biology Group. 2024 May 28;5(5):474-487. doi: 10.37871/jbres1917.
Traditional and Novel Foods as Vectors for Human Parasitic Diseases
Divyashri G*, Harini H, Likitha V, Lalitha Prasanna MV, Mahima ED and Shreya M
- Food
- Contamination
- Parasites
- Mechanism
- Transmission
- Sanitation
Abstract
Consumption of food contaminated with parasites pose a significant threat to public health particularly in underprivileged communities with inadequate access to sanitation and food safety practices. Unlike bacterial and viral infections, parasitic infections often develop slowly and are reported cause chronic health issues. However, due to extended incubation time, it is difficult to determine the source of the infection. The type and number of parasites in food, the temperature at which food is prepared and stored, as well as a person's immune system, all of these contribute significantly to the onset of parasitic infection. This mini-review focuses on common parasitic infections, their emergence with traditional and novel foods, transmission mechanisms, epidemiological trends, and effective prevention and control measures. Understanding these aspects is crucial for developing strategies to reduce the impact of foodborne parasitic infections on public health, particularly in vulnerable populations.
Introduction
While many foods are culturally significant and offer distinct flavors, they can also pose a risk: parasitic infections [1]. Foodborne parasites, often overlooked compared to bacteria and viruses, present a significant public health threat, potentially leading to severe and chronic health problems [2]. This is especially concerning for disadvantaged communities with inadequate access to sanitation and effective food safety practices [3]. In contrast to other foodborne illnesses that result in immediate illness, parasitic infections often develop slowly and lead to chronic issues. This delayed onset, combined with their prevalence in disadvantaged communities, has resulted in them being somewhat overlooked. Furthermore, identifying the source of infection is challenging due to the prolonged incubation period [4]. Several factors contribute to the likelihood of infection, including the specific type and quantity of parasite present in the food item, the temperature at which the food is stored or prepared, and the individual's immune response [5]. This mini-review discusses the common parasitic infections and their emergence with traditional and novel foods; their mechanism of transmission; analyzes the significant public health impact concerning epidemiological trends, and concludes by exploring effective prevention and control measures.
Parasitic Diseases Transmitted by Traditional Foods
Traditional foods are deeply rooted in cultural practices and are often celebrated for their unique flavors and historical significance [1]. However, these foods can also pose health risks, as they may be sources of parasitic infections. Foodborne parasites have historically received less attention as pathogens compared to other infectious agents, largely because they often result in subtle, long-term effects rather than immediate, acute illnesses [6]. These parasites are often associated with underprivileged or marginalized communities. Foodborne parasites can lead to severe and even fatal diseases in humans. Sources of infection include undercooked or raw food, processed items containing parasitic stages, and contaminated drinking water. Some of the most threatening infections include paragonimiasis, fascioliasis, clonorchiasis, opisthorchiasis, and other intestinal fluke infections [7]. These parasites are commonly found in freshwater fish, shellfish, and various aquatic plants [8]. Additionally, wildlife meat, freshwater and marine fish, snails, amphibians like frogs and snakes, as well as livestock such as cattle and pigs, can also be potential sources of parasitic infection [9].
Meat
Meat, particularly pork and beef, can transmit various parasitic infections, including Taeniasis, Trichinosis, Toxoplasmosis, and Sarcocystosis [10]. Taeniasis is caused by Taenia spp. such as Taenia solium and Taenia saginata. Their life cycles involve a connection between humans and either cattle (T. saginata) or pigs (T. saginata asiatica and T. solium). T. solium, the pork tapeworm, is mainly found in humans and pigs. It causes taeniasis in its adult form and cysticercosis in its larval form. This disease is associated with poor sanitation, open defecation, and the presence of free-roaming pigs. The life cycle of T. solium typically involves the transmission of eggs to scavenging pigs through human feces [11]. These eggs hatch into oncospheres that penetrate the intestinal wall and develop into cysticerci (cysts) in the muscle. Humans can become infected by consuming undercooked pork meat containing the cysticerci, which then develop into adult worms in the gut. Cysticerci may accumulate in the brain, leading to neurocysticercosis, a significant cause of acquired epilepsy in endemic areas. Intestinal taeniosis may be associated with abdominal discomfort, nausea, weight loss and anal pruritis [12]. Trichinellosis occurs when humans ingest Trichinella larvae encysted in the muscle tissue of domestic or wild animal meat. The primary source of human infection worldwide is the domestic pig, although meats from wild boars and horses have also contributed to outbreaks [13]. Clinical symptoms of trichinellosis include headaches, myalgia, fever, diarrhea, and facial edema [14].
Fish
Raw or undercooked freshwater fish are a significant source of parasitic infections, particularly those caused by trematodes (such as Opisthorchis spp., Clonorchis sinensis, and minute intestinal flukes), cestodes (including Diphyllobothrium spp. and Spirometra), and nematodes (like Gnathostoma spp. and Anisakidae) [15]. About 50 species of helminth parasites can cause zoonotic infections from consuming raw or undercooked aquatic foods like fish, crabs, crayfish, snails, and bivalves [16]. Common raw seafood dishes known to transmit these parasitic infections include Japanese sushi, sashimi, and salad (which contain raw fish), Hawaiian lomi lomi salmon, tako poki (a dish made from cephalopods), Dutch green herring, Scandinavian gravalax, Philippine bagoong (made from uncooked fish viscera), and Pacific island poisson cru (fish fillets marinated in coconut juice) [17].
Diphyllobothriosis: Diphyllobothriosis caused by Diphyllobothrium latum, is transmitted through the consumption of raw, undercooked, or mildly smoked fish, such as salmon or pike. These parasites are prevalent in Europe, North and South America, and Asia [18]. Severe D. latum infection can lead to megaloblastic anemia by dissociating the vitamin B12 intrinsic factor complex in the gut lumen, thus depriving the host of B12 [19]. Proper cooking or freezing of fish at -12°C for at least 24 hours are effective control methods. Additionally, appropriately controlled and treated sewage is crucial to prevent contamination [20].
Anisakiasis: Traditional fermented foods, such as raw fish or shellfish preparations, can transmit parasitic infections like anisakiasis, primarily caused by the worm species Anisakis simplex [4]. Larvae of Anisakis, commonly found in marine fish, can induce gastrointestinal discomfort or allergic reactions when consumed raw oundercooked. Proper freezing or cooking of fish can effectively eliminate the risk of anisakiasis.
Liver fluke infections: Liver flukes, including Clonorchis sinensis and various Opisthorchis species, can also cause parasitic diseases from raw fish [21]. Clonorchiasis, caused by C. sinensis, is transmitted through the consumption of raw or undercooked freshwater fish, particularly in traditional Asian cuisines [22]. Opisthorchiasis, caused by species like Opisthorchis viverrini and O. felineus, is another liver fluke infection [23]. Consuming raw or undercooked freshwater fish, commonly practiced in traditional cuisines of Southeast Asia and Eastern Europe, can lead to opisthorchiasis [24].
Crabs and crayfish
Paragonimiasis is caused by lung flukes belonging to the genus Paragonimus, commonly known as "lung flukes." These parasites typically infect freshwater and brackish-water crustaceans, including freshwater crabs and crayfish [25]. Paragonimiasis is a foodborne illness that can result from consuming raw or undercooked infected crabs, which are often used in traditional dishes such as raw crab salads or soy soups. The metacercarial larvae are released when the crustaceans are eaten raw, penetrating the intestinal wall and diaphragm before reaching the lungs [26].
