General Science. 2024 February 15;5(2):147-151. doi: 10.37871/jbres1881.
Autoimmune Hemolytic Anemia as a Rare Presentation of HIV Infection in an Infant
Surekha Tony1*, Hatem Masoud Al Rawahi2 and Roshan Mevada3
2Department of Child Health, Sultan Qaboos University Hospital, Muscat, Oman
3Father Muller Medical College Mangalore, India
- Infant
- Autoimmune hemolytic anemia
- Human Immunodeficiency Virus
- Oman
Abstract
In patients infected with Human Immunodeficiency Virus (HIV), cytopenia and bone marrow dysplasia are the most common hematologic complications. Autoimmune Hemolytic Anemias (AIHAs) are rare and heterogeneous disorders characterized by the destruction of red blood cells through warm or cold antibodies. Despite Autoimmune Hemolytic Anemia (AIHA) being a rare disease in children, and anemia in HIV patients being multifactorial, HIV infection can predispose to AIHA. Literature review reveals the occurrence of AIHA in People Living with HIV (PLWH) and most of the reported cases are in adults on treatment with HAART (Highly active antiretroviral therapy).
We aim to present a rare case of an Omani infant presenting with fever and progressive pallor. AIHA was identified as the cause of anemia and subsequently the patient was diagnosed to have underlying HIV infection.
Introduction
The clinical expression of Human Immunodeficiency Virus (HIV) in children is highly variable. Many HIV-infected children develop severe HIV-related signs and symptoms in the first year of life. Other HIV-infected children remain asymptomatic or mildly symptomatic for more than a year and may survive for several years [1]. In addition to developing immunosuppression, opportunistic infections, and malignancies, patients infected with (HIV) also develop many hematologic disorders [2].
Although cytopenia and bone marrow dysplasia are common hematologic complications in patients infected with HIV [3]; anemia is the most prevalent hematological disorder observed in children and adults with HIV infection [4].
The etiology of anemia in HIV is complex and multifactorial. Opportunistic infections, malignancies, micronutrient deficiencies, drug toxicity, immune-mediated destruction of cells and direct cytopathic effect of virus can contribute to anemia in HIV infected patients [5]. Despite Autoimmune Hemolytic Anemia (AIHA) being a rare disease in children, and anemia in HIV patients being multifactorial, HIV infection can predispose to AIHA [2].
AIHA can present with pallor, respiratory difficulty, jaundice, lethargy, cyanosis, abdominal pain, low-grade fever, dark urine (severe hemolysis), and hepatosplenomegaly [6]. We report an interesting case of a very young 11-month old infant presenting with AIHA, with subsequent evaluation leading to the underlying diagnosis of HIV.
Case Presentation
A 11-month old female infant presented with fever for 10 days and progressive pallor. The infant was the first offspring born to a non-consanguineous couple. The family history was negative for hematologic disorders. Examination showed generalized lymphadenopathy and organomegaly without icterus. Initial investigations (Table 1) revealed anemia (Hemoglobin [Hb] 6.5 g/dl Reticulocyte count 124 Reticulocyte 4.6% MVC 78.4 fL MCH 24.2 pg), normal platelet count (157 x 10^9/L), elevated white blood cell count (18.8 x 10^9/L), elevated Lactate Dehydrogenase (LDH) level (797 U/L; range 120-300 U/L) and normal haptoglobin level (1.67 g/L; range 0.3-2 g/L). Glucose 6 Phosphate Dehydrogenase (G6PD) level was normal. Patient was stabilized with oxygen support and received packed red blood cell transfusion overnight. The blood film demonstrated microcytic hypochromic Red Blood Cells (RBC) with nucleated RBCs, early erythroid precursors, mild polychromasia and RBC agglutination with positive Direct Antiglobulin Test (DAT). DAT monospecific was positive for RBCs coated with IgG and C3d.
