Biology Group. 2024 April 26;5(4):360-372. doi: 10.37871/jbres1902.
The Role of the RPD3 Complex of Saccharomyces cerevisiae Yeast in the Activation of UV-Induced Expression of RNR Complex Genes
Elena Anatolievna Alekseeva*, Tatiyana Anatolievna Evstyukhina, Vyacheslav Timofeevich Peshekhonov, Irina Igorievna Skobeleva and Vladimir Gennadievich Korolev
Laboratory of Molecular Genetic and Recombination Technologies, Kurchatov Genome Center-Petersburg Nuclear Physics Institute, 188300 Orlova Roscha, Gatchina, Leningrad District, Russia
- RPD3
- Saccharomyces cerevisiae
- Yeast
- RNR complex genes
Abstract
Reparative chromatin assembly is an important step in maintaining the stability of the genome. Proper chromatin assembly is provided by histone chaperones. Violation of the function of these proteins can lead to the development of various forms of cancer and to a number of hereditary diseases in humans. One of the key processes in the reparative assembly of chromatin is acetylation and deacetylation of histones, complexes NuB4 and RPD3. Results presented in this work demonstrate that inactivation of RPD3 and Sin3 subunits of the RPD3 complex blocks super activation of RNR3 gene. The phenotypes of RPD3Δ and sin3Δ mutants differ due to different activation of Rad53 kinase in these mutants, which may be due to deacetylation of Rad53 by RPD3 deacetylase. Our findings revealed that the contribution of deacetylation of Rad53 kinase makes a significant contribution to the regulation hyperactivation RNR complex after UV irradiation. In addition, this work provides indirect evidence that the Him1 protein may participate as a histone chaperone in chromatin repair assembly.
Introduction
The chromatin assembly of a genome places restrictions on numerous cellular processes which necessitate the ability to access chromosomal DNA. Histone chaperones have key roles in chromatin dynamics, controlling the processes of its disassembly and assembly [1,2]. An important condition for genome stability is proper chromatin assembly.
The repair of DNA damage takes place within the chromatin environment, and studies have demonstrated that the removal of histones from DNA and their subsequent reassembly onto DNA are associated with DNA repair [3-5]. It has been observed that after the repair of UV-induced dimers through Nucleotide Excision Repair (NER), the newly-repaired DNA is assembled into chromatin with the assistance of histone chaperones Asf1, CAF-1, and NuB4 complex [6,7].
The process of chromatin repair assembly is closely linked to the checkpoint pathway, as the vital histone chaperone Asf1 interacts with the primary checkpoint kinase Rad53 [8]. Existing literature suggests that the Hat1p subunit of the NuB4 histone acetylase complex also interacts with Asf1 [9-12]. Within a yeast cell, Hat1p is primarily localized in the nucleus as part of the NuB4 complex, comprising Hat1p, Hat2p, and Hif1p subunits [13,14]. Moreover, Hat1p has been demonstrated to interact with other chaperones, namely Asf1 and Hsm3, in addition to Hif1p and Hat2p [7,15,16]. Thus, the structure of the NuB4 complex consists of a core, including the Hat1p and Hat2p subunits, H3 and H4 histones, and the auxiliary subunits Hif1p and Hsm3p. Hif1p binds to histone H3 and Hat2p, while Hsm3p binds to H4 and Hat2p. Earlier, we demonstrated that the genes HSM3and Hif1 participates in the control of replicative and reparative spontaneous mutagenesis, and that hsm3Δ and Hif1Δ mutants increase the frequency of mutations induced by different mutagens [16]. Hif1p plays a role in the deposition of histones onto DNA [13], thereby providing direct evidence of Hat1p's involvement in the chromatin assembly process through its association with the histone chaperone Hif1p and the NuB4 complex. Previous studies from our group have shown that the partial suppression of UV-induced expression of RNR complex genes occurs upon inactivating the accessory subunits Hsm3p and Hif1p, while a complete suppression is observed upon inactivating both subunits [7]. Simultaneously, the hat1Δ mutation leads to increased expression of RNR complex genes both prior to and following irradiation.
To complete chromatin assembly, the acetylation pattern must be removed from the N-terminal tails of histones. Histone Deacetyltransferases (HDACs) are responsible for this process, and one of these HDACs is the RPD3 complex. This complex is a class I HDAC in S. cerevisiae and exists as the RPD3L or RPD3S complex, both of which share the RPD3, Sin3, and Ume1 subunits [17,18]. These complexes may exhibit histone chaperone properties during reparative chromatin assembly [19].
RPD3L contains numerous additional subunits is targeted to promoters [20]. The RPD3S complex contains two additional subunits, Rco1 and Eaf3, which target it to H3K36 trimethylated nucleosomes in the ORF [21]. From the data of genetic analysis, it follows that RPD3 and SIN3 function in one regulatory pathway and the phenotypes of their mutations are similar.
SIN3 gene product acts as a repressor of a large number of genes in the yeast S. cerevisiae [22-24]. This large protein with a mass of about 175 kDa is a member of the RPD3 protein complexes with assessed a mass greater than 2 million Da [25]. Double mutants at the genes that code for RPD3 and Sin3 proteins do not differ from single mutants by their phenotypes thus showing epistatic interaction. It is supposed that Sin3p recruits RPD3p on specific promoters by means of interaction with certain DNA binding proteins and, thus, per forms the transcriptional regulation of the controlled genes [26]. It was previously shown that RPD3 gene mutants influence recombination and repair processes in S. cerevisiae [27-29]. The inactivation of the RPD3 complex in human and yeast cells leads to the suppression of trinucleotide repeat expansion [30], what can lead to the development of various forms of cancer and a number of hereditary diseases?
Mutations of the HIM1 gene exhibit the same phenotype as mutations of genes encoding the Hif1 and HSM3subunits of the NuB4 complex [7,31]. It is shown that the RPD3 complex, as well as the NuB4 complex, exhibits the properties of a histone chaperone and takes part in the assembly or stabilization of nucleosomes [19].
In this study, we focused on the role of the core subunits of the RPD3 complex in the regulation of the activity of the Rib Nucleotide Reductase (RNR) complex under normal cell growth conditions and after UV irradiation. We have shown that inactivation of RPD3 and Sin3 subunits of the RPD3 complex blocks super activation of RNR3 gene. The phenotypes of RPD3Δ and sin3Δ mutants differ due to different activation of Rad53 kinase, which may be due to deacetylation of Rad53 by RPD3 deacetylase. Our findings revealed that the contribution of deacetylation of Rad53 kinase makes a significant contribution to the regulation hyperactivation RNR complex after UV irradiation [32]. Based on the data obtained, we assumed that the Him1 protein is a new histone chaperone participating in the repair assembly of chromatin.
Materials and Methods
Strains
Yeast strains- Yeast cultivation and genetic manipulation were carried out by standard methods [33]. Gene deletions were obtained by PCR-mediated destruction of a genes using a specific marker. The genotypes of the yeast strains used in this study are shown in table 1.
| Table 1: Strains used in the study. | ||
| Strains | Genotype | Origin |
| wt (2D-3034) | MATα ade2∆-248 leu2-3.112 ura3-160.188 trp1∆ | [34] |
| sin3Δ | 2D-3034 sin3::kanMX | [34] |
| rad1Δ | 2D-3034 rad1::LEU2 | [34] |
| rad1Δ sin3Δ | 2D-3034 rad1::LEU2 sin3∆::kanMX | [34] |
| RPD3Δ | 2D-3034 RPD3:: kanMX | This study |
| him1Δ | 2D-3034 him1::ura4+ | [31] |
| him1Δ RPD3Δ | 2D-3034 him1::ura4+ RPD3:: kanMX | This study |
| him1Δ sin3Δ | 2D-3034 him1::ura4+ sin3:: kanMX | This study |
| hsm3Δ | 2D-3034 hsm3:: kanMX | [35] |
| hsm3Δ sin3Δ | MATa hsm3:: kanMX×2D-3034 sin3::kanMX | This study |
| hsm3Δ RPD3Δ | MATa hsm3:: kanMX×2D-3034 RPD3:: kanMX | This study |
Media
The composition of the minimum medium used as a selective one, as well as the YPD medium for growing crops and taking into account survival, are given in [33]. A special medium with the addition of 96% ethanol (15 ml/l) and 40% glucose (4 ml/l), excluding the growth of mutants with respiratory insufficiency, was used to evaluate induced mutagenesis at the ADE4–ADE8 loci [36]. To determine the frequency of spontaneous mutations of resistance to canavanin, a medium of minimal composition [33] was used, enriched with a full set of amino acids (except arginine) and nitrogenous bases necessary for the growth of the tested strains, and various concentrations of canavanin (Sigma-Aldrich, St. Louis, USA).