Aquatic plants
Water serves as a primary vehicle for several parasites, which can contaminate fruits, vegetables, and seafood. Certain water plant species are commonly consumed raw or fresh. Among the emerging waterborne parasitic infections that can be acquired through food are Fasciola, Giardia, Cyclospora cayetanensis, Cryptosporidium and Fasciolopsis [27]. Fascioliasis, caused by two species of liver fluke, Fasciola hepatica and F. gigantica, can be transmitted through the consumption of aquatic plants contaminated with infective cysts (metacercariae) [28]. Traditional dishes that include raw or undercooked aquatic plants, like watercress or water chestnuts, may pose a risk of fascioliasis transmission. Metacercariae can also be found floating in water, thus infection can be acquired through drinking water [29].
Protozoan infections
Protozoan parasites like Giardia spp. and Cryptosporidium spp. can contaminate aquatic plants, especially those grown in or near contaminated water sources [29]. If these plants are consumed raw or not washed thoroughly, they can transmit parasites and cause gastrointestinal infections like giardiasis and cryptosporidiosis. Traditional dishes that use raw or minimally processed aquatic plants, such as salads or garnishes, can pose a risk of protozoan infection [30].
Parasites transmitted by faecal contamination of food and drinks
Parasites can be transmitted through food and drinks contaminated with fecal matter [31]. Vegetables, fruits, and fruit juices are examples of food items that can become contaminated by infectious parasite stages released into the environment through excrement. Toxoplasmosis is caused by the protozoan parasite T. gondii. Humans can acquire the disease by consuming raw or undercooked meat containing tissue cysts, or by accidentally ingesting sporulated oocysts excreted by Felidae [32]. Traditional foods, particularly raw or undercooked meats and unpasteurized dairy products, are significant vehicles for T. gondii transmission. Dishes like raw minced meat, cured meats, and unpasteurized cheeses may contain T. gondii cysts. Congenital toxoplasmosis, resulting from maternal infection during pregnancy, can lead to miscarriage, stillbirth, or severe neurological and ocular abnormalities in newborns [32]. Monitoring farms and adjusting farm management practices can play a crucial role in controlling Toxoplasma infection [29].
Emerging Trends in Novel Food Sources and Parasitic Risks
Novel food categories encompass products with limited historical consumption, often requiring safety assessments. These foods may be derived from innovative production methods or unconventional biological sources (animal, plant, microbial, or mineral) not previously used for human consumption, as defined by national regulations. Ensuring safety remains a critical requirement for introducing these products to the market. However, there is a growing emphasis on sustainable food systems, prompting the exploration of alternative food sources. These sources should ideally be cost-effective and promote efficient resource utilization [33]. Microorganisms have a long history of safe usage in food and can be employed to create enriched food items and protein sources [34]. Technological advancements in cell culture, particularly in economic approaches, are crucial for large-scale production of whole food sources from non-traditional biological sources, which are increasingly favored over concentrated extracts of natural components [35]. In line with this, plant and animal cell cultures hold promise for producing high-quality, safe, and nutritious food items [36]. However, despite seaweed and microalgae being excellent nutrient sources, safety risks such as heavy metal contamination and toxin presence should be considered [37]. Duckweed, with its high protein content, shows potential as a sustainable food source, but concerns about bioaccumulation safety issues need to be addressed [38]. Canola protein, derived from rapeseed variants with reduced toxic ingredients, is emerging as a new protein source [39]. Insects are also part of this trend as a novel protein source, offering a nutritious and environmentally friendly option with high food conversion efficiency [33]. Edible insects have reported either replace or enhance other high-protein feed components. It has been discovered that the amino acid contents of fish proteins and insects such as Hermetia illucens, Musca domestica, and Tenebrio molitor are comparable [40]. Additionally, the rearing and utilization of edible insects can have a positive impact on the environment by reducing greenhouse gas emissions and requiring less land and water compared to traditional livestock [41]. Insects are a sustainable and environmentally benign source of protein for both people and animals, and research suggests that eating them could help alleviate world hunger [42]. However, consumption of insects is linked to parasitic risks. As the world faces challenges related to food security and nutrition, edible insects offer a sustainable and nutritious alternative protein source that can help address these issues and contribute to achieving the UN’s Sustainable Development Goals [43]. In more than 80 nations worldwide, approximately 2000 different insect species are ingested, with subtropical and tropical climates being the most popular places for people to consume insects [44]. There is a higher rate of consumption in countries like those in Africa and the Latin America region. The Asian market is also becoming popular for using insects not only as a food source but also in the pharmaceutical industry [45].
The practice of eating insects, known as entomophagy, is increasing in popularity, especially in specific regions. However, a potential health risk associated with entomophagy is the transmission of parasitic infections. Wild-harvested insects pose a higher risk compared to farmed insects. This difference is due to the uncontrolled environment and diet of wild insects, which can expose them to a greater variety of parasites [43]. Insects can act as intermediate hosts, carriers, mechanical vectors, and reservoirs for various parasites that can harm vertebrates [46]. Examples of these insect-transmitted parasites include protozoans (Balantidium spp., Cryptosporidium spp., Entamoeba spp.), trematodes (Dicrocoelium spp., Lecithodendriidae), cestodes (Hymenolepis spp., Raillietina spp.), nematodes (Gordius spp., Spirocerca spp.), and mites. Additionally, insects like yellow mealworms (T. molitor) and lesser mealworms (Alphitobius diaperinus) from the Tenebrionidae family can be sources of canthariasis [42]. Zoonotic parasites, such as Dicrocoelium dendriticum, have been documented to be transmitted to humans through the consumption of specific edible insects, like ants [47]. Additionally, the isolation of foodborne and waterborne parasites, including Entamoeba histolytica, Giardia lamblia, and Toxoplasma spp., from insects like cockroaches further highlights the potential risk, particularly with wild-harvested insects [43].
The risk of parasitic infection is further heightened by the potential presence of entomopathogenic parasites within the insects themselves [48]. These entomopathogens include sporozoa (Leidyana spp., Gregarine spp., Septatorina spp.), ciliates (Tetrahymena spp., Nyctotherus spp.), nematodes (Thelastoma spp., Steinernema spp., Heterorhabditis spp.), and various mite species, including predatory mites (Cheyletus eruditus), opportunistic mites (Dermatophagoides spp., Glicephagus spp.), and storage mites (Acarus spp., Rhizoglyphus spp.). These parasites have the potential to induce digestive issues, allergic reactions, and other health complications in both humans and livestock [42]. Furthermore, studies have identified bacteria such as Vibrio, Streptococcus, Staphylococcus, Clostridium, and Bacillus in edible insects. These bacteria can lead to microbial foodborne illnesses, especially in regions where wild harvesting is prevalent. Furthermore, allergic reactions to insect consumption have been documented, with some cases resulting in fatalities [43].
The potential health risks associated with consuming insects underscore the importance of ensuring proper food safety measures, particularly in the context of entomophagy [49]. Strict regulations and guidelines for the farming, processing, and consumption of insects are necessary to mitigate these risks [50]. As the consumption of insects continues to gain popularity as a sustainable protein source, addressing these health concerns is crucial to ensure the safety of consumers. Despite these potential drawbacks, research is crucial to fully understand and mitigate these risks. A critical aspect involves ensuring proper processing and sanitation practices throughout the insect production chain to minimize contamination with foodborne pathogens, including those transmitted by parasites [51]. Furthermore, thorough research on the microbiological safety of edible insects is essential before authorizing large-scale production. This research should encompass potential risks associated with parasitic infections, entomopathogenic fungi and mycotoxins, and pesticide residues, especially in wild-harvested insects [42].