| Table 1: Initial investigations. | |
| Laboratory Data | Value |
| Hemoglobin | 6.5 g/dl |
| Reticulocyte count | 127 x 10^9/L |
| Platelet count | 157 x 10^9/L |
| White Blood Cell Count | 18.8 x 10^9/L |
| Lactate dehydrogenase | 797 U/L |
| Haptoglobin | 1.6 g/L |
| DAT | Positive (RBCs coated with IgG and C3d) |
| CD3 Count | 0.750 x10^9/L |
| CD4 Count | 0.371 x10^9/L |
| CD19 Count | 0.242 x10^9/L |
| HIV RNA by PCR | 10000000 copies/ml (log 7.0 copies/ml). |
| Lymph Node Biopsy IHC | CD4+ CD8+ |
| DAT: Direct Antiglobulin Test; CD: Cluster of Differentiation; HIV: Human Immunodeficiency Virus; RNA: Ribonucleic Acid; PCR: Polymerase Chain Reaction; IHC: Immunohistochemistry | |
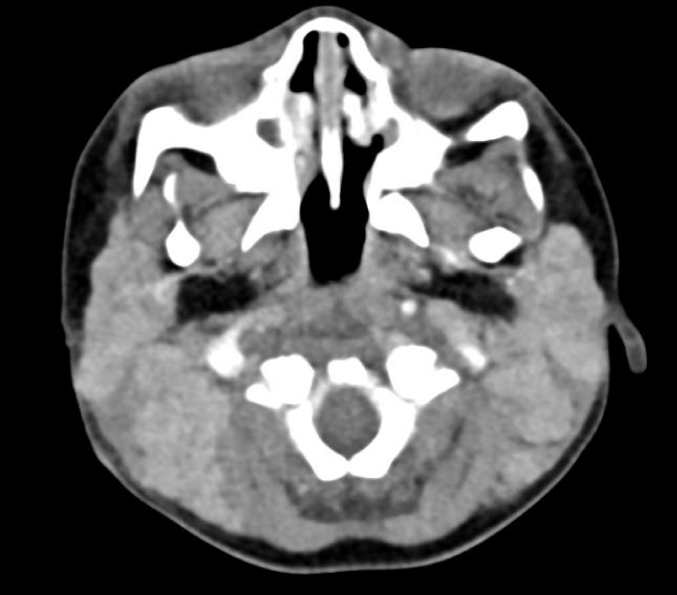
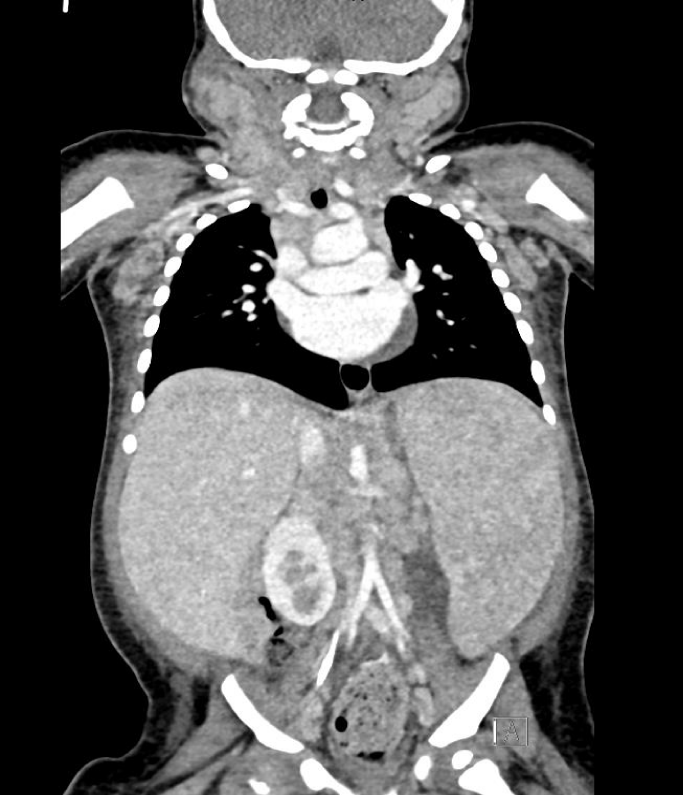
Computerized Tomography (CT) revealed extensive lymphadenopathy in the neck, mediastinum, bilateral axilla, retroperitoneum, pelvic and inguinal location along with massive non-focal hepatosplenomegaly with mild abdominal ascites and mild pericardial effusion with features favoring lymphoproliferative disease process (Figures 1,2). Immediate Bone Marrow Aspirate (BMA) with trephine biopsy and simultaneous cervical lymph node biopsy were done under general anesthesia, following which patient was intubated for 2 days with successful extubation.
BMA/trephine biopsy showed reactive marrow with mild increase in polyclonal population of lymphocytes (CD3 and CD20). Lymph node biopsy revealed florid/atypical lymphoid hyperplasia. Detailed immunohistochemistry was done to rule out autoimmune lymphoproliferative syndrome. The follicles were BCL6+, CD10+, and BCL2-. CD4+ cells were more than CD8+ cells. CD30, CD15, TdT, MPX, and CD1a were negative. HHV-8 CMV and EBER-ISH were all negative. Infectious disease work up revealed positive HIV Ag/Ab screen in two different immunoassays with HIV RNA by PCR showing 10000000 copies/ml (log 7.0 copies/ml). Lymphocyte subset revealed reduced CD3, CD4 and CD19 counts (0.750, 0.371 and 0.242 x10^9/L) respectively.
The infant was started on Injection Methyl prednisolone (2 mg/kg) from day 2 of admission for a total of 3 days and shifted to oral prednisolone dose (stable Hb level of 8 g/dl) at 2 mg/kg dose for 14 days with a plan to taper prednisolone slowly based on response to therapy. Simultaneously antiretroviral therapy was initiated within a week with Lamivudine (5 mg/kg twice daily), Zidovudine (12 mg/kg twice daily) and Nevirapine (200 mg/ m2 of BSA twice daily) along with cotrimoxazole prophylaxis three days a week. Patient is currently 14 months of age, clinically well without lymphadenopathy, regressing organomegaly, on antiretroviral therapy, off prednisolone 2 weeks and maintaining Hb of 10 g/dl. Informed consent from the legal guardian was obtained for data collection and publication.
Discussion
HIV infection leads to a multisystem disease with prevailing hematological abnormalities comprising defective hematopoiesis, cytopenias and abnormalities of coagulation [4]. Although some reports have shown that HIV infection can induce the production of autoantibodies, the association between HIV infection and subsequent development of AIHA has not been extensively studied [7].
AIHA is a rare acquired form of hemolytic anemia in which autoantibodies target Red Blood Cell (RBC) membrane antigens, inducing cell rupture [8,9]. A positive Direct Antiglobulin Test (DAT) confirms the presence of immunoglobulins (most often of the IgG class, sometimes IgM and IgA and/or complement-usually C3d) attached to erythrocytes and is used for diagnosis of AIHA [10].
The structural antigen similarity between HIV proteins and RBC antigens can induce autoantibody production [11,12] and might be associated with the development of incident AIHA. The molecular mimicry between HIV proteins and RBC antigens and the defective tolerance to self-antigens lead to the production of anti-RBC antibodies and may consequently induce the development of AIHA [7]. Moreover, HIV infection is an independent risk factor for AIHA and increases the incidence of AIHA more than 20-fold [7]. Yen et al published the largest cohort study to address the association between HIV infection and subsequent development of AIHA and noted that PLWH had a 28-fold higher risk of AIHA than controls. the incidence rate of AIHA was noted to be 23.76 events/100000 person-years among PLWH (n: 19052) in the age group of 33.4 ± 10.2 years. PLWH receiving HAART were more likely to develop incident AIHA than PLWH not receiving HAART [7].
Recent studies indicate the involvement of T and B cell dysregulation, reduced CD4+ and CD25+ Tregs, increased clonal expansions of CD8 + T cells, imbalance of Th17/Tregs and Tfh/Tfr, and impaired lymphocyte apoptosis [10]. T-cell–mediated immunity has been shown to play a critical role in the development of AIHA [10]. When HIV infects the host, it causes the dysregulation of CD4+ T-cell immunity, which could lead to defects in the body’s tolerance to self-antigens [13].
AIHA can be primary, when the underlying disease has not been demonstrated, or secondary. In approximately 50% of cases, the primary form of AIHA is diagnosed, while in other cases the autoantibodies are related to autoimmune diseases, lymphoproliferative diseases, infections (also SARS-CoV-2 infection), solid tumors or solid organ transplantation [10].
The serological types of AIHA include warm Autoimmune Hemolytic Anemia (wAIHA), Cold Agglutinin Disease (CAD), mixed type AIHA (mixed AIHA) and Paroxysmal Cold Hemoglobinuria (PCH). Recently, an atypical form of AIHA with DAT negative and the presence of IgA and warm IgM has been distinguished [14]. Previous case reports also found that typical warm antibodies can be detected in HIV infected individuals with hemolytic anemia [15].
In AIHA, as in the course of other hemolytic anemias, normocytic anemia with spherocytes is found in the peripheral blood smear. Reticulocytosis (although reticulocytopenia sometimes occurs) is a typical finding in hemolytic anemia but not a specific marker and indicates an active and accelerated, compensatory production of erythrocytes in the bone marrow in response to hemolysis. Increased unconjugated bilirubin levels, low or absent serum haptoglobin, elevated LDH and an increase in urinary urobilinogen are all hallmarks of hemolysis. Hemoglobinuria, which is an early symptom, indicates intravascular hemolysis. DAT confirms the immune mechanism. Sometimes low autoantibody titres below the threshold of the test or no appropriate antibody testing can give a negative DAT despite the presence of AIHA. 5–10% of AIHA cases fail to obtain a DAT positive despite highly sensitive tests [10].
An acute presentation of autoimmune hemolytic anemia is frequently a life-threatening, fast-progressive disease and requires prompt diagnosis, initiation of treatment, and close monitoring. Therefore, the first question to be answered on presentation of a patient with evidence of hemolytic anemia, is if this is an immune-mediated hemolytic anemia by ruling out other potential causes [9].
Screening for underlying causes is necessary for every child presenting with w-AIHA.A high index of suspicion for a primary immune disorder is advised and baseline testing of lymphocyte subpopulations with flow cytometry is recommended; of note, the blood sample for this test should optimally be obtained before initiating treatment with steroids [9].
Aladjidi et al reviewed 265 patients with a median age of 3.8 years at diagnosis and noted a prevalence of 21% of AIHA in infants with female preponderance. Consanguinity (8%) was observed in specific ethnic groups and a history of immunological diseases in first-degree relatives (14%) in all ethnic groups. Their review suggested a genetic predisposition to the development of AIHA in a subgroup of patients and the possibility of an underlying immune deregulation in more than half of cases. The insight into genetic basis of autoimmune disorders stemmed from the comprehension of rare defects in immune homeostasis, such as impaired apoptosis of auto-reactive lymphocytes in autoimmune lymphoproliferative syndrome, disruption of thymic negative selection in Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy (APECED) syndrome, and absence of regulatory T cells in Immunodysregulation Polyendocrinopathy Enteropathy X-linked (IPEX) syndrome. Hence, complete immunological investigations are recommended even if a well-defined infection is diagnosed, and treatment monitoring and long-term follow-up are essential [16].
Treatment depends on the type of AIHA, the presence and severity of clinical symptoms, the underlying diseases that caused the AIHA, and the presence of concomitant comorbidities. In general, symptomatic anemia is primarily an indication for therapy in both newly diagnosed and persistent AIHA [10]. The initial goals are decreasing hemolysis, stabilizing hemoglobin levels, and increasing safety and tolerability of packed RBC transfusion, if needed. RBC transfusions should be limited only to critical cases with severe anemia (Hb < 6 g/dl) and in hemodynamically unstable patients. The mainstay of the treatment is centered on the inhibition of autoantibody production by mono-or combination therapy [17,18].
In the treatment of secondary AIHA, it is crucial to treat any underlying autoimmune diseases [10]. The first line treatment for warm AIHA is oral prednisolone [19]. The steroid response rate is high, up to 80%, and is usually apparent within 24 to 72 h after initiation. After normalization of hemoglobin, the steroids should be tapered slowly over approximately 6 months, since quick tapering or abrupt discontinuation have been associated with disease relapse [20]. The preferred second line therapy is rituximab. Third line treatment options include splenectomy, azathioprine, cyclosporin and mycophenolate and should be based on the individual assessment of the benefit over risk ratio. Other lines of therapy to be considered are cyclophosphamide, continuous low dose prednisone, danazol, hematopoietic stem cell transplantation, and bortezomib [19].
Our patient tolerated transfusion which resulted in symptomatic improvement, responded successfully to steroid therapy and received prompt treatment with antiretroviral therapy. In conclusion, the reported cases of HIV-related AIHA in the pediatric age group are limited. To our knowledge, this is the first reported case of HIV infection presenting as AIHA in Oman. HIV as an etiology merits testing in the initial evaluation of children with autoimmune hemolytic anemia.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Manual on Paediatric HIV Care and Treatment for District Hospitals: Addendum to the Pocket Book of Hospital Care of Children. Geneva: World Health Organization; 2011. PMID: 26131545.
- Saif MW. HIV-associated autoimmune hemolytic anemia: an update. AIDS Patient Care STDS. 2001 Apr;15(4):217-24. doi: 10.1089/10872910151133783. PMID: 11359664.
- Dhurve SA, Dhurve AS. Bone Marrow Abnormalities in HIV Disease. Mediterr J Hematol Infect Dis. 2013 Jun 3;5(1): e2013033. doi: 10.4084/MJHID.2013.033. PMID: 23795271; PMCID: PMC3684351.
- Bhardwaj S, Almaeen A, Ahmed Wani F, Thirunavukkarasu A. Hematologic derangements in HIV/AIDS patients and their relationship with the CD4 counts: a cross-sectional study. Int J Clin Exp Pathol. 2020 Apr 1;13(4):756-763. PMID: 32355524; PMCID: PMC7191136.
- Redig AJ, Berliner N. Pathogenesis and clinical implications of HIV-related anemia in 2013. Hematology Am Soc Hematol Educ Program. 2013; 2013:377-81. doi: 10.1182/asheducation-2013.1.377. PMID: 24319207.
- Kukreja S, Baker SA, Ochani S, Lohana S, Kalwar A, Memon K, et al Autoimmune hemolytic anemia, a rare disease in newborns: a case report. Ann Med Surg (Lond). 2023 Apr 18;85(5):2212-2215. doi: 10.1097/MS9.0000000000000681. PMID: 37229037; PMCID: PMC10205368.
- Yen YF, Lan YC, Huang CT, Jen IA, Chen M, Lee CY, Chuang PH, Lee Y, Morisky DE, Chen YA. Human Immunodeficiency Virus Infection Increases the Risk of Incident Autoimmune Hemolytic Anemia: A Population-Based Cohort Study in Taiwan. J Infect Dis. 2017 Nov 15;216(8):1000-1007. doi: 10.1093/infdis/jix384. PMID: 29149339.
- Liebman HA, Weitz IC. Autoimmune Hemolytic Anemia. Med Clin North Am. 2017 Mar;101(2):351-359. doi: 10.1016/j.mcna.2016.09.007. Epub 2016 Dec 14. PMID: 28189175.
- Voulgaridou A, Kalfa TA. Autoimmune Hemolytic Anemia in the Pediatric Setting. J Clin Med. 2021 Jan 9;10(2):216. doi: 10.3390/jcm10020216. PMID: 33435309; PMCID: PMC7828053.
- Michalak SS, Olewicz-Gawlik A, Rupa-Matysek J, Wolny-Rokicka E, Nowakowska E, Gil L. Autoimmune hemolytic anemia: current knowledge and perspectives. Immun Ageing. 2020 Nov 20;17(1):38. doi: 10.1186/s12979-020-00208-7. PMID: 33292368; PMCID: PMC7677104.
- Tsiakalos A, Routsias JG, Kordossis T, Moutsopoulos HM, Tzioufas AG, Sipsas NV. Fine epitope specificity of anti-erythropoietin antibodies reveals molecular mimicry with HIV-1 p17 protein: a pathogenetic mechanism for HIV-1-related anemia. J Infect Dis 2011; 204:902–11. doi: 10.1093/infdis/jir433. PMID: 21849287.
- Russo S, Lopalco L. Is autoimmunity a component of natural immunity to HIV? Curr HIV Res 2006; 4:177–90. doi: 10.2174/157016206776055011. PMID: 16611056.
- Barcellini W. New Insights in the Pathogenesis of Autoimmune Hemolytic Anemia. Transfus Med Hemother. 2015 Sep;42(5):287-93. doi: 10.1159/000439002. Epub 2015 Sep 7. PMID: 26696796; PMCID: PMC4678320.
- Barcellini W, Fattizzo B, Zaninoni A. Current and emerging treatment options for autoimmune hemolytic anemia. Expert Rev Clin Immunol. 2018;14(10):857–872. doi: 10.1080/1744666X.2018.1521722.Epub 2018 Oct 8. PMID: 30204521.
- Iordache L, Launay O, Bouchaud O, Jeantils V, Goujard C, Boue F, et al. Associated authors. Autoimmune diseases in HIV-infected patients: 52 cases and literature review. Autoimmun Rev. 2014 Aug;13(8):850-7. doi: 10.1016/j.autrev.2014.04.005. Epub 2014 Apr 18. PMID: 24747058.
- Aladjidi N, Leverger G, Leblanc T, Picat MQ, Michel G, Bertrand Y, et al. New insights into childhood autoimmune hemolytic anemia: a French national observational study of 265 children. Haematologica 2011;96(5):655-663; https://doi.org/10.3324/haematol.2010.036053. Epub 2011 Jan 12. PMID: 21228033; PMCID: PMC3084911.
- Sigler E, Shvidel L, Yahalom V, Berrebi A, Shtalrid M.et al. “Clinical significance of serologic markers related to red blood cell autoantibodies production after red blood cell transfusion-severe autoimmune hemolytic anemia occurring after transfusion and alloimmunization: Successful treatment with rituximab.” Transfusion, Vol. 49, No. 7, 2009, pp. 1370-74. doi: 10.1111/j.1537-2995.2009.02163.x. Epub 2009 Apr 3. PMID: 19374728.
- Park SH, Choe WH, Kwon SW. Red Blood Cell Transfusion in Patients With Autoantibodies: Is It Effective and Safe Without Increasing Hemolysis Risk? Ann Lab Med. 2015 Jul;35(4):436-44. doi: 10.3343/alm.2015.35.4.436. Epub 2015 May 21. PMID: 26131416; PMCID: PMC4446583.
- Jäger U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2020 May; 41:100648. doi: 10.1016/j.blre.2019.100648. Epub 2019 Dec 5. PMID: 31839434.
- Naithani R, Agrawal N, Mahapatra M, Kumar R, Pati HP, Choudhry VP. Autoimmune hemolytic anemia in children. Pediatr Hematol Oncol. 2007 Jun;24(4):309-15. doi: 10.1080/08880010701360783. PMID: 17613874.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.