the filtering efficiency by>5% for four of the six respirators tested [9]. Kumar et al. also reported that low-temperature HPGP using STRERRAD 100NX (Advanced Sterilization Products, Irvine, CA) reduced the filtration efficiency to <95% for three of the six respirators tested [10]. Wigginton KR, et al. [11] reported that the minimum filtration efficiency of an N95 respirator after five cycles of HPGP was >74% [11]. The reason for the discrepancy in results of HPGP is unclear, but repeated HPGP will damage the N95 integrity significantly, as our data showed more than 50% permeability change with a single HPGP treatment. HPGP is not recommended for decontamination of N95 respirators.
Another strongly damaging decontamination method is immersion in a 0.2% alkyldiaminoethyl glycine hydrochloride aqueous solution. The solution (0.1%) is recommended for disinfection and sterilization of healthcare facilities (CDC, 2008) [12]. In our search in PubMed, we could not find any report using alkyldiaminoethylglycine hydrochloride solution for the decontamination of N95 respirators. Because it is an amphoteric surfactant, it may induce electrostatic discharge and damage the N95 integrity [13].
Other immersive decontamination procedures were also harmful to N95 integrity, except 0.1% hypochlorous acid. The use of 70% ethanol clearly reduced the filtration efficiency by approximately 40%, even with a single treatment. Two mechanisms have been proposed for this degradation: electrostatic discharge and surface tension interactions [14,15]. If the electrostatic discharge hypothesis is the major mechanism of N95 degradation, 0.1% hypochlorous acid solution treatment would also have shown similar degradation in this study, but no significant change in N95 integrity was observed in our study. This may support the surface tension interaction hypothesis, although we did not acquire sufficient detailed data to make this determination.
No N95 integrity degradation was observed after immersion in 0.1% hypochlorous acid. Hydrogen Peroxide Vapour (HPV) has been reported to be an effective decontaminant for maintaining N95 integrity [16]. Hydrogen peroxide may not damage N95 integrity, although we did not repeatedly test 0.1% hypochlorous acid immersion. Water absorption may damage the N95 integrity after multiple treatment cycles.
It was difficult to extract microorganisms from the N95 filter using mechanical dispersion (vortex mixing). We stapled the filter sample (2 × 2 cm) to prevent the separation of multiple layers of the N95 respirator. Once the microorganisms were trapped in the filter, approximately 1% of the inoculated bacteria were recovered in the buffer solution, and the exact decontamination capacity of each method was unclear. However, other in-vitro studies have demonstrated that many methods tested in this study have efficient decontamination capacity against viruses and bacteria, including M. tuberculosis [12,17-19]. However, the microorganism trapping capacity of the N95 respirator is high and may not release the captured microorganisms from the fibre filtration system. This may explain why trapped microorganisms do not cause aerosol infection to the N95 user.
In this study, we confirmed that conventional pasteurisation is the most effective decontamination method. Iki S, et al. [20] also reported the efficiency of high-heat (75 °C) disinfection [20]. Although we did not evaluate the proper fitting after the decontamination process, the pasteurisation process does not affect the form of the respirator. We also confirmed that five cycles of pasteurisation did not affect the N95 integrity. Pasteurisation is recommended in many hospital settings where highly toxic materials such as HPV are not readily available.
Conclusion
The conventional pasteurisation method was most effective for the sterilization of N95 respirators without losing any particle collection efficiency, whereas the immersion methods were generally degenerative to the N95 respirators tested. Pasteurisation is easy to perform in general hospitals and is cost effective. Although the N95 respirator is generally disposable in clinical practice, it can be reused after appropriate sterilization, such as pasteurisation.
Acknowledgement
The authors are grateful to Mr. Yu Rong and Mr. Shuta Minagawa for their dedicated contribution to this study.
Author contributions
Satoshi Mitarai: Conceptualisation, Methodology, Writing, Jun Noda: Methodology, Reviewing and Editing, Satoshi Gondaira: Methodology, Reviewing and Editing, Ikuo Uchida: Sample preparation, Methodology, Reviewing and Editing, Rikio Kirisawa: Sample preparation, Methodology, Reviewing and Editing.
Competing Interests
The authors do not have any conflict of interest related to this study.
Funding Statement
This work was supported by the Japan Agency for Medical Research and Development (AMED; grant number 20he0622004h).
References
- Nogee D, Tomassoni AJ. Covid-19 and the N95 respirator shortage: Closing the gap. Infect Control Hosp Epidemiol 2020; 41: 958. https://doi.org/10.1017/ice.2020.124. [Epub 2020 April 13]. PMID: 32279694, PMCID: PMC7205548.
- Schelhorn JE. Herbers JM. 24Profile: Peter Tsai. Beyond discovery: Moving academic research to the market. Schelhorn JE, Herbers JM, editors. Oxford University Press; 2022. https://doi.org/10.1093/oso/9780197512715.003.0004.
- van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020; 382: 1564-7. https://doi.org/10.1056/NEJMc2004973. [Epub 2020 March 17]. PMID: 32182409, PMCID: PMC7121658.