Mechanism of Transmission
The mechanism of transmission for human parasitic diseases is a complex process often involving the consumption of infected meat that is either uncooked or undercooked. Not all consumption of parasitic-infected food leads to infection; several criteria must be met for an individual to become infected. These include the presence of the parasite in sufficient quantities to cause infection, the ability of the parasite to subsist on the food, the maintenance of the food at an appropriate temperature for the parasite to survive, and the ingestion of a sufficient portion of the food containing the pathogen to surpass the individual's immunological response [52]. The most common disease-causing parasites are Taenia solium, Toxoplasma gondii, Cryptosporidium, and Trichinella spiralis.
Taenia solium
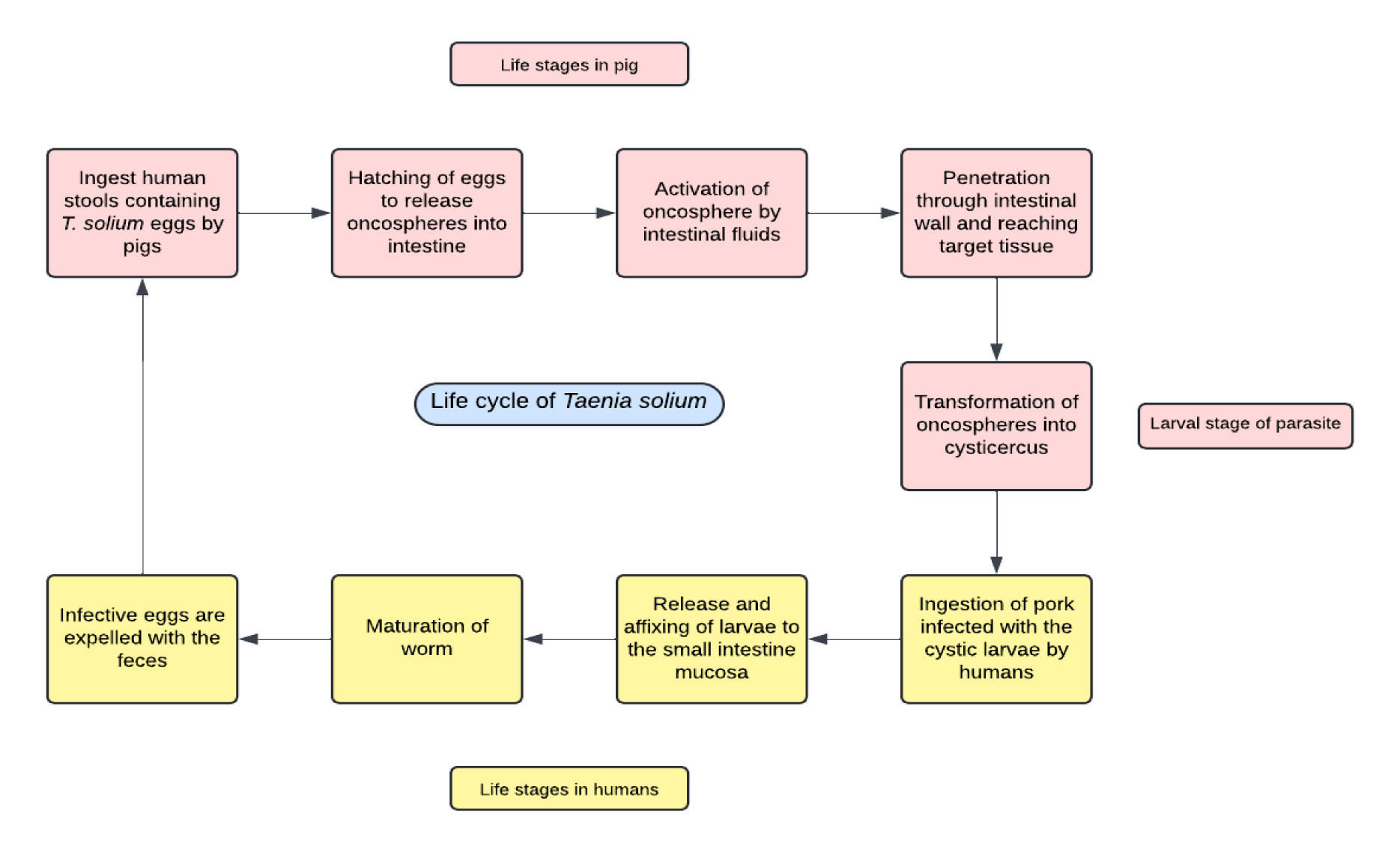
The mechanism of transmission for T. solium involves a complex life cycle between humans and pigs. Humans, the definitive hosts, harbor the adult tapeworm, while pigs act as intermediate hosts, hosting the larval stage (cysticercus). Humans acquire T. solium tapeworm infection (taeniasis) by consuming undercooked pork containing viable cysticerci (Figure 1). Pigs, on the other hand, contract porcine cysticercosis by ingesting T. solium eggs present in human feces from tapeworm carriers. The larval stage establishes in the pig's central nervous system. The cycle begins with pigs ingesting T. solium eggs from human stool. Once ingested, the eggs release a six-hooked larva, the oncosphere, which is activated by the intestinal fluid, penetrates the intestinal wall, and reaches the target tissues where it transforms into a cysticercus. Humans can acquire intestinal taeniasis by ingesting pork infected with the cystic larvae. In humans, the cyst's scolex attaches to the small intestine mucosa, where it matures and produces eggs. These eggs are then expelled with the feces, infecting pigs and continuing the cycle.
Toxoplasma gondii
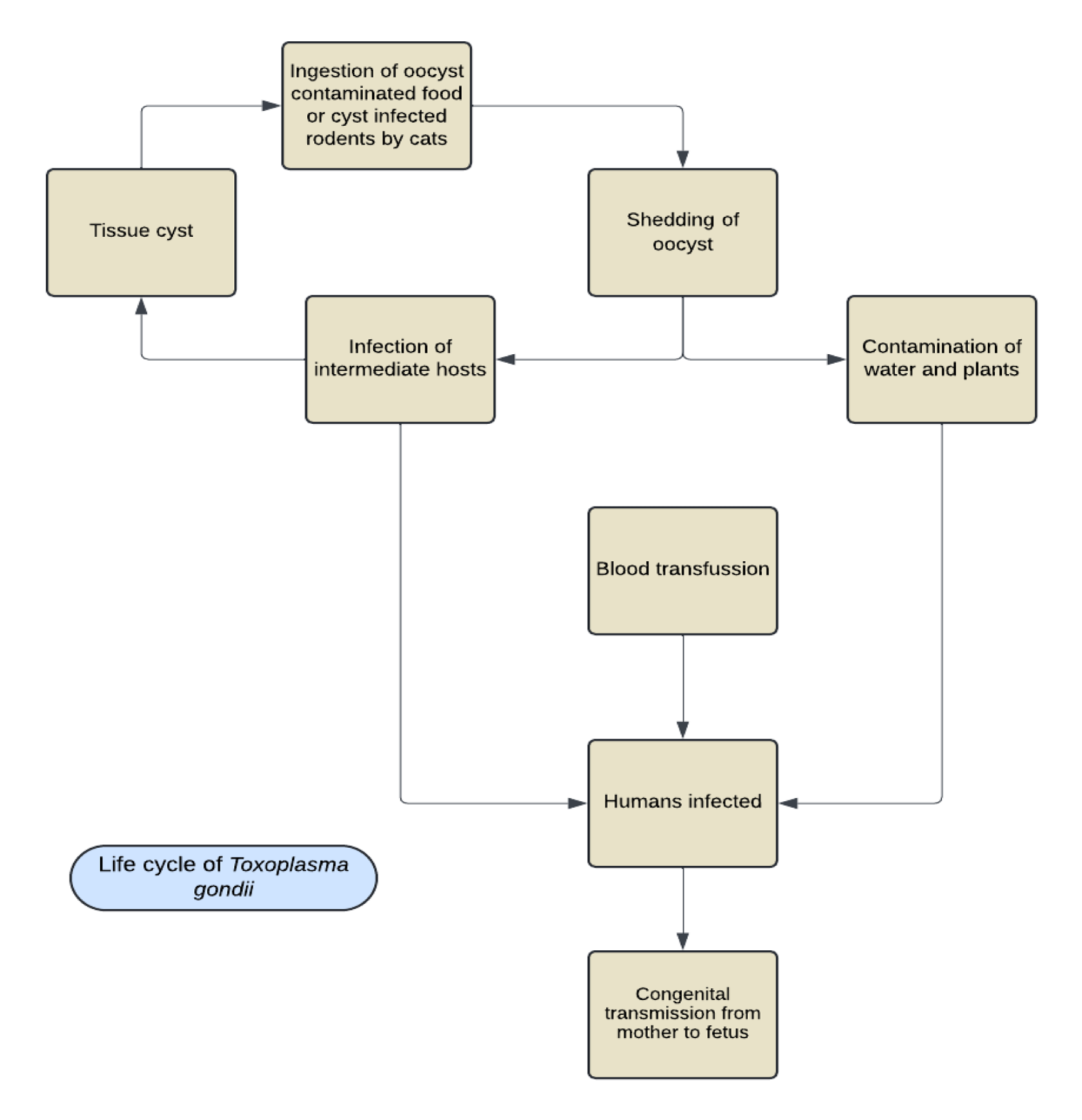
Toxoplasma gondii is a common obligate intracellular protozoan parasite that infects a significant portion of the global population. It is acquired through the consumption of undercooked or raw meat contaminated with cysts or by ingesting food contaminated with oocysts shed by cats. While the disease is often asymptomatic in adults, it can lead to severe complications in congenitally infected children, including blindness and mental retardation. Immunocompromised individuals are also at risk of developing encephalitis, the most severe manifestation of the disease. The lifecycle of T. gondii is distinctive and includes both asexual and sexual reproduction (Figure 2). There are three infectious stages for all hosts: sporozoites (found in sporulated oocysts, which are environmentally resistant), tachyzoites (rapidly multiplying individually or in groups), and bradyzoites (slowly multiplying in tissue cysts). Cats, the definitive hosts, can shed millions of oocysts after consuming just one bradyzoite or tissue cyst, with many tissue cysts potentially present in an infected mouse. Cats can contract toxoplasmosis by consuming contaminated prey, such as birds or rodents, and serve as hosts for continued sexual reproduction, enteroepithelial cycles, and oocyst production. These oocysts are excreted by the cats and can contaminate soil, water, and plants. Intermediate hosts, including rodents, birds, pigs, and deer, become infected by consuming the contaminated soil, water, and plants, where the eggs release parasites in the form of tachyzoites. These tachyzoites can spread throughout the animal's body and form cysts in nerve and muscle tissue. The cycle continues when cats consume infected rodents (Figure 2). Humans can contract toxoplasmosis by consuming contaminated plants and water, ingesting tissue cysts in raw or uncooked meat, or through blood transfusion. Congenital transmission is also possible, where tachyzoites from the mother cross the placenta and infect the fetus. The ingested tissue cyst is digested in the host organism's stomach, releasing the parasite in the form of bradyzoites, which are highly resistant to proteases and can survive in the host organism's small intestine.
Cryptosporidium
Cryptosporidium is an enteric protozoan parasite that can be transmitted to humans from animals, other humans, contaminated food, or water, often leading to waterborne outbreaks. The parasite's cysts and oocysts are typically excreted in large quantities in the feces of infected hosts. Cryptosporidium follows a unique life cycle that involves growth in a single host, with both asexual and sexual stages [55]. The oocyst stage is crucial for its survival in harsh environments. Upon ingestion, oocysts excyst in the small intestine, releasing sporozoites that attach to microvilli. These sporozoites then develop into trophozoites and undergo asexual reproduction through schizogony. The resulting Type 1 or Type 2 merozoites can either cause autoinfection or attach to epithelial cells. These merozoites then mature into macrogamonts or microgamonts, the male and female forms of the parasite, respectively. Sexual reproduction occurs, leading to the formation of zygotes, which develop into oocysts. These oocysts can either reinfect the host or be excreted into the environment.
Trichinella spiralis
The nematode parasites of the genus Trichinella are responsible for causing trichinellosis, a zoonotic parasitic disease that occurs when raw or undercooked meat from infected animals is consumed [56]. Trichinellosis develops when encysted larvae in the meat are released in the stomach [2]. Upon exposure to gastric acid and pepsin, the larvae are liberated from the cysts and invade the small intestine. Within a week, the larvae mature into adults and reproduce. The resulting larvae then migrate to the body's striated muscles, where they encyst themselves.
Public Health Impact and Epidemiological Concern
Numerous risks can contaminate food, leading to over 200 acute and chronic illnesses. These risks include microbiological organisms such as bacteria, viruses, fungus, or parasites, as well as chemical hazards from human pollution, food processing or packaging, and naturally occurring poisons in raw materials [57]. The prevalence of foodborne illness and their effects on societies vary locally, nationally, and internationally. Measuring this impact is crucial to pinpoint issues with food safety and set priorities at different levels, from national to international [57]. Annually, foodborne illness-causing microorganisms affect approximately one-third of the population in numerous nations, including wealthy ones. In the year 2020 alone, over 3,000 foodborne disease outbreaks and more than 30,000 foodborne illnesses are expected to be reported across twenty-one European Union member states [58]. Parasitic infections rank among the most perilous global public health challenges. With over 100 parasite species currently posing risks to public health [24], these infections often go overlooked due to their stigmatized nature and subtle, long-term impacts compared to acute illnesses [4]. One notable group of neglected tropical diseases is foodborne trematode infections, which have persisted, causing 2 million disability-free years and fatalities annually [59]. These diseases are particularly endemic in regions like Southeast Asia and South America, where poverty remains widespread [60]. Foodborne parasitic illnesses are leading to significant outbreaks, causing widespread illness and even fatalities [61]. They also strain healthcare systems and can result in significant economic losses due to healthcare costs and productivity losses. From an epidemiological standpoint, tracking and controlling foodborne parasitic illnesses can be challenging [62]. Parasites can be transmitted through various routes, including contaminated food, water, and soil, making it difficult to pinpoint the exact source of an outbreak [63]. Additionally, many parasitic infections have long incubation periods, further complicating efforts to identify and contain outbreaks [64]. WHO reports indicate that parasites account for 7% of foodborne illnesses globally, underscoring the persistent threat posed by these pathogens [63]. Despite advancements in food technology, safety policies, and alarm systems, parasitic foodborne infections remain a major concern for consumers and public health authorities, with profound impacts on the economy and society [65].
Efforts to address foodborne parasitic illness must consider the broader context of food safety and its impact on public health. Collaboration between stakeholders at all levels is essential to improve food safety measures, enhance surveillance systems, and raise awareness among consumers and food producers. Ensuring food safety is essential for achieving global food security. Several Sustainable Development Goals (SDGs) focused on reducing inequality, advancing socioeconomic development, and promoting health rely on access to safe and nutritious food [66]. Despite increased political attention, parasitic foodborne infections remain a significant global health, economic, and social burden, particularly in low- and middle-income countries [47].
In a 2020 prospective study, individuals in Kashmir, India who consumed raw or undercooked beef were found to be infected with Taenia saginata (beef tapeworm). The study revealed a higher infection rate among males (3.40%) compared to females (2.05%), with an overall frequency of 2.74%. Moreover, individuals over 60 years old showed a higher prevalence, with 2.68 % among females and 7.21% among males [67]. Due to a lack of investigation, Trichinella nematode worms are still widely ignored in India. Recent human outbreaks of trichinellosis linked to pig meal in northern India have highlighted the increasing threat to food safety posed by this overlooked parasite. A recent investigation found that 3.77% of pooled pig samples and 1.26% of individual pig samples tested positive for Trichinella [68]. The detection of Trichinella in slaughtered pigs signals the potential onset of foodborne illness from this neglected parasite. Given the importance of pigs as a meat source and their role as a reservoir for Trichinella, there is a pressing need for thorough meat inspection and comprehensive research on neglected parasites in India [68]. Toxoplasmosis, caused by the protozoan parasite T. gondii, infects both humans and livestock. T. gondii is considered one of the most important foodborne and waterborne parasites in terms of veterinary and medical importance [69]. Consuming contaminated undercooked or raw meat from farm animals is a major risk factor for acquiring T. gondii infection in humans [70]. The European Food Safety Authority (EFSA) recently suggested that meat-borne transmission accounts for approximately 60% of T. gondii infections, with contaminated beef, pork, and small ruminant meat being the major contributing sources [71].
Prevention and Control Measures
Preventive measures
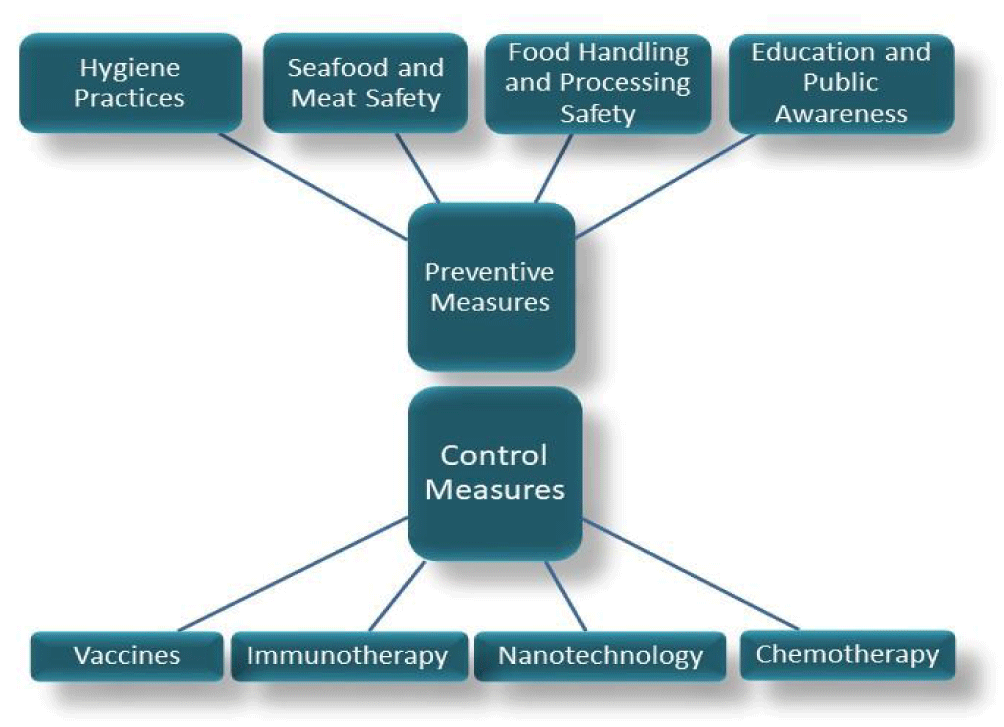
Parasitic diseases pose a considerable threat to individual and public health, predominantly affecting regions with low socio-economic conditions due to inadequate sanitation and dense populations, particularly where food is processed [72]. This issue is exacerbated in developing countries by the rapid urban migration that fuels the proliferation of food outlets. Effective preventive and controlling strategies are required to combat these infections (Figure 3). The spread of intestinal parasites is closely linked to the hygiene practices of food handlers. Parasitic eggs, originating from soil, can adhere to fruits and vegetables and may be transferred to human hands. Consequently, the failure to thoroughly wash either the produce or the hands of those handling the food can lead to parasitic transmission. Furthermore, examining the fingernails of food handlers can reveal the presence of parasitic ova. Studies have shown that regular handwashing with soap and the use of gloves during food preparation significantly minimize the risk of food contamination [73,74]. Studies have reported significantly higher contamination levels in fruits and vegetables sold in open-air markets than those in enclosed supermarkets, highlighting the substandard sanitary conditions prevalent in these markets, which serve as breeding grounds for disease transmission. Furthermore, vegetables stored in farmers' storage rooms have been found to be contaminated with T. gondii [75]. Additionally, river waters in varied locations, including the Kathmandu Valley in Nepal, Brazil, and Poland, are reported to be frequently contaminated with various infectious stages of parasitic species [76]. Using this contaminated river water to wash vegetables facilitates the transmission of parasites to humans, particularly when these vegetables are consumed raw. These practices highlight the findings of Wang M, et al. [77], which emphasize the health risks associated with inadequate hygiene and sanitation in the handling and storage of food.
Multi-drug resistant pathogens frequently transfer from aquatic species to humans through seafood, acting as zoonotic agents. In the seafood industry, it is crucial to regularly clean and sterilize the ponds or lakes where fish are raised to prevent the proliferation of parasitic species in seafood. Implementing periodic sanitation measures before refilling tanks is vital for decreasing the population of intermediate hosts that carry parasites and for interrupting their life cycles [78]. To prevent contamination, it is crucial to ensure that every meal is thoroughly cooked. Cooking fish to an internal temperature of 62°C for 15 seconds can effectively kill parasites [78]. Poultry should be cooked to at least 74°C, ground or minced meat to at least 71°C, and other cuts of meat to at least 63°C, with a subsequent three-minute resting period before consumption. Additionally, an effective strategy for preventing contamination is to freeze meat at -12°C or lower before cooking [79]. Parasites, in their various stages including oocytes, eggs, cysts, and spores, can be accidentally consumed. In a commercial setting, the best way to prevent this is by maintaining stringent hygiene throughout all stages from production to consumption, including processing, post-processing, storage, and trade [77]. At the production stage, pre-harvest measures should include limiting the presence of cats and rodents and ensuring a clean water supply for irrigation. Since oocytes are generally resistant to strong acids and chemical treatments, filtration techniques are used for sterilization. Post-harvest measures focus on removing or inactivating parasitic oocytes in meat tissue through techniques such as freezing, irradiation, and high-pressure processing [77].
The increasing availability of pre-packaged food items, such as ready-to-eat salads and raw fish products like sushi or sashimi, presents potential health threats and should be classified as biohazards [80]. To mitigate these risks, governments should enforce stringent regulations to monitor the safety and quality of these food products. Such measures could include the implementation of Good Manufacturing Practices (GMP) and Hazard Analysis and Critical Control Points (HACCP) protocols, ensuring that food items are produced and handled in ways that minimize the risk of contamination [3]. At the individual level, thorough washing of fruits and vegetables before consumption is essential. It's equally important to wash hands thoroughly before preparing food and after handling uncooked meat. Additionally, kitchen equipment such as crockery, cutlery, and cutting boards should be meticulously cleaned to remove contaminants. Drinking untreated water should be avoided. Regular medical check-ups and treatments are also crucial for maintaining health and preventing disease [79]. There is a notable correlation between education levels and the incidence of parasitic infections. Research indicates that there is generally lower awareness of these infections in South Asian countries compared to more developed nations such as Italy. Consequently, improving literacy rates may lead to a reduction in the prevalence of these diseases [73]. However, the effectiveness of educational initiatives depends on the accuracy of the information provided and how well it is understood by the public. Comprehensive evaluation programs are essential for effective public education. France serves as a successful example, having implemented mandatory periodic screening tests during pregnancy and offering treatment options [79].
Control Strategies
Controlling strategies for parasitic infections typically involve a multi-faceted approach, which are detailed as below.
Vaccines: Vaccination stands out as a highly effective medical measure for controlling and preventing foodborne infectious diseases in both animals and humans. An ideal human vaccine should provide protection against both acute and chronic infections, while livestock vaccines should aim to prevent cyst formation to reduce transmission to humans through undercooked food [5]. The efficiency of vaccine delivery hinges on several factors, including the parasite's infectious stage, the target host, the specific parasitic antigen, and the method of administration [79]. However, the development of vaccines against foodborne parasites is challenging due to their diverse nature and complex host-pathogen interactions [81]. Despite these obstacles, the vaccines design for these parasites remains a prominent area of medical research. Most vaccines for parasite control in livestock fall into three categories: live attenuated vaccines, inactivated vaccines, and recombinant subunit vaccines [5]. Among these, subunit vaccines offer several advantages, including ease of storage, lack of contaminants, and absence of toxic side effects. However, their effectiveness is limited by the selection of antigenic proteins and their weak ability to stimulate the innate immune response, often requiring the use of adjuvants [5]. Registered anti-parasitic vaccines for direct human use are scarce. One notable example is a highly efficient recombinant vaccine designed to combat a Taeniid cestodes parasites. These vaccines offer protection against infections in the intermediate hosts of various Taenia and E. granulosus species [82].
Chemotherapy: Chemotherapy options for parasitic infections are currently very limited. Drugs, Pyrimethamine and sulfadiazine are combined to target two specific steps in the folic acid metabolism of Taxoplasma [83]. Other treatments include pyrimethamine with clindamycin, azithromycin, adovaquone, or trimethoprim with sulfamethoxazole [84]. However, these treatments can lead to adverse side effects such as rash, fever, and hematological toxicity during prolonged courses. Current chemotherapeutic agents target various pathways including folic acid metabolism, mitochondrial electron chain, calcium-dependent protein kinase-1, fatty acid synthesis, histone synthesis, isoprene biosynthesis, and protein synthesis [79]. Ongoing research aims to identify novel drugs and chemotherapies using high-throughput screening methods. Ideally, new anti-parasitic drugs should be safe for human administration and for its use during pregnancy. They should also be more effective and less toxic than current treatments [79].
Immunotherapy: While there are currently limited immunotherapeutic strategies for combating parasitic infections, their lower propensity for resistance and side effects has positioned them as a crucial area of research for controlling parasitic diseases. Some potential immunotherapies include passive immunization, monoclonal antibody therapy, CAR-T and NK cell therapy, cytokines and their complexes, chemokines and receptors, immunomodulatory drugs, and trained immunity [79].
Nanotechnology: Nanoparticles possess unique physicochemical properties that make them promising carriers for drugs and vaccines [85]. They can facilitate slower drug delivery, enhance target specificity and efficacy, and minimize side effects [86]. One notable example is the use of chitosan nanoparticles combined with anti-T. gondii drugs, which has been shown to enhance the effectiveness of parasite eradication [87]. Additionally, the administration of chitosan nanoparticles alongside silver nanoparticles has led to a significant reduction in parasitic burden in mouse models [52]. Plant extract nanoparticles have also been employed as stabilizing or reducing agents to enhance biocompatibility in mouse models. In line with this, biogenic selenium has demonstrated a reduction in parasitic burden [88]. Nonetheless, a major challenge with metal nanoparticles is their non-biodegradability, which can lead to toxicity [79]. DNA vaccines can be coupled with nanoparticles to prevent the degradation of DNA by extracellular nucleases during delivery. Encapsulation of DNA vaccines in liposomal carriers has shown promising results in providing significant protection against T. gondii in mice [89].
References
- Surya R, Lee AGY. Exploring the philosophical values of kimchi and kimjang culture. Journal of Ethnic Foods. 2022;9(1). doi: 10.1186/s42779-022-00136-5.
- Mohamed A, Oumer N, Abdi D, Musa A, Adem M. A review on ruminant’s meat-born helminth zoonoses. Annals of Infectious Diseases & Preventive Medicine. 2024;4(1):1009.
- Awuchi CG. HACCP, quality, and food safety management in food and agricultural systems. Cogent Food & Agriculture. 2023;9(1). doi: 10.1080/23311932.2023.2176280.
- Robertson LJ. Parasites in Food: From a neglected position to an emerging issue. In: Advances in Food and Nutrition Research.2018:71-113. doi:10.1016/bs.afnr.2018.04.003. PMID: 30077225; PMCID: PMC7129657.
- Sander VA, López EFS, Morales LFM, Duarte VAR, Corigliano MG, Clemente M. Use of veterinary vaccines for livestock as a strategy to control foodborne parasitic diseases. Frontiers in Cellular and Infection Microbiology. 2020;10. doi:10.3389/fcimb.2020.00288. PMID: 32670892; PMCID: PMC7332557.
- Garcia LS, Arrowood M, Kokoskin E, et al. Practical Guidance for Clinical Microbiology Laboratories: Laboratory Diagnosis of Parasites from the Gastrointestinal Tract. Clinical Microbiology Reviews. 2018;31(1). doi:10.1128/cmr.00025-17. PMID: 29142079; PMCID: PMC5740970.
- Salim NM, Masroor NMS, Parween NS. An overview on trematodes developing disease complications causing cancer in human. World Journal of Advanced Research and Reviews. 2021;12(1):362-374. doi: 10.30574/wjarr.2021.12.1.0535.
- Paladini G, Longshaw M, Gustinelli A, Shinn AP. Parasitic diseases in aquaculture: Their biology, diagnosis and control. Diagnosis and Control of Diseases of Fish and Shellfish. 2017;37-107. doi: 10.1002/9781119152125.ch4.
- Chhabra MB, Pathak KML, Muraleedharan K. Food-borne parasitic zoonoses: Status, emerging risk factors and issues: An overview. J. Foodborne Zoonotic Dis. 2017;5(2):16-31.
- Abuseir S. Meat-borne parasites in the Arab world: a review in a One Health perspective. Parasitol Res. 2021;120(12):4153-4166. doi:10.1007/s00436-021-07149-0. Epub 2021 Apr 15. PMID: 33856533.
- Symeonidou I. Human taeniasis/cysticercosis: a potentially emerging parasitic disease in Europe. Annals of Gastroenterology. Published online January 1, 2018. doi:10.20524/aog.2018.0260. PMID: 29991885; PMCID: PMC6033766.
- Uslu U, Şenlik B. Meatborne parasitic zoonosis. In: Veterinary Pathobiology and Public Health. 2019;96-113. doi: 10.47278/book.vpph/2021.001.
- Diaz JH, Warren RJ, Oster MJ. The disease ecology, epidemiology, clinical manifestations, and management of trichinellosis linked to consumption of wild animal meat. Wilderness & Environmental Medicine. 2020;31(2):235-244. doi:10.1016/j.wem.2019.12.003. PMID: 32169338.
- Dupouy-Camet J, Raffetin A, Rosca EC, Yera H. Clinical picture and diagnosis of human trichinellosis. In: Elsevier eBooks. 2021;333-352. doi: 10.1016/b978-0-12-821209-7.00010-x.
- Gay M, Verrez‐bagnis V. Fish parasites and associated risks. Current Challenges for the Aquatic Products Processing Industry. 2023;147-186. doi: 10.1002/9781394264728.ch6.
- John HC. Fish- and Invertebrate-Borne helminths. Taylor & Francis. 2018. doi: 10.1201/9781351072106-12.
- Furuya K, Nakajima H, Sasaki Y, Urita Y. Anisakiasis: The risks of seafood consumption. Nigerian Journal of Clinical Practice. 2018;21(11):1492. doi:10.4103/njcp.njcp_256_17. PMID: 30417849.
- Ikuno H, Akao S, Yamasaki H. Epidemiology of Diphyllobothrium Nihonkaiense Diphyllobothriasis, Japan, 2001–2016. Emerging Infectious Diseases. 2018;24(8). doi:10.3201/eid2408.171454; PMID: 30016246; PMCID: PMC6056094.
- Sharma K, Wijarnpreecha K, Merrell N. Diphyllobothrium latum Mimicking Subacute Appendicitis. Gastroenterology Research. 2018;11(3):235-237. doi:10.14740/gr989w. PMID: 29915635; PMCID: PMC5997473.
- Santos CAMLD. The possible use of HACCP in the prevention, and control of food-borne treinatode infections in aquacultured fish. In: CRC Press eBooks. 2020;53-64. doi: 10.1201/9781003075899-8.
- Murrell KD, Pozio E. The liver flukes: Clonorchis sinensis, Opisthorchis spp, and Metorchis spp. In: Michigan State University eBooks. 2019. doi: 10.14321/waterpathogens.44.
- Zhang Y, Gong Q, Lv Q, et al. Prevalence of Clonorchis sinensis infection in fish in South‐East Asia: A systematic review and meta‐analysis. Journal of Fish Diseases. 2020;43(11):1409-1418. doi:10.1111/jfd.13245. PMID: 32880984.
- Pakharukova MY, Mordvinov VA. Similarities and differences among the Opisthorchiidae liver flukes: insights from Opisthorchis felineus. Parasitology. 2022;149(10):1306-1318. doi:10.1017/s0031182022000397;PMID: 35570685; PMCID: PMC11010525.
- Chai J-Y, Jung B-K. General overview of the current status of human foodborne trematodiasis. Parasitology. 2022;149(10):1262-1285. doi:10.1017/S0031182022000725. PMID: 35591777; PMCID: PMC10090779.
- Yoshida A, Doanh PN, Maruyama H. Paragonimus and paragonimiasis in Asia: An update. Acta Tropica. 2019;199:105074. doi:10.1016/j.actatropica.2019.105074. PMID: 31295431.
- Silachamroon U, Wattanagoon Y. Paragonimiasis. In: Elsevier eBooks. 2020;928-931. doi: 10.1016/b978-0-323-55512-8.00129-0.
- Hikal W, Ahl HAHSA. Food related parasitic infection: A review. American Journal of Food Science and Health. 2017;3(2):30-34.
- Lalor R, Cwiklinski K, Calvani NED, et al. Pathogenicity and virulence of the liver flukes Fasciola hepatica and Fasciola Gigantica that cause the zoonosis Fasciolosis. Virulence. 2021;12(1):2839-2867. doi:10.1080/21505594.2021.1996520. PMID: 34696693; PMCID: PMC8632118.
- Mas-Coma S, Bargues MD, Valero MA. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures – CORRIGENDUM. Parasitology. 2020;147(5):601. doi:10.1017/s0031182020000256. PMID: 32052720; PMCID: PMC7174693.
- Hadjilouka A, Tsaltas D. Cyclospora Cayetanensis—Major Outbreaks from Ready to Eat Fresh Fruits and Vegetables. Foods. 2020;9(11):1703. doi:10.3390/foods9111703; PMID: 33233660;PMCID: PMC769973.
- Gizaw Z, Yalew AW, Bitew BD, Lee J, Bisesi M. Fecal indicator bacteria along multiple environmental exposure pathways (water, food, and soil) and intestinal parasites among children in the rural northwest Ethiopia. BMC Gastroenterology. 2022;22(1). doi:10.1186/s12876-022-02174-4; PMID: 35220951;PMCID: PMC8882269.
- Dixit B, Meshram S, Jha AK, Khare R. Parasitic fauna associated with reproductive disorders. Principles and Practices of Canine and Feline Clinical Parasitic Diseases. 2024;161-172. doi: 10.1002/9781394158256.ch16.
- Calabrese MG, Ferranti P. Novel foods: New food sources. In: Elsevier eBooks. 2019;271-275. doi: 10.1016/b978-0-08-100596-5.22128-8.
- Binda S, Hill C, Johansen E, et al. Criteria to qualify microorganisms as “Probiotic” in foods and dietary supplements. Frontiers in Microbiology. 2020;11. doi:10.3389/fmicb.2020.01662. PMID: 32793153; PMCID: PMC7394020.
- Wikandari R, Manikharda, Baldermann S, Ningrum A, Taherzadeh MJ. Application of cell culture technology and genetic engineering for production of future foods and crop improvement to strengthen food security. Bioengineered. 2021;12(2):11305-11330. doi:10.1080/21655979.2021.2003665. PMID: 34779353; PMCID: PMC8810126.
- George AS. Cultivating sustainability: The development and potential of cell-cultured beef rice as a novel high-protein food alternative. Zenodo. 2024. doi: 10.5281/zenodo.10800816.
- Cavallo G, Lorini C, Garamella G, Bonaccorsi G. Seaweeds as a “Palatable” challenge between innovation and sustainability: A systematic review of food safety. Sustainability. 2021;13(14):7652. doi: 10.3390/su13147652.
- Prada OJ, Diaz OL, Rocha KT. Common duckweed (Lemna minor): Food and environmental potential. Review. Revista Mexicana De Ciencias Pecuarias. 2024;15(2):404-424. doi: 10.22319/rmcp.v15i2.6107.
- Wanasundara JPD, Kapel R, Albe-Slabi S. Proteins from canola/rapeseed-current status. In: Elsevier eBooks.. 2024;285-309. doi: 10.1016/b978-0-323-91652-3.00004-6.
- Mastoraki M, Ferrándiz PM, Vardali SC, et al. A comparative study on the effect of fish meal substitution with three different insect meals on growth, body composition and metabolism of European sea bass (Dicentrarchus labrax L.). Aquaculture. 2020;528:735511. doi: 10.1016/j.aquaculture.2020.735511.
- Halloran A, Roos N, Eilenberg J, Cerutti A, Bruun S. Life cycle assessment of edible insects for food protein: a review. Agronomy for Sustainable Development. 2016;36(4). doi:10.1007/s13593-016-0392-8; PMID: 32010238; PMCID: PMC6961468.
- Gałęcki R, Bakuła T, Gołaszewski J. Foodborne diseases in the edible insect industry in Europe—New challenges and old problems. Foods. 2023;12(4):770. doi:10.3390/foods12040770. PMID: 36832845 PMCID: PMC9956073.
- Imathiu S. Benefits and food safety concerns associated with consumption of edible insects. NFS Journal. 2020;18:1-11. doi: 10.1016/j.nfs.2019.11.002.
- Raheem D, Carrascosa C, Oluwole OB, et al. Traditional consumption of and rearing edible insects in Africa, Asia and Europe. Critical Reviews in Food Science and Nutrition. 2018;59(14):2169-2188. doi:10.1080/10408398.2018.1440191. PMID: 29446643.
- Selaledi L, Hassan Z, Manyelo TG, Mabelebele M. Insects’ Production, Consumption, Policy, and Sustainability: What Have We Learned from the Indigenous Knowledge Systems? Insects. 2021;12(5):432. doi:10.3390/insects12050432. PMID: 34064777; PMCID: PMC8150288.
- Wilson AJ, Morgan ER, Booth M, et al. What is a vector? Philosophical Transactions - Royal Society Biological Sciences. 2017;372(1719):20160085. doi:10.1098/rstb.2016.0085. PMID: 28289253; PMCID: PMC5352812.
- Anisuzzaman N, Hossain MdS, Hatta T, et al. Food- and vector-borne parasitic zoonoses: Global burden and impacts. Advances in Parasitology/Advances in Parasitology. Published online January 1, 2023:87-136. doi:10.1016/bs.apar.2023.02.001; PMID: 36948728
- Jones RS, Fenton A, Speed MP. “Parasite-induced aposematism” protects entomopathogenic nematode parasites against invertebrate enemies. Behavioral Ecology. 2015;27(2):645-651. doi:10.1093/beheco/arv202; PMID: 27004015; PMCID: PMC4797382.
- Cantalapiedra F, Juan-Garcia A, Juan C. Perception of food risk of entomophagy among higher education students: Exploring insects as a novel food source. Foods. 2023. doi: 10.20944/preprints202311.0462.v1.
- Belluco S, Bertola M, Montarsi F, et al. Insects and Public Health: An Overview. Insects. 2023;14(3):240. doi:10.3390/insects14030240. PMID: 36975925; PMCID: PMC10059202.
- Vandeweyer D, De Smet J, Van Looveren N, Van Campenhout L. Biological contaminants in insects as food and feed. Journal of Insects as Food and Feed. 2021;7(5):807-822. doi:10.3920/jiff2020.0060. PMID: 29510743; PMCID: PMC5840809.
- Gaafar, Mady RF, Diab RG, Shalaby ThI. Chitosan and silver nanoparticles: Promising anti-toxoplasma agents. Experimental Parasitology. 2014;143:30-38. doi:10.1016/j.exppara.2014.05.005. PMID: 24852215.
- Chávez-Ruvalcaba F, Chávez-Ruvalcaba MI, Santibañez KM, Muñoz-Carrillo JL, Coria AL, Martínez RR. Foodborne Parasitic Diseases in the Neotropics – a review. Helminthologia. 2021;58(2):119-133. doi:10.2478/helm-2021-0022. PMID: 34248373; PMCID: PMC8256457.
- Al-Malki ES. Toxoplasmosis: stages of the protozoan life cycle and risk assessment in humans and animals for an enhanced awareness and an improved socio-economic status. Al-Mi’galaẗ Al-sa’udiyaẗ Lī-ulum Al-ḥayaẗ. 2021;28(1):962-969. doi:10.1016/j.sjbs.2020.11.007. PMID: 33424388; PMCID: PMC7783816.
- Pinto DJ, Vinayak S. Cryptosporidium: Host-Parasite interactions and Pathogenesis. Current Clinical Microbiology Reports. 2021;8(2):62-67. doi:10.1007/s40588-021-00159-7; PMID: 33585166; PMCID: PMC7868307
- Gottstein B, Pozio E, NöCkler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clinical Microbiology Reviews. 2009;22(1):127-145. doi:10.1128/cmr.00026-08. PMID: 19136437; PMCID: PMC2620635.
- Pires SM, Thomsen ST, Nauta M, Poulsen M, Jakobsen LS. Food safety implications of transitions toward sustainable healthy diets. Food and Nutrition Bulletin. 2020;41(2_suppl):104S-124S. doi:10.1177/0379572120953047. PMID: 33356595.
- Almaary KS. Food-Borne diseases and their impact on health. Biosciences Biotechnology Research Asia/Biosciences Biotechnology Research Asia. 2023;20(3):745-755. doi: 10.13005/bbra/3129.
- Ganasegeran K, Abdulrahman SA. Epidemiology of neglected tropical diseases. Neglected Tropical Diseases and Phytochemicals in Drug Discovery. 2021;1-36. doi: 10.1002/9781119617143.ch1.
- Wang YC, Namsanor J, Law A, Sithithaworn P. A socio-ecological framework for examining foodborne parasitic infection risk. Acta Tropica. 2023;244:106957. doi:10.1016/j.actatropica.2023.106957. PMID: 37269890.
- Scharff RL. Food Attribution and Economic Cost Estimates for Meat- and Poultry-Related Illnesses. Journal of Food Protection. 2020;83(6):959-967. doi:10.4315/jfp-19-548. PMID: 32032420.
- Gabriël S, Dorny P, Saelens G, Dermauw V. Foodborne parasites and their complex life cycles challenging food safety in different food chains. Foods. 2022;12(1):142. doi:10.3390/foods12010142. PMID: 36613359; PMCID: PMC9818752.
- Song L, Xie Q, Lv Z. Foodborne parasitic diseases in China: A scoping review on current situation, epidemiological trends, prevention and control. Asian Pacific Journal of Tropical Medicine. 2021;14(9):385. doi: 10.4103/1995-7645.326252.
- Alvar J, Alves F, Bucheton B, et al. Implications of asymptomatic infection for the natural history of selected parasitic tropical diseases. Seminars in Immunopathology. 2020;42(3):231-246. doi:10.1007/s00281-020-00796-y. PMID: 32189034; PMCID: PMC7299918.
- Aragrande M, Canali M. Integrating epidemiological and economic models to identify the cost of foodborne diseases. Experimental Parasitology. 2020;210:107832. doi:10.1016/j.exppara.2020.107832. PMID: 32004854.
- Banik D. Achieving food security in a sustainable development era. Food Ethics. 2019;4(2):117-121. doi: 10.1007/s41055-019-00057-1.
- Lateef M, Nazir M, Zargar SA, Tariq KA. Epidemiology of Taenia saginata taeniasis with emphasis on its prevalence and transmission in a Kashmiri population in India: A prospective study. International Journal of Infectious Diseases. 2020;98:401-405. doi: 10.1016/j.ijid.2020.06.088.
- Kalambhe DG, Kaur H, Gill JPS. Trichinella spp. in slaughtered pigs of India: From neglected disease to an emerging food safety threat for public health. Transboundary and Emerging Diseases. 2024;2024:1-9. doi: 10.1155/2024/7550006.
- Almeria S, Dubey JP. Foodborne transmission of toxoplasma gondii infection in the last decade. An overview. Research in Veterinary Science. 2021;135:371-385. doi: 10.1016/j.rvsc.2020.10.019.
- Innes EA, Hamilton C, Garcia JL, Chryssafidis A, Smith D. A one health approach to vaccines against Toxoplasma gondii. Food and Waterborne Parasitology. 2019;15:e00053. doi:10.1016/j.fawpar.2019.e00053; PMID: 32095623;PMCID: PMC7034027.
- Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, et al. Public health risks associated with food‐borne parasites. EFSA Journal. 2018;16(12). doi:10.2903/j.efsa.2018.5495. PMID: 32625781; PMCID: PMC7009631.
- Li J, Wang Z, Karim R, Zhang L. Detection of human intestinal protozoan parasites in vegetables and fruits: a review. Parasites & Vectors. 2020;13(1). doi:10.1186/s13071-020-04255-3. PMID: 32727529; PMCID: PMC7392835.
- Hajare ST, Gobena RK, