Biology Group. 2024 April 06;5(4):296-302. doi: 10.37871/jbres1895.
Chemical-Biological Activity of Nanodiamond Colloids
Stepan S Batsanov1*, Sergey M Gavrilkin1, Dmitry A Dankin2, Alexander V Kurakov3 and Andrei S Batsanov4
2Fritsch Laboratory Instruments, Moscow Branch, Moscow, Russia
3Biological Faculty, Moscow State University, Moscow, Russia
4Chemistry Department, Durham University, Durham, United Kingdom
- Nanodiamond
- Suspension
- Nitrogen fixation
- Bacteria
- Ammonium nitrate
Abstract
The study of the Nanoscale Diamonds (NDs) and their water suspensions has opened huge opportunities for their technological and biomedical applications. Therefore, the interaction of ND with water is actively studied. However, insufficient attention has been paid to the biochemical behavior of suspensions with low carbon content, despite their great scientific prospects. This work aims to fill this gap in scientific literature.
Introduction
The nanoscale diamond is intensely studied for its biochemical and biomedical applications [1-5]. Since ND is often used in the form of concentrated aqueous suspensions, many patents, scientific studies and reviews are devoted to this topic, but insufficient attention is paid to the study of the physico-chemical and biological properties of dilute colloidal solutions (henceforth called ‘Diamond Water’, DW), which show unique properties of great scientific significance. The present work describes chemical and biological behavior of ND particles in DW.
The ND used in this work, with typical crystal (primary particle) sizes of 4-5 nm, was produced in our laboratory by detonating a 1:1 blend of TNT and RDX enclosed in an ice shell, designed for fast quenching of the detonation products and thus preventing the annealing of diamond into graphite by the residual heat of the explosion. Explosive experiments were carried out in a vacuumated chamber. The detonation soot was then subjected to intense oxidation to remove the graphite component and isolate the ND powder [6].
Aqueous ND suspensions with high carbon contents (up to 3.1 [7], 8.6 [8], 10 [9], 50 [10] wt%) have been reported; these are colored (even if transparent) liquids or non-transparent slurries. On the contrary, DWs containing only ca. 0.01-0.1 wt% of ND [6 -11], are colorless liquids, apparently clear (to naked eye). We prepared DW by dispersing (by stirring and sonication) 1 % of ND in high-purity water, intense centrifugation of the mixture and removal of the solid precipitate. DLS studies showed the particle sizes in DW to vary from tens to hundreds of nanometers [11,12], indicating a very strong tendency of primary ND particles to agglomerate. However, colloids of mono-dispersed ND (i.e. separated primary particles) are also known, particularly used (with grafted Gd or Mn atoms) as MRI contrast reagents [13-15].
Results
Biological nature of ‘diamond-water fibers’ (DWF)
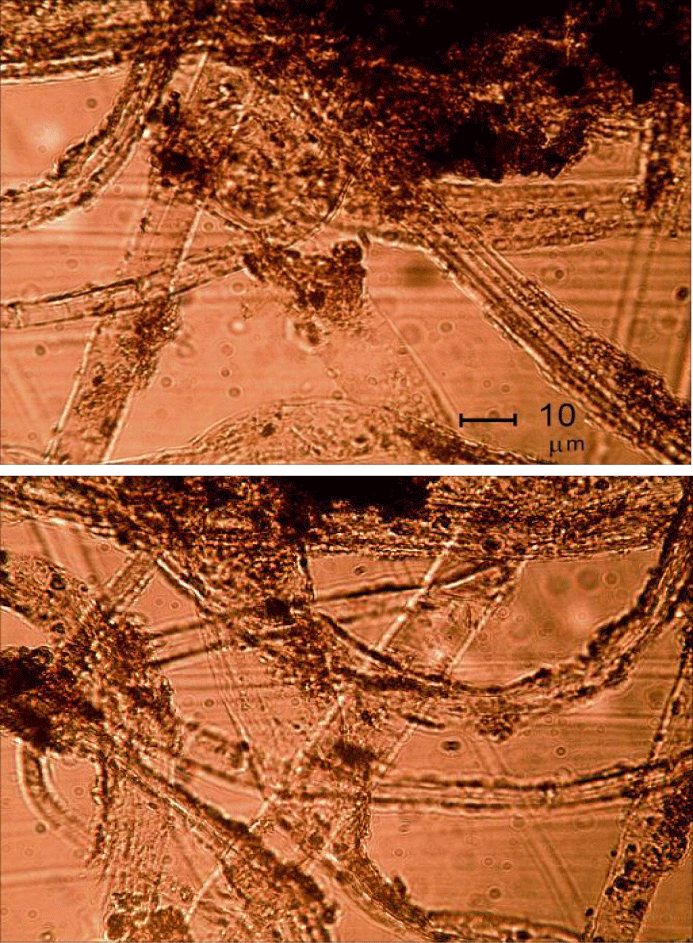
In vials of DW exposed to atmospheric air at room temperature, after periods of time ranging from several days to weeks, cloudy and then cotton Wool-like Fibers (DWF) emerged and gradually increased in size. Evolution of DWF shapes and sizes in DW begins with globular features ca. a few µm in diameter, which then coagulated into larger quasi-spherical clusters of the order of 100 µm. A microscopic study showed that these agglomerates contain entangled light-brown or grey fibers, on the order of 1-2 µm thick and up to 1 mm in length, emerged and ultimately grew to several mm, sometimes up to 1 cm long. Earlier, biochemical (staining) tests proved the biological nature of DWF [16,17].
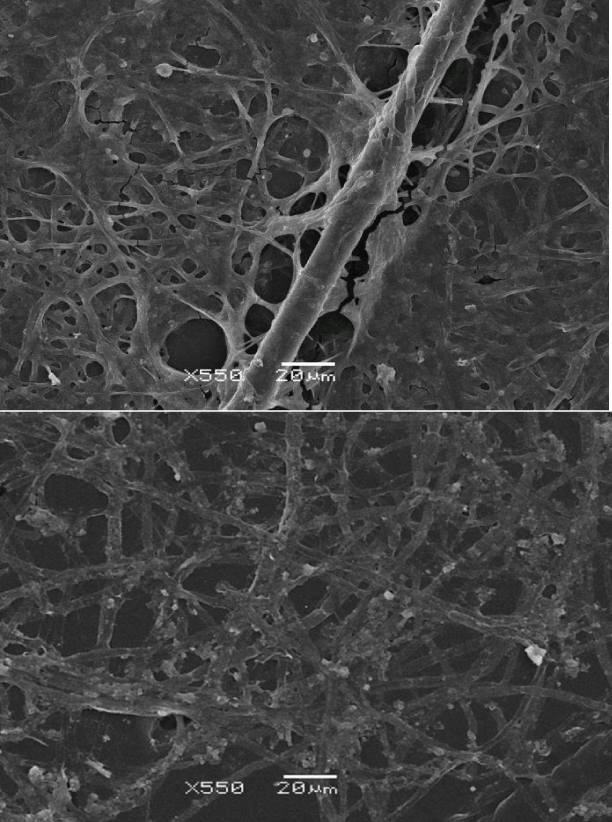
In the present work, we studied the DWFs with optical (Figure 1) and scanning electron microscopy (Figure 2) and found them to consist of mycelial hyphae, fungal spores and bacterial cells (thin bacilli- and cocci-like structures) with the diameters in the usual ranges of 1.5 to 18.0 μm, for hyphae, 2.5 to 4.5 μm for spores, and 0.25 to 0.75 μm for cocci, while the bacilli had width of 1 to 2 μm and length 2 to 4 μm. The hyphae were attached to, and apparently ‘irradiated’ from, agglomerates of ND particles (Figure 1). The fungi formed conidiophores with individual spores. The spores and bacteria were both entangled on the surface of the fungal mycelia and free-floating in solution.