- Fischer RJ, Morris DH, van Doremalen N, Sarchette S, Matson MJ, Bushmaker T, Yinda CK, Seifert SN, Gamble A, Williamson BN, Judson SD, de Wit E, Lloyd-Smith JO, Munster VJ. Effectiveness of N95 Respirator Decontamination and Reuse against SARS-CoV-2 Virus. Emerg Infect Dis. 2020 Sep;26(9):2253–5. doi: 10.3201/eid2609.201524. Epub 2020 Jun 3. PMID: 32491983; PMCID: PMC7454118.
- Paul D, Mondal SK, Mandal SM. Biologia Futura: Use of biocides during COVID-19-global reshuffling of the microbiota. Biol Futur 2021; 72: 273-80. https://doi.org/10.1007/s42977-021-00069-1. [Epub 2021 February 1]. PMID: 34554548, PMCID: PMC7848239.
- COVID-19 epidemiological update. World Health Organization. 2024.
- Kanno T, Ishihara R, Hatama S, Uchida I. Antigenic variation among recent Japanese isolates of bovine coronaviruses belonging to phylogenetically distinct genetic groups. Arch Virol 2013; 158: 1047-53. https://doi.org/10.1007/s00705-012-1587-1. [Epub 2012 December 27]. PMID: 23269444, PMCID: PMC7086937.
- Viscusi DJ, Bergman MS, Eimer BC, Shaffer RE. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg 2009; 53: 815-27. https://doi.org/10.1093/annhyg/mep070. [Epub 2009 October 4]. PMID: 19805391, PMCID: PMC2781738.
- Bergman MS, Viscusi DJ, Heimbuch BK, Wander JD, Sambol AR, Shaffer RE. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. J Engineered Fibers Fabr. 2010;5. doi: 10.1177/155892501000500405.
- Kumar A, Kasloff SB, Leung A, Cutts T, Strong JE, Hills K et al. Decontamination of N95 masks for re-use employing 7 widely available sterilization methods. PLoS One 2020; 15: e0243965. https://doi.org/10.1371/journal.pone.0243965. PMID: 33326504, PMCID: PMC7744046.
- Wigginton KR, Arts PJ, Clack HL, Fitzsimmons WJ, Gamba M, Harrison KR et al. Validation of N95 filtering facepiece respirator decontamination methods available at a large University Hospital. Open Forum Infect Dis 2021; 8: ofaa610. https://doi.org/10.1093/ofid/ofaa610. PMID: 33575418, PMCID: PMC7863868.
- Rutala WA, Weber DJ. The Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for disinfection and sterilisation in healthcare facilities. 2008.
- Soontravanich S, Munoz JA, Scamehorn JF, Harwell JH, Sabatini DA. Interaction between an anionic and an amphoteric surfactant. Part I: Monomer-micelle equilibrium. J Surfact Deterg. 2008;11:251-61. doi: 10.1007/s11743-008-1080-8.
- Nazeeri AI, Hilburn IA, Wu D-A, Mohammed KA, Badal DY, Chan MHW, Kirschvink JL. Ethanol-drying regeneration of N95 respirators. medRxiv. 2020. doi: 10.1101/2020.04.12.20059709.
- Faridi-Majidi R, Norouz F, Boroumand S, Nasrollah Tabatabaei S, Faridi-Majidi R. Decontamination assessment of nanofiber-based N95 masks. Environ Sci Pollut Res Int 2022;29:80411-21. https://doi.org/10.1007/s11356-022-20903-w. [Epub 2022 June 18]. PMID: 35716305, PMCID: PMC9206400.
- Cooper J, Csapó A, Ranasinghe R, Jeronimo M, Brockington-Tyhy T, Alawfi S et al. Filtration performance of three models of N95 filtering facepiece respirators following clinical usage and vaporized hydrogen peroxide decontamination. J Hosp Infect 2022; 131: 122-5. https://doi.org/10.1016/j.jhin.2022.09.026. [Epub ahead of print]. PMID: 36272553.
- Brooks JP, Lupfer C, Yang W, Hao W, Kapiamba KF. The effect of hypochlorous acid on the filtration performance and bacterial decontamination of N95 filtering facemask respirators. Am J Infect Control 2022; S0196-6553(22)00540-5. https://doi.org/10.1016/j.ajic.2022.07.013. [Epub ahead of print]. PMID: 35870660.
- Derr TH, James MA, Kuny CV, Patel DR, Kandel PP, Field C et al. Aerosolized hydrogen peroxide decontamination of N95 respirators, with fit-testing and viral inactivation, demonstrates feasibility for reuse during the COVID-19 pandemic. mSphere 2022; 7: e0030322. https://doi.org/10.1128/msphere.00303-22. [Epub 2022 August 30]. PMID: 36040048, PMCID: PMC9599425.
- Sharma S, Wang F, Rao PVK, Agrawal AK, Jassal M, Szenti I et al. Unfolding the effects of decontamination treatments on the structural and functional integrity of N95 respirators via numerical simulations. Sci Rep 2022; 12: 4191. https://doi.org/10.1038/s41598-022-08150-y. PMID: 35264706, PMCID: PMC8906365.
- Iki S, Sekiguchi K, Kurata Y, Shimizu E, Sugiura A, Yuasa H et al. Effects of various physical and chemical disinfection methods on the fine particle collection efficiency of N95 respirators and surgical masks. Jpn J Infect Dis 2022; 75: 341-6. https://doi.org/10.7883/yoken.JJID.2021.663. [Epub 2021 December 28]. PMID: 34980707.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.