Sensitivity against UV irradiation
Cell survival tests were performed by growing a culture of the corresponding strain overnight in liquid YPD at 30°C. The cells were washed and resuspended in water with a density of 1 × 107 cells/ml. The cell suspension was irradiated with a UV lamp BUV-30 (UV-C range) with a dose rate of 1.4 J/m2×sec. Aliquots were selected after various doses of irradiation, diluted and placed on cups with YPD to determine the number of survivors [31].
Mutation frequency
Mutation tests were performed on Petri dishes by overnight culture of the corresponding strain in liquid YPD at 30°C. The cells were washed and resuspended in water at a density of 1 × 107 cells/ml. The cells were irradiated with a BUV-30 UV lamp. Aliquots were taken at different times, diluted, and seeded on Petri dishes with YPD to determine the number of survivors. To determine the mutation frequency, undiluted aliquots were placed on YPD medium with alcohol instead of glucose, the composition of which was described earlier [36].
Mutation rates
Spontaneous mutagenesis was determined using the standard median method (fluctuation test), which mainly registers replication errors [7,37]. After three days of incubation, 12 separate colonies were selected, each of which was suspended in 1 ml of water and seeded on a selective medium with canavanin in a concentration that excluded the possibility of growth of canavanin-sensitive cells. When estimating the number of cells sown, we diluted the suspensions and sowed them on a full medium. After incubation, the number of canavanin-resistant colonies and the total number of canavanin-sensitive colonies on a Petri dish were calculated for three to four days. The frequency of spontaneous mutations was estimated using a special formula [7,37].
In other experiments, the rate of spontaneous mutations of resistance to canavanin was estimated using the Khromov-Borisov ordered seeding method [7,38]. This method allows you to measure the rate of reparative mutagenesis. In these experiments, the tested yeast cultures were grown on cups with a full medium during the day. Then 5 ml of suspension (1×106 cells/ml) was prepared. A special 150-pin replicator was immersed in this suspension and transferred to a Petri dish with a medium containing canavanin. The replicator transferred 150 equal drops of yeast suspension (about 2 µl each) at equal distances from each other, each drop contained approximately 2000 cells. The concentration of canavanin to assess the rate of mutations of antibiotic resistance was determined in special preliminary experiments for all strains and amounted to 50 mg/l. Mutants have faster growth, which manifests itself in the form of “warts” on spots with limited growth of the culture under study. After 14-15 days of incubation, the warts of canavanin-resistant mutants and the total number of cells were counted. The latter was done after flushing cells from a number of replica drops devoid of warts. The mutation rates per cell division were determined by dividing the number of warts by the total number of cells per cup. The results are presented as the average values of 3-5 independent experiments with 95% confidence intervals.
Real-time PCR
RT-PCR CFX96 detection system (Bio-Rad, UK) was used for real-time PCR. The reactions were carried out in 25 µl volumes consisting of 10 µl of a 2.5-fold reaction mixture for RT-PCR in the presence of the dye SYBR Green I and the reference dye Rox (Syntol, Russia), 13.8 µl of water, 1 µl of cDNA and 0.1 (2 mm) corresponding primers (primers for the RNR3 gene: ForRNR3 5'-ACACCTTTCATGGTTTATAAG-3' and RevRNR3 5'-CGACGATTTCACAACATAA-3'; for the ACT1 gene: ForACT1 5'-GAAGGTCAAGATCATTGC-3' and RevACT1 5'- GTTGGAAGGTAGTCAAAG-3').
The PCR amplification conditions were as follows: 1 cycle of 5 minutes at 95°C, followed by 39 cycles of 15 seconds at 95°C and 20 seconds at 52°C. Analysis of the melting curve showed a 5-second increase of 1°C from 55 to 95°C.
Control reactions with a primer and without a matrix of reaction mixtures were included. Two biological and three technical repeats were performed for each sample. The results were processed using the CFX Manager program [31].
Statistical analysis
Experimental data are presented in the form of average standard deviations from at least three biological repeats, and statistical differences were determined using the Student's t-test. The significance was determined at the level of p < 0.05 [31].
Results
RPD3 and NuB4 complex
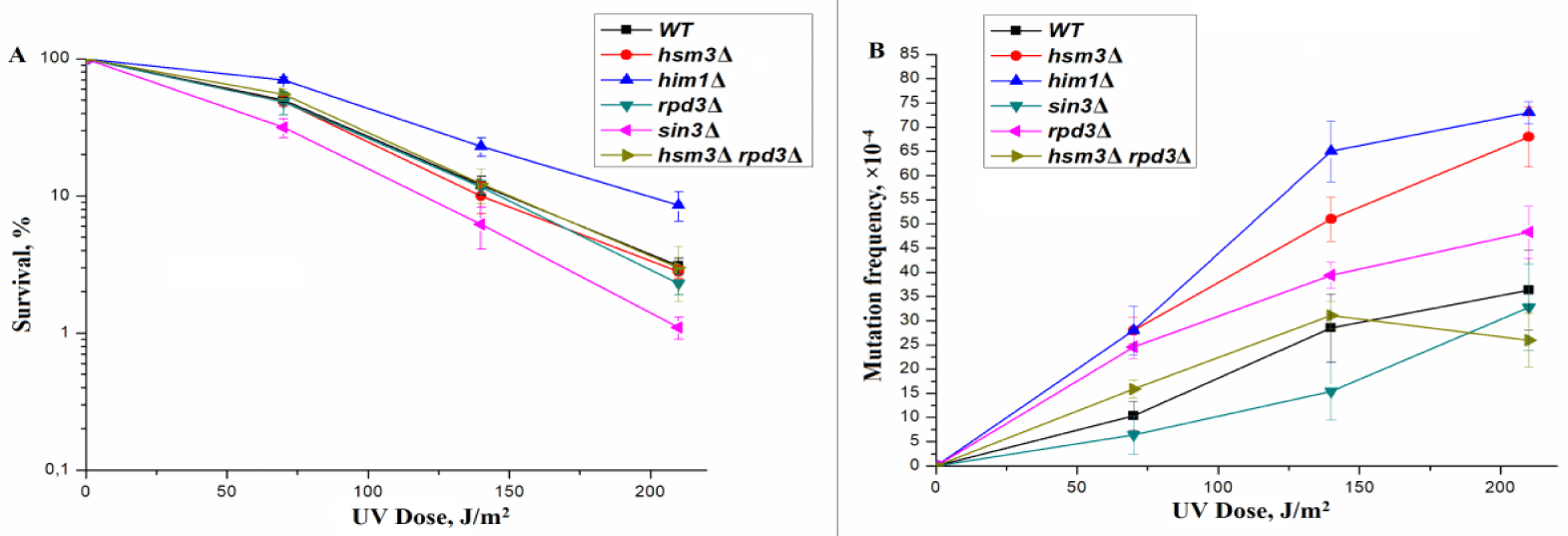
In the process of reparative assembly of chromatin, the RPD3 complex must be connected after the NuB4 complex; therefore, epistatic interaction can be observed between the genes encoding the subunits of these complexes. In this regard, we conducted a comparative analysis of the effect of mutations in the genes encoding the core subunits of the RPD3 and NuB4 complexes on UV-induced mutagenesis and expression of the RNR complex genes. RPD3p is the catalytic subunit of RPD3 complex. Therefore, we first tested for the genetic properties of RPD3Δ mutant. We defined the UV sensitivity of wild-type strain and RPD3Δ single mutant. As can be seen from figure 1A, a single mutant showed a sensitivity to UV comparable to the sensitivity of a wild-type strain. The frequency of direct mutations, in the loci of ADE4-ADE8, induced by UV rays in a wild-type strain and a single RPD3Δ mutant was measured. The data presented in figure 1B suggests that RPD3Δ mutation increases the frequency of UV-induced mutagenesis compared to the wild type strain.
Thus, there is a parallel in the sensitivity of mutants in genes controlling subunits of the RPD3 and NuB4 complexes to the mutagenic effect of UV light. Based on these data, we hypothesized that between the genes that control the subunits of both complexes, there may be a genetic interaction. To test this hypothesis, we deleted HSM3gene from RPD3Δ mutant and the wild-type strain. The UV resistance of the double mutant RPD3Δ hsm3Δ practically did not differ from the single mutant RPD3Δ (Figure 1A). A double mutant exhibits the same mutagenesis as the wild-type strain. Consequently, RPD3Δ is epistatic with respect to hsm3Δ, and induced hsm3Δ mutagenesis depends on RPD3.
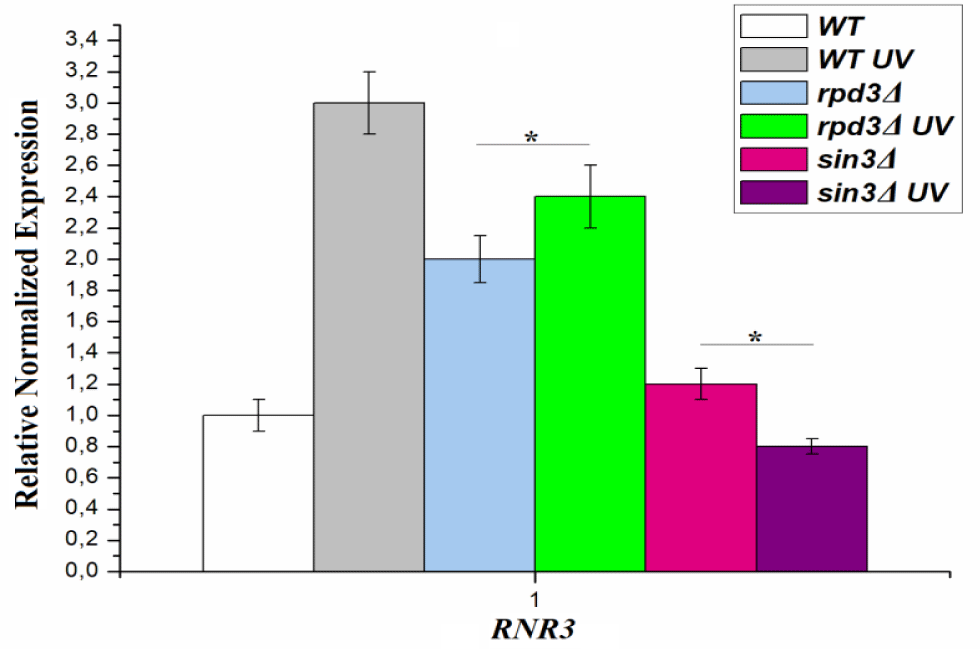
We have previously shown that hsm3Δ-dependent mutagenesis is regulated by the level of dNTPs [7]. Acetylation plays an important role in checkpoint activation. It has been shown that Rad53 is a target of RPD3 in the regulation of adaptation and that deacetylation of Rad53 by RPD3 reduces its kinase activity [32]. To check which level of RPD3 expression corresponds to RPD3Δ-dependent mutagenesis, we examined UV-irradiated RPD3Δ cells for increased RNR3 expression. We irradiated with UV light the wild-type and RPD3Δ mutant cells and, after 4 hours, measured the mRNA RNR3 gene levels in the irradiated cells. As can be seen from figure 2, RNR3 gene mRNA level in wild-type cells increased almost 3 times, while in the RPD3∆ mutant, the expression level of RNR3 gene was almost two-fold increased without irradiation compared with this indicator for the wild type strain and increased by another 25% after irradiation. Thus, when grown under normal conditions, RPD3Δ mutant shows an increased level of expression of the RNR3 gene. After UV irradiation, the expression level of RPD3 further increases slightly. Increased dNTPs levels lead to an increased rate of replicative mutagenesis. We confirmed this statement in recent work using sml1Δ mutant that increases the level of dNTP several fold.
We measured the rate of spontaneous replicative mutagenesis (Fluctuation test) in RPD3Δ mutant. As can be seen from table 2, the rate of spontaneous replicative mutagenesis in RPD3Δ mutant is two times higher than in wild-type cells. This result is consistent with the statement made above.
| Table 2: Spontaneous mutagenesis of resistance to canavanine. | ||
| Strains | Ordered seeding, ×10−7 (Spontaneous reparative mutagenesis) | Fluctuation test, ×10−7 (Spontaneous replicative mutagenesis) |
| wt | 3.6 ± 0.8 | 3.4 ± 0.5 |
| sin3Δ | 1.1 ± 0.4 | 1.6 ± 0.2 |
| rad1Δ | 26.7 ± 3.5 | 13.4 ± 2.0 |
| sin3Δ rad1Δ | 2.9 ± 0.8 | 2.3 ± 1.1 |
| hsm3Δ | 15.8 ± 0.5 | 5.7 ± 0.3 |
| sin3Δ hsm3Δ | 9.3 ± 1.3 | 1.3 ± 0.4 |
| him1Δ | 12.8 ± 1.4 | 7.0 ± 1.3 |
| sin3Δ him1Δ | 9.6 ± 1.1 | 11.6 ± 1.1 |
| RPD3Δ | 7.9 ± 2.2 | 9.5 ± 2.6 |
| RPD3Δ him1Δ | 4.1 ± 1.7 | 2.8 ± 0.8 |
Previously, we showed that a decrease in the level of UV-induced RNR3 expression stimulates hsm3Δ-specific mutagenesis [7]. As can be seen in figure 1B, the frequency of UV-induced mutagenesis in the RPD3Δ mutant is lower than that of the hsm3Δ mutant, which is explained by a higher level of RNR3 expression in the RPD3Δ mutant (Figure 2), approaches the level of the wild-type strain. This is especially noticeable at high UV-doses.
Mutations arising both as replication errors and errors in repair are registered by the method of ordered plating, when cells are grown for long periods on selective medium and many spontaneous lesions accumulate in DNA during one generation [39]. If cells have a defect in the system of DNA damage repair, this might significantly increase the level of spontaneous mutagenesis. The rate of spontaneous mutagenesis measured by both tests in RPD3Δ mutant was the same (Table 2). This result means that the rate of reparative spontaneous mutagenesis in the RPD3Δ mutant is not altered or suppressed.
We studied the epistatic interaction of RPD3Δ mutation with him1Δ mutation. It can be seen from table 2 that the presence of him1Δ mutation in RPD3Δ cells leads to suppression of both types of spontaneous mutagenesis to the level of the wild-type strain. Here we observe an additive suppression of the expression level of the RNR3 gene, which leads to the disappearance of RPD3Δ- and him1Δ -specific mutagenesis.
Sin3 and NuB4 complex
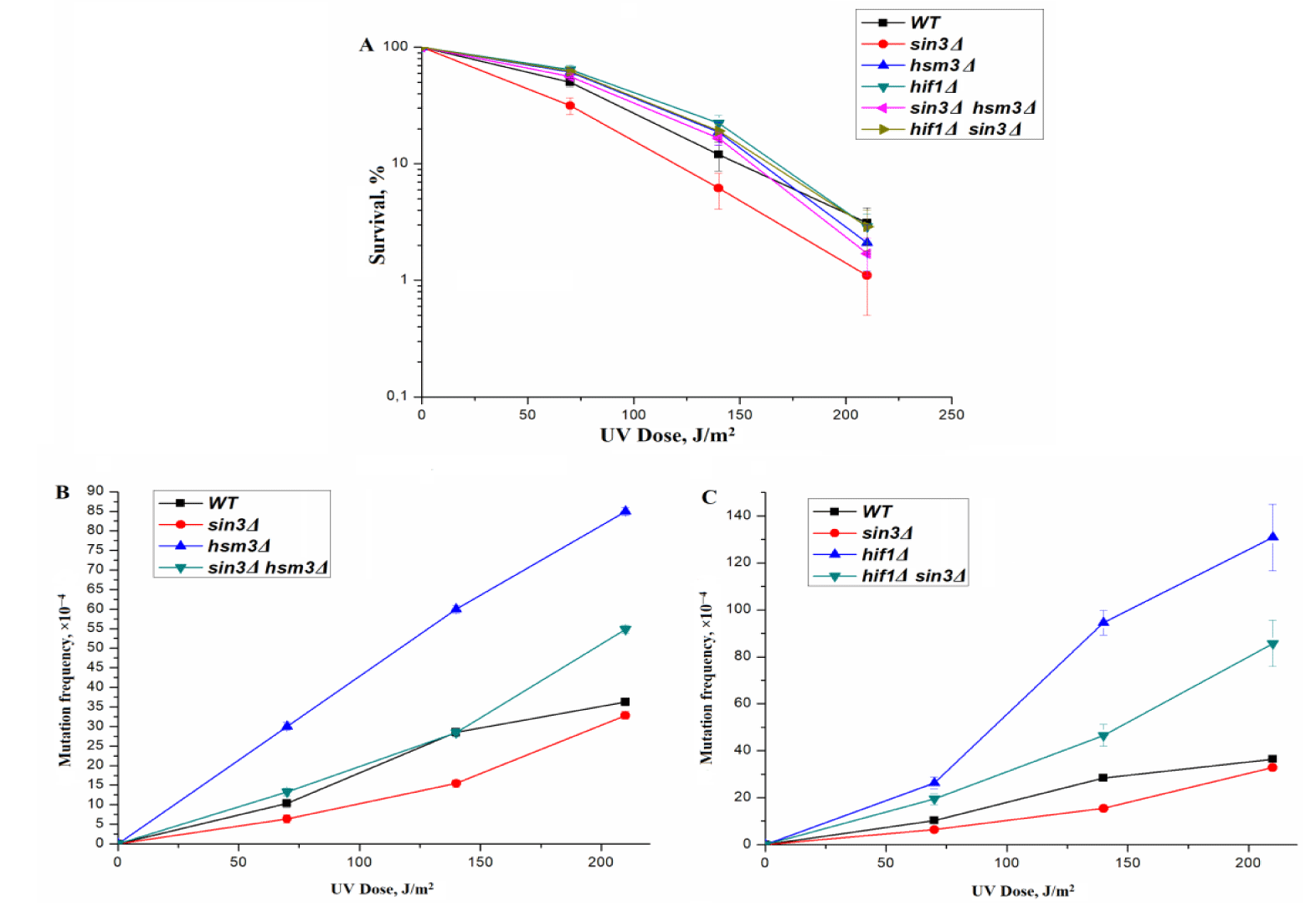
Sin3 subunit, together with Ume1 and RPD3 subunits, form a platform for the assembly of both RPD3 complexes; therefore, the properties of mutations in the genes encoding these subunits largely coincide. A sin3Δ mutation was previously identified during the quantitative assessment of genes that involved a UV-induced DNA damage response, the deletion of which renders the yeast sensitive to UV light treatment [34,40]. Early we studied the impact of the sin3Δ mutation to UV-induced mutagenesis in budding yeast cells [34]. The deletion of the SIN3 gene causes UV sensitivity of mutant cells and reduced UV-induced mutagenesis as compared to the wild type strain (Figure 3).
The rate of spontaneous mutations of canavanine resistance measured by the use of fluctuation test and method of ordered plating for the sin3Δ mutant was lower compared to the wild type strain (Table 2). At the same time, the level of spontaneous mutagenesis, measured by the method of ordered seeding, decreased to a greater extent compared to the result of measurement by the fluctuation test. As in the case of the RPD3Δ mutant, the rate of spontaneous mutagenesis in sin3Δ mutant measured by both tests is practically the same, this indicates that the repair part of spontaneous mutagenesis in both mutants is suppressed and a branch of the mutation process is functioning that reveals replication errors on an intact template. We observed the same nature of the interaction of mutations in the double him1Δ sin3Δ mutant. To confirm this result, we measured the rates of both types of spontaneous mutagenesis in sin3Δ rad1Δ double mutant. In the rad1Δ mutant, nucleotide excision repair is blocked and during growth, under normal conditions and during slow growth, damage accumulates in the DNA of the mutant, which is a substrate for NER. In rad1Δ sin3Δ mutant, the rate of spontaneous mutagenesis was no different from the wild-type strain. Thus, we can conclude that the RPD3Δ and sin3Δ mutations suppress the repair branch of spontaneous mutagenesis.
As in the case of RPD3Δ mutant, we studied the UV-induced RNR3 expression in sin3Δ mutant (Figure 2). In experiments without UV irradiation, the level of RNR3 expression in sin3Δ strain did not differ from that of the wild type strain. However, after UV irradiation, the RNR3 expression level in this mutant was reduced to a level below that of the wild-type strain without UV irradiation. Summarizing the data obtained, we can conclude that the absence of histone deacetylation after repair chromatin assembly suppresses DNA damage induced induction of expression genes of the RNR complex.
We studied the epistatic interaction of sin3Δ mutation with mutation in gene, coding for subunit of the NuB4 complex. It can be seen from table 2 that the presence of hsm3Δ mutation in cells leads to a weakly change in the rate of replicative spontaneous mutations compared to wild-type cells. In the Ordered seeding test, hsm3Δ mutation led to a sharp increase in the rate of spontaneous mutagenesis. In the double mutant, it can be seen that the sin3Δ mutation significantly suppresses the high level of spontaneous mutagenesis characteristic of the hsm3Δ mutant in both tests. However, in this case, the level of suppression of reparative mutagenesis was less than in the double mutants described above. We observed the same type of interaction between sin3Δ mutation and mutations in the HSM3and Hif1 genes, encoding the subunits of the NuB4 complex, in UV-induced mutagenesis (Figure 3). In these experiments, the sin3Δ mutation only partially suppressed hsm3Δ - and Hif1Δ-specific UV-induced mutagenesis.
Him1 and RPD3 complex
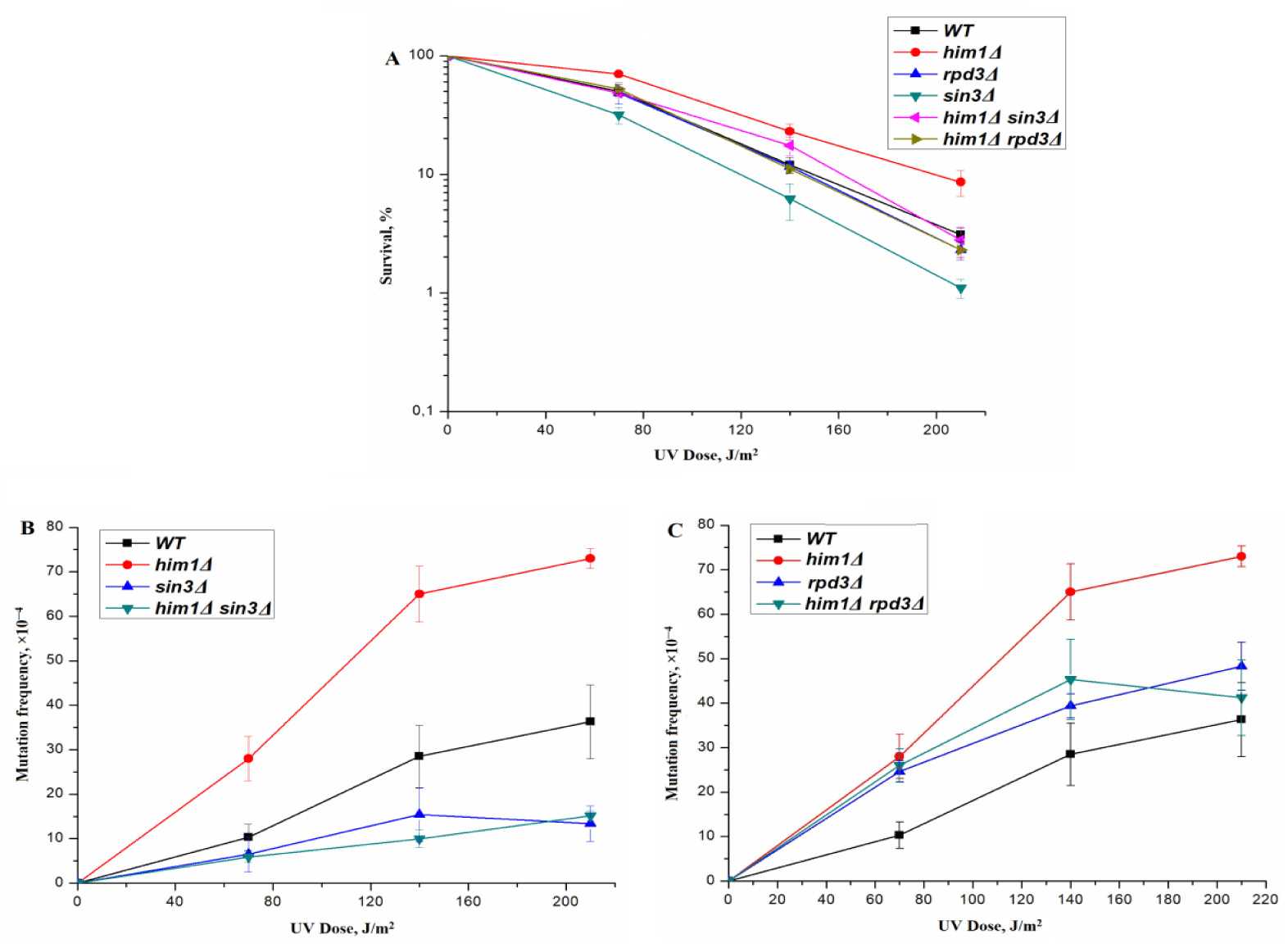
The him1Δ mutant phenotype is similar to the hsm3Δ phenotype [31]. The interaction between him1Δ and hsm3Δ mutations is epistatic in nature [41,42]. Based on these data, we examined potential epistatic relationships between mutations of SIN3 and HIM1 genes. The sin3Δ mutation suppresses the him1Δ-dependent UV resistance of the him1Δ strain to the level of the wild-type strain (Figure 4A). At the same time, as can be seen from figure 4B, sin3Δ mutation completely blocks him1Δ-dependent UV-induced mutagenesis.
Next, we deleted RPD3 gene from him1Δ and hsm3Δ mutants. UV-induced mutagenesis in RPD3Δ him1Δ (Figure 4С) and RPD3Δ hsm3Δ (Figure 1В) strains is close to the level of mutagenesis in single RPD3Δ strain (Figures 1В, 4С). Thus, the RPD3Δ mutation, like the sin3Δ mutation, epistates hsm3Δ and him1Δ mutations. Taken together, these results suggest that him1Δ- like hsm3Δ-dependent UV-induced mutagenesis is completely controlled by genes encoding the core subunits of the RPD3 complex.
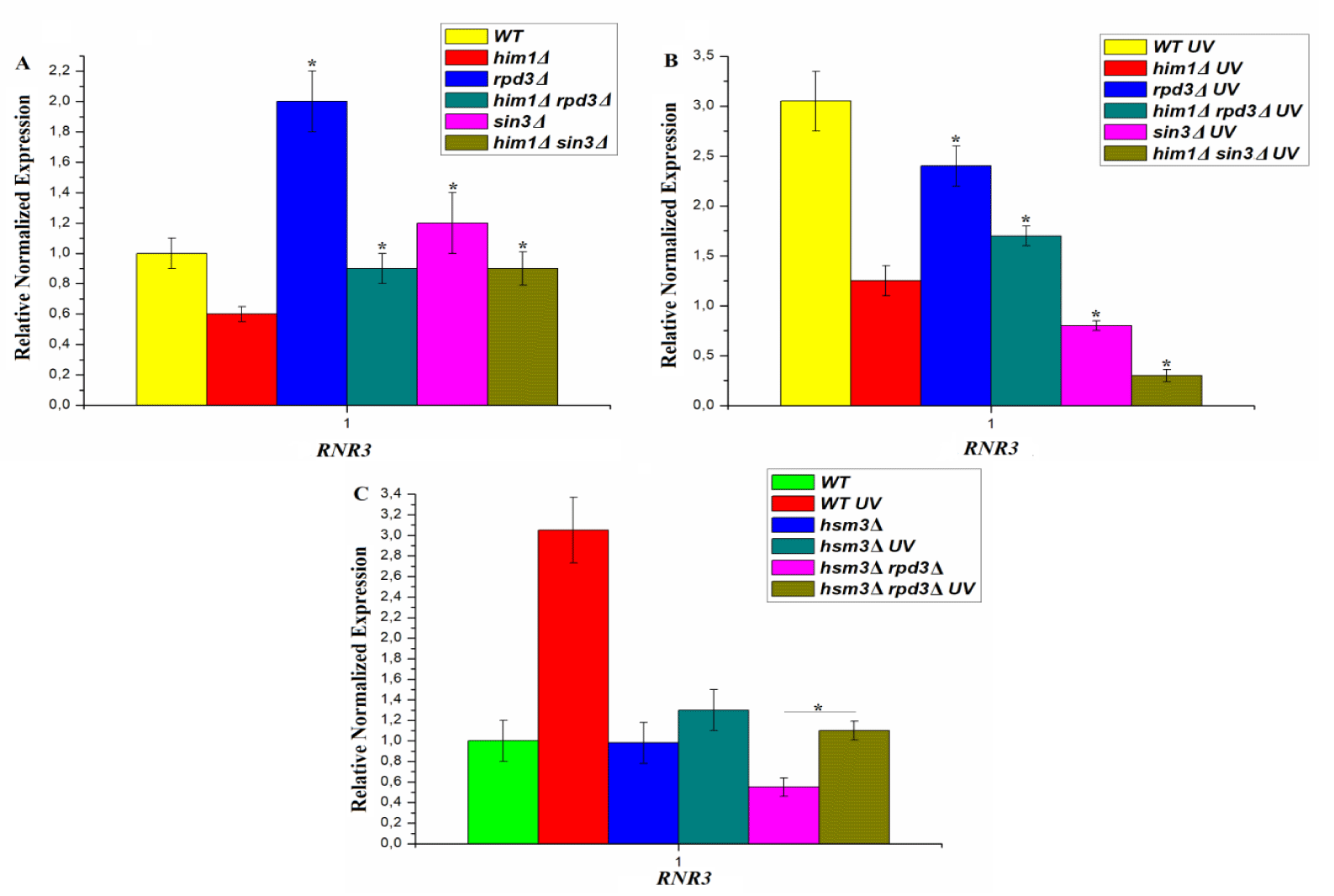
The mutator phenotype of HIM1 gene deletion is associated with a decrease in the rate of DNA synthesis during error-free bypass of DNA damage during post-replicative repair [31]. The rate of DNA synthesis is influenced by the level of dNTPs in the cell. The expression of the RNR complex genes responsible for the level of dNTPs in the cell is controlled by a checkpoint, namely the checkpoint kinases Mec1/Ddc2, Rad53, Dun1. We have shown that the him1Δ and hsm3Δ mutations leads to suppression of the expression of the RNR3 gene, both before and after UV- irradiation [7,31]. Single mutants in the genes encoding subunits of the RPD3 complex also suppress the expression of the RNR3 gene after UV irradiation (Figures 5A,B). However, under normal growth conditions, the expression of the RNR3 gene in sin3Δ tended to increase, while in RPD3Δ strain it is significantly higher. We studied the effect of combinations of mutations in genes, encoding subunits of the RPD3 complex, with him1Δ and hsm3Δ mutations (Figure 5).
Him1Δ mutation leads to suppression of RNR3 gene expression in him1Δ sin3Δ mutant both before and after UV-irradiation. This result is consistent with data on UV-induced mutagenesis in this strain. The expression level of RNR3 in him1Δ sin3Δ mutant is several times lower than in the wild-type strain, which is the reason for the sharp suppression of him1Δ - specific mutagenesis. Him1Δ mutation also leads to suppression of RNR3 gene expression in double him1Δ RPD3Δ mutant both before and after UV-irradiation. Under normal growth conditions, expression of the RNR3 gene in the double him1Δ RPD3Δ mutant dropped to the level of the wild type strain. However, after UV irradiation, the expression level of RNR3 in this strain decreased to an intermediate level, which maintained UV-specific mutagenesis (Figure 4С). Thus, we observe an additive interaction between him1Δ and sin3Δ and RPD3Δ mutations. Formally, this means that the HIM1 and SIN3 genes control parallel pathways process of UV-induced mutagenesis.
Discussions
In contrast to replicative chromatin assembly, the process of repair assembly is less fully understood. This is due to the fact that not all participants have yet been identified and not all connections between the stages of this process have been established. In this work, we made an attempt to fill some of these gaps, which will help to reconstruct a complete version of the process of repair chromatin assembly in yeast S. cerevisiae.
The first step of the assembly process is the assembly of the histone core of nucleosomes and its delivery to DNA. At this stage, the histone acetylase complex NuB4 plays a key role. We previously showed that this complex includes a novel subunit Hsm3p [7]. Earlier, we demonstrated that the genes encoding two auxiliary subunits of this complex HSM3and Hif1 participates in the control of replicative and reparative spontaneous mutagenesis, and that hsm3Δ and Hif1Δ mutants increase the frequency of mutations induced by different mutagens [16]. A direct physical association between Asf1p and the NuB4 complex was reported suggesting the possibility that the NuB4 complex may directly transfer newly synthesized histones to Asf1p [43], which acts as a carrier of the NuB4 complex to DNA. Measurement of the rate of spontaneous reparative mutagenesis showed a 25-fold increase in asf1Δ mutant compared to the wild type strain [44]. These results indicate that the process of nucleosome assembly influences the mutation process in yeast cells. Significant increase and suppression in the dNTP levels suppress him1Δ-, hsm3Δ- and Hif1Δ-dependent mutagenesis [7,31]. Our findings show that Polη responsible for him1Δ-, hsm3Δ- and Hif1Δ-dependent mutagenesis. A mechanism has been proposed by which, in the process of recombination bypass of DNA damage, due to a decrease in the concentration of dNTPs caused by a decrease in the level of expression of the RNR complex, premature termination of the D-loop and incomplete bridging of the single-strand gap occurs. The resulting single-stranded region is filled with highly erroneous Polη [31].
Phosphorylated Rad9 interacts with the COOH-terminal fork head homology-associated domain of Rad53. Inactivation of this domain abolished DNA damage-dependent Rad53 phosphorylation, G2/M cell cycle phase arrest, and increase of RNR3 transcription [45]. We changed the C-terminal sequence of the Rad53 protein by adding the 6-His (6 histidines) and HA-F (influenza virus epitope hemagglutinin) sequences to the reading frame. Rad53 + HA-F mutation does not increase expression of the RNR3 compared to wild-type cells under normal growth conditions. After high dose UV irradiation (252 J/m2), the expression of the RNR3 gene increased by three times, while in the cells of Rad53 + HA-F mutant, the level of expression of the RNR3 gene did not change. Rad53 + HA-F mutation epistatized to the hsm3Δ and asf1Δ mutations, reducing the level of UV-induced and spontaneous mutagenesis to the level of a wild type strain that is identical to the level of single mutant Rad53+HA-F. Thus, mutations on the C-terminal domain of the Rad53 gene suppress hsm3Δ- and asf1Δ-specific mutagenesis [44]. Taken together, these results indicate that the increased mutagenesis in our mutants depends on the degree of activation of the RNR complex, which depends on the degree of activation of Rad53 kinase.
In this study, we report that there is a genetic interaction between the genes encoding the subunits of the NuB4 and RPD3 complexes. Mutations in genes encoding these complexes have a comparative effect on the mutation process in yeast. Wherein RPD3Δ and sin3Δ are epistatic with respect to hsm3Δ, and induced hsm3Δ-specific mutagenesis depends on RPD3 and Sin3.
Two HDACs, RPD3 and Hos2, are required for the activation of DNA damage-inducible RNR3 gene [46]. Deletions of both genes have virtually no effect on the level of expression of the RNR3 gene. However, the combination of these mutations in one cell leads to an intermediate decrease in the RNR3 expression level. Thus, a single RPD3Δ mutation has no effect on the transcription of the RNR3 gene. At the same time, RPD3Δ mutation blocks the deacetylation of Rad53 kinase and thereby delays its deactivation [32]. Since on the one hand, the RPD3 deletion is involved in transcription activation RNR3, on the other hand, this deletion suppresses the transcription of the RNR3 gene, thereby reducing the activity of the Rnr3 protein. We compared the total expression level of the RNR3 gene in the RPD3Δ mutant and the wild type strain after UV irradiation. Total expression level of the RNR3 gene after UV irradiation in the RPD3Δ mutant was 25% lower than in the wild type strain. For sin3Δ mutant this difference was more than 3-fold. These results confirm in vitro data on the role of RPD3 deacetylase in the deactivation of Rad53 kinase [32].
RPD3 and Sin3 proteins constitute the core part of the RPD3 complex, so inactivation of each protein leads to loss of function of the entire complex. The difference in the effects of sin3Δ and RPD3Δ mutants demonstrated above shown for the first time that the ability of RPD3 to function independently of RPD3 complex. It is surprising that the contribution of deacetylation of Rad53 kinase makes a significant contribution to the regulation of kinase hyperactivation.
Loss of the RPD3 complex, as a result of deletion of the SIN3 gene, likely leads to destabilization of the nucleosome as a result of a decrease in the efficiency of its assembly process and lack of effective histone deacetylation. As in the case of loss of both auxiliary subunits of the NuB4 complex, these events lead to complete suppression of hyperactivation of the RNR complex due to the absence of Rad53 hyperactivity. Deletion of the RPD3 gene also leads to the loss of the RPD3 complex, wherein, the Rad53 kinase will not be quickly deacytylated. As a result, we observe intermediate hyperactivation of the RNR complex and initiation RPD3Δ-specific mutagenesis (Figure 5). Thus, we see parallelism in the effects of mutations in genes that control NuB4 and RPD3 complexes on the mutation process.
Analysis of DNA sequences in the Gene Bank revealed ORF YDR317w with unknown functions, which is situated in the chromosome region where the HIM1 gene was mapped [42]. The phenotype of the him1Δ mutant is very similar to that of hsm3Δ mutant. Genetic analysis showed that him1Δ-dependent UV-induced mutagenesis is completely controlled by genes encoding the core subunits of the RPD3 complex. Under normal growth conditions, him1Δ mutation suppresses RNR3 gene expression in sin3Δ and RPD3Δ mutants to the level of the wild-type strain (Figure 5). After UV irradiation, the level of gene expression in double mutants decreased below the level of any single mutant. Thus, we observe an additive interaction of him1Δ mutation with the RPD3Δ and sin3Δ mutations. Formally, this means that HIM1 gene and genes of the RPD3 complex control parallel pathways, perhaps these are pathways for repair chromatin assembly.
Acknowledgement
The study was carried out with the financial support of the Russian Science Foundation grant Number 23-24-00119.
References
- De Koning L, Corpet A, Haber J.E, Almouzni G. Histone chaperones: an escort network regulating histone traffic. Nat Struct Mol Biol. 2007 Nov;14(11):997-1007. doi: 10.1038/nsmb1318. Epub 2007 Nov 5. PMID: 17984962
- Burgess R.J, Zhang Z, Histone chaperones in nucleosome assembly and human disease. Nat. Nat Struct Mol Biol. 2013 Jan;20(1):14-22. doi: 10.1038/nsmb.2461. PMID: 23288364 PMCID: PMC4004355
- Chen C.C, Carson J.J, Feser J, Tamburini B, Zabaronick S, Linger J, Tyler J.K. Acetylated lysine 56 on histone H3 drives chromatin assembly after repair and signals for the completion of repair. Cell. 2008 Jul 25;134(2):231-43. doi: 10.1016/j.cell.2008.06.035. PMID: 18662539 PMCID: PMC2610811
- Osley M.A, Shen X. Altering nucleosomes during DNA double-strand break repair in yeast. Trends Genet. 2006 Dec;22(12):671-7. doi: 10.1016/j.tig.2006.09.007. Epub 2006 Sep 25. PMID: 16997415
- Tsukuda T, Fleming A.B, Nickoloff J.A, Osley M.A. Chromatin remodelling at a DNA double-strand break site in Saccharomyces cerevisiae. Nature. 2005 Nov 17;438(7066):379-83. doi: 10.1038/nature04148. PMID: 16292314 PMCID: PMC1388271
- Gaillard P.H, Martini E.M, Kaufman P.D, Stillman B, Moustacchi E, Almouzni G. Chromatin assembly coupled to DNA repair: a new role for chromatin assembly factor 1. Cell. 1996 Sep 20;86(6):887-96. doi: 10.1016/s0092-8674(00)80164-6. PMID: 8808624
- Evstyukhina T.A, Alekseeva E.A, Fedorov D.V, Peshekhonov V.T, Korolev V.G. Genetic analysis of the Hsm3 protein function in yeast Saccharomyces cerevisiae NuB4 complex. Genes (Basel). 2021 Jul 17;12(7):1083. doi: 10.3390/genes12071083. PMID: 34356099 PMCID: PMC8307810
- Hu F, Alcasabas A.A, Elledge S.J. Asf1 links Rad53 to control of chromatin assembly. Genes Dev. 2001 May 1;15(9):1061-6. doi: 10.1101/gad.873201. PMID: 11331602 PMCID: PMC312686
- Jasencakova Z, Scharf A.N, Corpet A, Imhof A, Almouzni G, Groth A. Replication stress interferes with histone recycling and predeposition marking of new histones. Mol Cell. 2010 Mar 12;37(5):736-43. doi: 10.1016/j.molcel.2010.01.033. PMID: 20227376
- Das C, Tyler J.K, Churchill M.E. The histone shuffle: Histone chaperones in an energetic dance. Trends Biochem Sci. 2010 Sep;35(9):476-89. doi: 10.1016/j.tibs.2010.04.001. Epub 2010 May 3. PMID: 20444609 PMCID: PMC4004086
- Groth A, Corpet A, Cook AJ, Roche D, Bartek J, Lukas J, Almouzni G. Regulation of replication fork progression through histone supply and demand. Science. 2007;318(5858):1928-31. doi: 10.1126/science.1148992.
- Groth A, Ray-Gallet D, Quivy J.P, Lukas J, Bartek J, Almouzni G. Human Asf1 regulates the flow of S phase histones during replicational stress. Mol Cell. 2005 Jan 21;17(2):301-11. doi: 10.1016/j.molcel.2004.12.018. PMID: 15664198
- Ai X, Parthun M.R. The nuclear Hat1p/Hat2p complex: A molecular link between type B histone acetyltransferases and chromatin assembly. Mol Cell. 2004 Apr 23;14(2):195-205. doi: 10.1016/s1097-2765(04)00184-4. PMID: 15099519
- Poveda A, Pamblanco M, Tafrov S, Tordera V, Sternglanz R., Sendra R. Hif1 is a component of yeast histone acetyltransferase B, a complex mainly localized in the nucleus. J Biol Chem. 2004 Apr 16;279(16):16033-43. doi: 10.1074/jbc.M314228200. Epub 2004 Feb 3. PMID: 14761951
- Qin S, Parthum M.R. Histone H3 and the histone acetyltransferase Hat1 contribute to DNA double-srand break repair. Mol Cell Biol. 2002 Dec;22(23):8353-65. doi: 10.1128/MCB.22.23.8353-8365.2002. PMID: 12417736 PMCID: PMC134061
- Campos E.I, Fillingham J, Li G, Zheng H, Voigt P, Kuo W.H, Seepany H, Gao Z, Day L.A, Greenblatt J.F, Reinberg D. The program for processing newly synthesized histones H3.1 and H4. Nat Struct Mol Biol. 2010 Nov;17(11):1343-51. doi: 10.1038/nsmb.1911. Epub 2010 Oct 17. PMID: 20953179 PMCID: PMC2988979
- Carrozza M.J, Li B, Florens L, Suganuma T, Swanson S.K, Lee K.K, Shia W.J, Anderson S, Yates J, Washburn M.P, Workman J.L. Histone H3 methylation by Set2 directs deacetylation of coding regions by RPD3S to suppress spurious intragenic transcription. Cell. 2005 Nov 18;123(4):581-92. doi: 10.1016/j.cell.2005.10.023. PMID: 16286007
- Keogh M.C, Kurdistani S.K, Morris S.A, Ahn S.H, Podolny V, Collins S.R, Schuldiner M, Chin K, Punna T, Thjmpson N.J, Boone C, Emili A, Weissman J.S, Hughes T.R, Strahl B.D, Grunstein M, Greenblatt J.F, Buratowski S, Krogan N.J. Cotranscriptional set2 methylation of histone H3 lysine 36 recruits a repressive RPD3 complex. Cell. 2005 Nov 18;123(4):593-605. doi: 10.1016/j.cell.2005.10.025. PMID: 16286008
- Chen X-F, Kuryan B, Kitada T, Tran N, Li J-Y, Kurdistani S, Grunstein M, Li B, Carey M. The RPD3 core complex is a chromatin stabilization module. Curr Biol. 2012 Jan 10;22(1):56-63. doi: 10.1016/j.cub.2011.11.042. Epub 2011 Dec 15. PMID: 22177115 PMCID: PMC3758238
- Florens L, Carrozza M.J, Swanson S.K, Fournier M, Coleman M.K, Workman J.L, Washburn M.P. Analyzing chromatin remodeling complexes using shotgun proteomics and normalized spectral abundance factors. Methods. 2006 Dec;40(4):303-11. doi: 10.1016/j.ymeth.2006.07.028. PMID: 17101441 PMCID: PMC1815300
- Li B, Carey M, Workman J.L. The role of chromatin during transcription. Cell. 2007 Feb 23;128(4):707-19. doi: 10.1016/j.cell.2007.01.015. PMID: 17320508
- Bowdish K.S, Mitchell A. Bipartite structure of an early meiotic upstream activation sequence from Saccharomyces cerevisiae. Mol Cell Biol. 1993 Apr;13(4):2172-81. doi: 10.1128/mcb.13.4.2172-2181.1993. PMID: 8455605 PMCID: PMC359538
- Vidal M, Strich R.E, Gaber R.F. RPD1 (SIN3/UME4) is required for maximal activation and repression of diverse yeast genes. Mol Cell Biol. 1991 Dec;11(12):6306-16. doi: 10.1128/mcb.11.12.6306-6316.1991. PMID: 1944290 PMCID: PMC361824
- Yoshimoto H, Yamashita I. The Saccharomyces cerevisiae GAM2/SIN3 protein plays a role in both activation and repression of transcription. Mol Gen Genet. 1992 May;233(1-2):327-30. doi: 10.1007/BF00587597. PMID: 1603074
- Kasten M.M, Dorland S, Stillman D.J. A large protein complex containing the yeast Sin3p and RPD3p transcriptional regulators. Mol Cell Biol. 1997 Aug;17(8):4852-8. doi: 10.1128/MCB.17.8.4852. PMID: 9234741 PMCID: PMC232337
- Wolffe A.P. Histone acetylase: a regulator of transcription. Science. 1996 Apr 19;272(5260):371-2. doi: 10.1126/science.272.5260.371. PMID: 8602525
- Esposito M.S, Brown J.T. Conditional hypo-recombination mutants of three REC genes of Saccharomyces cerevisiae. Curr Genet. 1990 Jan;17(1):7-12. doi: 10.1007/BF00313242. PMID: 2178786
- Dora E.G, Rudin N, Martell J.R, Esposito M.S, Ramírez R.M. RPD3 (REC3) mutations affect mitotic recombination in Saccharomyces cerevisiae. Curr Genet. 1999 Mar;35(2):68-76. doi: 10.1007/s002940050434. PMID: 10079324
- Scott K.L, Plon S.E. Loss of Sin3/RPD3 histone deacetylase restores the DNA damage response in checkpoint-deficient strains of Saccharomyces cerevisiae. Mol Cell Biol. 2003 Jul;23(13):4522-31. doi: 10.1128/MCB.23.13.4522-4531.2003. PMID: 12808094 PMCID: PMC164854
- Debacker K, Frizzell A, Gleeson O, Kirkham-McCarthy L, Mertz T, Lahue R.S. Histone deacetylase complexes promote trinucleotide repeat expansions. PLoS Biol. 2012 Feb;10(2):e1001257. doi: 10.1371/journal.pbio.1001257. Epub 2012 Feb 21. PMID: 22363205 PMCID: PMC3283555
- Alekseeva E.A, Evstyukhina T.A, Peshekhonov V.T, Korolev V.G. Participation of the HIM1 gene of yeast Saccharomyces cerevisiae in the error-free branch of post-replicative repair and role Polη in him1-dependent mutagenesis. Curr Genet. 2021 Feb;67(1):141-151. doi: 10.1007/s00294-020-01115-6. Epub 2020 Oct 31. PMID: 33128582 PMCID: PMC7886746
- Tao R, Xue H, Zhang J, Liu J, Deng H, Chen Y-G. Deacetylase RPD3 facilitates checkpoint adaption by preventing Rad53 overactivation, Mol Cell Biol. 2013 Nov;33(21):4212-24. doi: 10.1128/MCB.00618-13. Epub 2013 Aug 26. PMID: 23979600 PMCID: PMC3811893
- Zakharov I.A, Kozhin S.A, Kozhina T.A, Fedorova I.V. Sbornik metodik po genetike drozhzhei-sakharomitsetov, Methods of yeast Saccharomyces cerevisiae genetics. Leningrad: Nauka. 1984. p144.
- Lebovka I.Yu, Kozhina T.N, Fedorova I.V, Peshekhonov V.T, Evstyukhina T.A, Chernenkov A.Yu, V.G. Korolev V.G. Sin3 histone deacetylase controls level of spontaneous and UV induced mutagenesis in yeast Saccharomyces cerevisiae. Genetika. 2014 Jan;50(1):5-11. PMID: 25711007
- Chernenkov A.Y, Gracheva L.M, Evstyukhina T.A, Koval’tsova S.V, Peshekhonov V.T, Fedorova I.V, Korolev V.G. HSM3 gene interaction with epistatic RAD6 group genes in yeasts Saccharomyces cerevisiae. Genetika. 2012 Feb;48(2):160-7. PMID: 22567994
- Kovaltzova S.V, Korolev V.G. The Saccharomyces cerevisiae yeast strain for testing environmental mutagens based on the interaction between rad2 and him1 mutations. Genetika. 1996 Mar;32(3):366-72. PMID: 8723629
- Lea D.E, Coulson C.A. The distribution of the numbers of mutants in bacterial populations. J Genet. 1949 Dec;49(3):264-85. doi: 10.1007/BF02986080. PMID: 24536673
- Khromov-Borisov NN, Saffi J, Henriques JAP. Perfect order plating: Principal and applications. TTO. 2002;1TO2638.
- Fedorova I.V, Kovaltzova S.V, Gracheva L.M, Evstyuhina T.A, Korolev V.G. Requirement of HSM3 gene for spontaneous mutagenesis in Saccharomyces cerevisiae. Mutat Res. 2004 Oct 4;554(1-2):67-75. doi: 10.1016/j.mrfmmm.2004.03.003. PMID: 15450405
- Hanway D, Ghin J.K, Xia G, Oshiro G, Winzeler E.A, Romesberg F.E. Previously uncharacterized genes in the UV- and MMS-induced DNA damage response in yeast. Proc Natl Acad Sci U S A. 2002 Aug 6;99(16):10605-10. doi: 10.1073/pnas.152264899. Epub 2002 Jul 29. PMID: 12149442 PMCID: PMC124988
- Fedorova I.V, Gracheva L.M, Kovaltzova S.V, Evstuhina T.A, Alekseev S.Y, Korolev V.G. The yeast HSM3 gene acts in one of the mismatch repair pathways. Genetics. 1998 Mar;148(3):963-73. doi: 10.1093/genetics/148.3.963. PMID: 9539417 PMCID: PMC1460053
- Kelberg E.P, Kovaltsova S.V, Alekseev S.Yu, Fedorova I.V, Gracheva L.M, Evstukhina T.A, Korolev V.G. HIM1, a new yeast Saccharomyces cerevisiae gene playing a role in control of spontaneous and induction mutagenesis. Mutat Res. 2005 Oct 15;578(1-2):64-78. doi: 10.1016/j.mrfmmm.2005.03.003. PMID: 15885712
- Keck K.M, Pemberton L.F. Histone chaperones link histone nuclear import and chromatin assembly. Biochim Biophys Acta. 2012 Mar;1819(3-4):277-89. doi: 10.1016/j.bbagrm.2011.09.007. Epub 2011 Oct 8. PMID: 22015777 PMCID: PMC3272145
- Evstyukhina T.A, Alekseeva E.A, Peshekhonov V.T, Skobeleva I.I, Korolev V.G. The role of chromatin assembly factors in induced mutagenesis at low levels of DNA damage. Genes (Basel). 2023 Jun 10;14(6):1242. doi: 10.3390/genes14061242. PMID: 37372422 PMCID: PMC10298286
- Sun Z, Hsiao J, Fay D.S, Stern D.F. Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science. 1998 Jul 10;281(5374):272-4. doi: 10.1126/science.281.5374.272. PMID: 9657725
- Sharma V.M, Tomar R.S, Dempsey A.E, Reese J.C. Histone deacetilases RPD3 and Hos2 regulate the transcriptional activation of DNA damage inducible genes. Mol Cell Biol. 2007 Apr;27(8):3199-210. doi: 10.1128/MCB.02311-06. Epub 2007 Feb 12. PMID: 17296735 PMCID: PMC1899941a
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.