The mycelia of the following fungal species were identified: P. cyclopium, P. aurantiogriseum, P. aurantiogriseum, P. chrysogenum, P. lilacinum, B. bassiana, as well as some species of the genera Mucor, Rhizopus, Trichoderma and Aspergillus. Some species distinguished by dark-colored mycelium with chlamidospores, or light-colored mycelium, as well as several types of bacterial mucous colonies (white, rosy and light yellow) and colonies characteristic of B. mycoides were observed, without full identification.
Inoculation with the original ND powder did not produce the growth and formation of colonies of fungi or bacteria for 10 days, although individual bacterial cells and fragments of mycelia were detected. Inoculation with original ND exposed to air and moisture for a short time, already after 1 week yielded isolated colonies of P. bacteria, particularly P. cyclopium, clear white mycelium, and B. mycoides; on the third week, fungal colonies evolved, distinguished by clear dark-coloured mycelium.
Neither fungi nor bacteria were isolated using ND previously sterilized in an autoclave (at 1 atm and 120°C), whether immediately before the tests or after kept hermetically sealed for 1 year. Even after a protracted (1 month) incubation of sterile ND in sterile water, fungi or bacteria did not emerge or grow. If the original ND was placed into sterile distilled water, 1 month later some isolated fungal hyphae and bacterial cells were detected. If a sterile ND powder was incubated in ordinary distilled water or a distilled water previously exposed (for 1 to 3 h) to atmospheric air, then the growth of fungal mycelia and bacterial cells was also observed. Note that in blank experiments ‒ identical vials of pure water kept alongside the DW vials no fibers were formed; moreover, if transplanted there, the already formed DWF failed to grow and soon died.
Chemical composition and evolution of DW and DWF
For elemental analysis, samples of DW with DWF of different ages were dried at 150°C and analyzed using Carlo Erba 1106 instrument (standard errors of 0.3, 0.1, 0.05% for C, H, N, respectively, each sample analysed twice). The balance can be attributed entirely to oxygen, for although the original ND contained small amounts of Fe (≤ 0.2%) and Si (≤ 0.3%) extracted by shock waves from the steel walls of the explosion chamber and yielding non-combustible residue, these heavier impurities were practically removed by intense centrifugation of DW. The results (Table 1) show a steady decline of the carbon content over time, while those of all other elements increase. During the growth of DWF (first two months) the C/N ratio was intermediate between those of the initial ND (40) and living organisms (5 to 10 for fungi and bacteria), as could be expected for the mixture of these. IR spectra of the DWF at this stage [16] show the stretching bands C-O at 1026 and 1154 cm-1 of carbohydrates; C = O 1746, CH2 or CH3 2853 (symm) and 2934 cm-1 (asymm) of lipids; C-N 1420 cm-1 of proteins, as well as amide bands of the latter at 1638, 1548 and 1316 cm-1, all typical for living micro-organisms, especially fungi [18]. Later, the lysis of the micro-organisms sets in, releasing carbon as CO2 (which escapes, hence the dwindling carbon content) and nitrogen as NH4+ or NO3‒ (which stay in solution). The high oxygen content, ultimately reaching 74.25%, is somewhat unusual, although NH4NO3 (which was in fact isolated, see below) contains 60%.
Actually, the atomic composition of samples 2 to 5 in table 1 can be rationalized respectively as:
С5.8 N0.3 H1.2 O1.55 → 28C•5CO•2H2O•NH4NO3
C4.1 N0.3 H1.7 O2.78 → 19C•3H2O•5CO2•NH4NO3
C1.3 N1.05 H3 O4.15 → CO•H2O•CO2•NH4NO3
C0.2 N1.25 H5.3 O4.65 → 2H2O2•NH4NO3
Admittedly, the presence of O-O bonds may seem far-fetched, but the products was indeed unstable and exploded under 23-25 k bar of static compression and under heating in argon at T > 200°C [19].
A faster evolution of the chemical composition was observed with DW prepared by bead-milling procedure. 6.5 g of ND and 50 ml of water were placed in a planetary bead mill with both the 80 ml pot and the 25 beads of 5 mm diameter made of WC-6 alloy (tungsten carbide doped with 6% of Co), which was rotated at 650 rpm for 30 min in either direction, After the milling, 10 ml of the liquid phase was placed in an open vial to dry at room temperature. The dry residue had the composition: C 42.55, H 3.02, N 3.36, О 51.07%. Repeated experiments have shown that during very slow evaporation of DW (from a vial with a pinhole in the lid), powders of white, yellow and reddish colors were consequently precipitated, with the compositions (%):
(i) Colorless C 15.35, Н 7.15, N 10.15, О (balance) 67.35
(ii) Yellow С 13.3, Н 3.89, N 9.02, О (balance) 73.79
(iii) Reddish С 0.58, Н 3.95, N 19.88, О (balance) 75.59
Recrystallization of the entire sediment from DW after prolonged (several weeks) evaporation, yielded a solid with the composition С 0.98; Н 4.64, N 26.70, O (balance) 67.68%. Remarkably, the compositions of the latter phase and the reddish solid correspond to NH4NO3•⅓ H2O and NH4NO3 •H2O2•O2, respectively. Since these formulae are close to the ammonium nitrate, we have extended the exposure of DW (40 ml) to air to 18 months, whereupon the liquid colloid converted into a solid mixture of powder (2.1 g) and colorless crystals (19.4 g) with content (%):
C H N O Formula
Powder 1.90 4.80 33.64 59.66 NH4NO3•C0.13
Crystals 0.22 5.10 33.75 60.93 NH4NO3
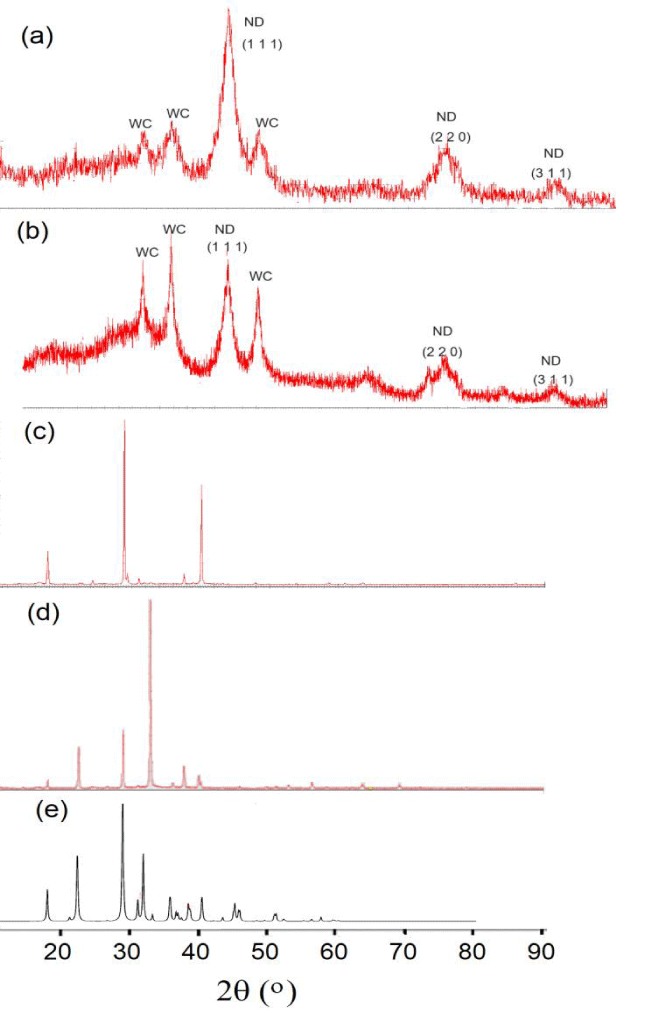
Interestingly, the carbon content in these solids (0.08 g) was close to that in the original DW (0.2% in 40 ml), indicating that ND participated in the formation of NH4NO3 as a catalyst. Results of the X-ray study of all above-mentioned samples are presented in figure 3.
Discussion
As shown above, DWFs are colonies of fungi and bacteria growing around ND particles. Obviously, bacterial and fungal spores are omnipresent in atmospheric air and infestation of water exposed to it is only a matter of time. However, we found that an admixture of ND, even in small concentration, greatly facilitates such infestation and subsequent growth of bacterial and fungal colonies, while no such colonization occurs under the same conditions in pure water – where the spores can get with equal probability.
The effects of ND on living cells have been studied extensively, but usually with the focus on biocompatibility, i.e. the absence of cytotoxicity. The possibility of ND encouraging cellular (including bacterial and fungal) growth has not attracted the attention it deserves, given the practical applications of ND in medicine (drug delivery, biocensors, etc.).
Is it possible that ND can supply the micro-organisms with some vital nutrients? ND particles have a unique chemical activity due to their small sizes. This ‘size effect’ is caused by increasing fraction of surface atoms with lower coordination numbers, e.g. by increasing number of dangling chemical bonds in fine powders [20], which results in reducing surface tension, melting point, heat capacity and cohesive energy in nanophases [21-23]. Experimental data and all theoretical models show that the cohesive energy of nanoparticles falls linearly with their sizes. According to [24] the cohesive (atomization) energy of a particle (Ep) of arbitrary size is related to that of the bulk solid (Eb) as
Ep = (Np/Nb) Eb (1)
Where Np is the average coordination number of atoms in the structure of particle and Nb is that in the bulk. Since Np < Nb, the atomization energy of particles will decrease, thus enhancing their reactivity. Calculations of Ep by Eq. (1) for Cu, Au, C, Si, Ge, Mn, Fe, Co, Ni, and Pt using atomization energies in bulk (Eb) and 5 nm-sized (Ep) elemental elements show that the average relation Ep/Eb = 13 ± 1% [23]. We also found experimentally [23] that Group 4 elements in nano-state, due to their increased reactivity, change their positions in the electrochemical sequence and replace hydrogen in water under ambient conditions (E = C, Si and Ge):
E + 2H2O = EO2 + 2H2 (2)
The natural conversion of N2 to NH3 is mostly realized by nitrogenases in bacteria, where the MoFe-protein or the Fe-protein work in tandem for the activation or reduction of N2 molecules, respectively [25]. This reaction has been realized by contact glow discharge under a voltage of 500-600 V in an aqueous solution, where N2 serves as both the electron donor and acceptor.
Alternatively, we can consider the use of ND to realize the direct reactions
2C + N2 = C2N2 (4)
C2N2 + 2H2O + ½ О2 = NH4NO3 + 2C (5)
Where C is nanocarbon in DW, and N2 is atmospheric nitrogen; the composition of sample 5 in table 1 is consistent with a combination of reactions (4) and (5). Under standard conditions, Eq. (4) is endothermic (ΔH = 309 kJ/mol) and requires heating to > 1400°C to proceed. However, this refers to bulk diamond with the atomization energy Ea = 716.7 kJ/mol; but this reaction would be exothermic for atomic carbon with ΔH = ‒ 1124.5 kJ/mol or with ΔH = ‒ 506.5 kJ/mol for C2 molecule. DSC experiments on ND powders showed the heat of combustion ΔHс (ND) = 37.7 kJ/g (or 43.3 kJ/g for the carbon content only), ca. 30% higher than for polycrystalline diamond, 33.0 kJ/g. Thus, reactions (4) and (5) for ND will proceed exothermically. Note that ND in reactions (4) and (5) acts as a catalyst for the formation of NH4NO3; catalytic properties of ND and WC in other reactions were reported earlier [26-28]. This explains why the process was facilitated if a colloid was prepared using bead-milling procedure (see above).
| Table 1: Composition of WDFs, depending on their age. | ||||||
| Sample | Age, | Elemental analysis, wt% | C/N ratio | |||
| No. | months | С | N | Н | O | |
| 1 | 0* | 86.5 | 2.15 | 0.5 | ca. 10 | 40.2 |
| 2 | 1 | 69.6 | 4.2 | 1.2 | 25.0 | 17.2 |
| 3 | 2 | 49.0 | 4.4 | 1.7 | 44.5 | 11.2 |
| 4 | 3 | 15.7 | 14.9 | 3.0 | 66.4 | 1.05 |
| 5 | 4 | 2.65 | 17.8 | 5.3 | 74.25 | 0.15 |
Thus, an interaction of ND particles with atmospheric nitrogen in water medium forms ammonium nitrate, e.g. a fixation of atmospheric nitrogen occurs. It is very important since nitrogen is an essential element for all living organisms. The importance of atmospheric nitrogen fixation, which has been carried out by the Haber-Bosch method for more than 100 years, is evident from the huge scale of global ammonia production (170 million tons per year), which accounts for up to 1-2% of the total global energy consumption. The high cost, high energy costs and environmental harm (the release of a huge amount of carbon dioxide into the atmosphere) in this method make us look for alternatives, to which up to 30,000 publications and citations for the topic of ammonia synthesis have been devoted annually [29].
An amazing feature of this process is the formation of the final product in the form of large crystalline grains of NH4NO3 (see the photograph in [30] as a result of a very long (1.5 years!) contact of ND particles in colloidal solution with air. A very small concentration of these particles, which served as centers of crystallization of the formed NH4NO3, remained in the form of 0.2% carbon in the composition of NH4NO3 crystals, as shown by chemical analysis. The slow kinetics of these reactions is explained by the same low concentration of ND, which is a carrier of fixed nitrogen from air to water.
Conclusion
Bacteria and fungi can flourish in highly dilute colloidal solutions of ND in the manner not observed in pure water. Their growth is facilitated by fixation of atmospheric molecular nitrogen on ND particles (which provides the microorganisms with a vital nutrient), made possible by high reactivity of ND which makes the reaction of carbon with N2 an exothermic process. The resulting enrichment of the media with bioaccessible nitrogen is also confirmed by formation of NH4NO3 crystals after long exposure to air.
Competing Interests
The authors declare no competing interests.
References
- Krueger A. New carbon materials: biological applications of functionalized nanodiamond materials. Chemistry. 2008;14(5):1382-90. doi: 10.1002/chem.200700987. PMID: 18033700.
- Shugalei IV, Voznyakovskii AP, Garabadzhiu AV, Tselinskii IV, Sudarikov AM, Ilyushin MA. Biological activity of detonation nanodiamond and prospects in its medical and biological applications. Russ J Gen Chem. 2013;83:851-883. doi: 10.1134/S1070363213050010.
- Shenderova OA, McGuire GE. Science and engineering of nanodiamond particle surfaces for biological applications (Review). Biointerphases. 2015 Sep 5;10(3):030802. doi: 10.1116/1.4927679. PMID: 26245200.
- Ju Y, Liao H, Richardson JJ, Guo J, Caruso F. Nanostructured particles assembled from natural building blocks for advanced therapies. Chem Soc Rev. 2022 Jun 6;51(11):4287-4336. doi: 10.1039/d1cs00343g. PMID: 35471996.
- Dekhtyar Y, Abols D, Avotina L, Stoppel A, Balakin S, Khroustalyova G, Opitz J, Sorokins H, Beshchasna N, Tamane P, Rapoport A. Effects of diamond nanoparticles immobilisation on the surface of yeast cells: A phenomenological study. Fermentation. 2023;9:162. doi: 10.3390/fermentation9020162.
- Batsanov SS, Gavrilkin SM, Batsanov AS, Poyarkov KB, Kulakova II, Johnson DW, Mendis BG. Giant dielectric permittivity of detonation-produced nanodiamond is caused by water. J Mater Chem. 2012;22:11166-11172. doi: 10.1039/C2JM30836C.
- Kulvelis Yu V, Shvidchenko AV, Aleksenskii AE, Yudina EB, Lebedev VT, Shestakov MS, Dideikin AT, Khozyaeva LO, Kuklin AI, Töröke GY, Rulev MI, Vul’ AYa. Stabilization of detonation nanodiamonds hydrosol in physiological media. Diam Relat Mater. 2018;87:78-89. doi: 10.1016/j.diamond.2018.05.012.
- Petit T, Yuzawa H, Nagasaka R, Yamanoi M, Osawa E, Kosugi N, Aziz EF. Probing interfacial water on nanodiamonds in colloidal dispersion. J Phys Chem Lett. 2015;6:2909-2912. doi: 10.1021/acs.jpclett.5b00820.
- Ozawa M, Inaguma M, Takahashi M, Kataoka F, Krüger A, Osawa E. Preparation and behavior of brownish, clear nanodiamond colloids. Adv Mater. 2007;19:1201-1206. doi: 10.1002/adma.200601452.
- Korobov MV, Batuk MM, Avramenko NV, Ivanova NI, Rozhkova NN, Osawa E. Aggregate structure of single-nano buckydiamond in gel and dried powder by differential scanning calorimetry and nitrogen adsorption. Diam Relat Mater. 2010;19:665-671. doi: 10.1016/j.diamond.2010.02.032.
- Williams OA, Hees J, Dieker C, Jäger W, Kirste L, Nebel CE. Size-dependent reactivity of diamond nanoparticles. ACS Nano. 2010 Aug 24;4(8):4824-30. doi: 10.1021/nn100748k. PMID: 20731457.
- Batsanov SS, Poyarkov KB, Gavrilkin SM, Lesnikov EV, Schlegel VR. Orientation of water molecules by the diamond surface. Russ J Phys Chem. 2011;85:712-715. doi: 10.1134/S0036024411040054.
- Panich AM, Salti M, Goren SD, Yudina EB, Aleksenskii AE, Vul’ AYa, Shames AI. Gd(III)-grafted detonation nanodiamonds for MRI contrast enhancement. J Phys Chem C. 2019;123:2627-2641. doi: :10.1021/acs.jpcc.8b11655.
- Panich AM, Salti M, Prager O, Swissa E, Kulvelis YV, Yudina EB, Aleksenskii AE, Goren SD, Vul' AY, Shames AI. PVP-coated Gd-grafted nanodiamonds as a novel and potentially safer contrast agent for in vivo MRI. Magn Reson Med. 2021 Aug;86(2):935-942. doi: 10.1002/mrm.28762. Epub 2021 Mar 16. PMID: 33724543.
- Panich AM, Salti M, Aleksenskii AE, Kulvelis YuV, Chizhikova A, Vul’ AYa, Shames AI. Suspensions of manganese-grafted nanodiamonds: preparation, NMR and MRI study. Diamond Relat Mater. 2023;131:109591. doi: 10.1016/j.diamond.2022.109591.
- Batsanov SS, Guriev DL, Gavrilkin SM, Hamilton KA, Lindsey K, Mendis BG, Riggs HJ, Batsanov AS. On the nature of fibres grown from nanodiamond colloids. Mater Chem Phys. 2016;173:325-332. doi: 10.1016/j.matchemphys.2016.02.019.
- Kurakov AV, Batsanov AS, Gavrilkin SM, Batsanov SS. Nitrogen Fixation and Biological Behavior of Nanodiamond Colloidal Solutions. Chempluschem. 2020 Aug;85(8):1905-1911. doi: 10.1002/cplu.202000437. PMID: 32845079.
- Salman A, Tsror L, Pomerantz A, Moreh R, Mordechai S, Huleihel M. FTIR spectroscopy for detection and identification of fungal phytopathogenes. Spectroscopy. 2010;24:261-267. doi: 10.3233/SPE-2010-0448.
- Batsanov SS, Gavrilkin SM, Shatalova TB, Mendis BG, Batsanov AS. Fixation of atmospheric nitrogen by nanodiamonds. New J Chem. 2018;42:11160-11164. doi: 10.1039/C8NJ01425F.
- Panich AM, Sergeev NA, Shames AI, Osipov VY, Boudou JP, Goren SD. Size dependence of 13C nuclear spin-lattice relaxation in micro- and nanodiamonds. J Phys Condens Matter. 2015 Feb 25;27(7):072203. doi: 10.1088/0953-8984/27/7/072203. Epub 2015 Feb 3. PMID: 25646270.
- Yang CC, Mai YW. Thermodynamics at the nanoscale: a new approach to the investigation of unique physicochemical properties of nanomaterials. Mater Sci Engin R. 2014;79:1-40. doi: 10.1016/j.mser.2014.02.001.
- Qi W. Nanoscopic Thermodynamics. Acc Chem Res. 2016 Sep 20;49(9):1587-95. doi: 10.1021/acs.accounts.6b00205. Epub 2016 Jun 29. PMID: 27355129.
- Batsanov SS, Dan’kin DA. Size effect in cohesive energy of elements. Mater Chem Phys. 2017;196:245-248. doi: 10.1023/A:1020904317133.
- Shandiz MA, Safaei A, Sanjabi S, Barber ZH. Modeling the cohesive energy and melting point of nanoparticles by their average coordination number. Solid State Commun. 2008;145:432-437. doi: 10.1016/j.jpcs.2008.03.014.
- Hoffman BM, Lukoyanov D, Yang ZY, Dean DR, Seefeldt LC. Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem Rev. 2014 Apr 23;114(8):4041-62. doi: 10.1021/cr400641x. Epub 2014 Jan 27. PMID: 24467365; PMCID: PMC4012840.
- Mironova EYu, Ermilova EE, Efimov MN, Zemtsov LM, Orekhova NV, Karpacheva GP, Bondarenko GN, Zhilyaeva NA, Muraviev DN, Yaroslavtsev AB. Detonation nanodiamonds as catalysts of steam reforming of ethanol. Russ Chem Bull. 2013;62:2317-2332.
- Varley TS, Hirani M, Harrison G, Holt RB. Nanodiamond surface redox chemistry: Influence of physicochemical properties on catalytic processes. Faraday Discuss. 2014;174:349-64. doi: 10.1039/C4FD00041B.
- Führer M, Haasterecht TV, Bitter JH. Molybdenum and tungsten carbides can shine too. Catal Sci Technol. 2020;10(18):6089-6097. doi:10.1039/D0CY01420F.
- Hosono H. Spiers memorial lecture: Catalytic activation of molecular nitrogen for green ammonia synthesis: introduction and current status. Faraday Discuss. 2023;243:9-26. doi: 10.1039/D3FD00070B.
- Batsanov SS, Gavrilkin SM, Dan'kin DA, Batsanov AS, Kurakov AV, Shatalova TB, Kulikova IM. Transparent Colloids of Detonation Nanodiamond: Physical, Chemical and Biological Properties. Materials (Basel). 2023 Sep 15;16(18):6227. doi: 10.3390/ma16186227. PMID: 37763505; PMCID: PMC10532683.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.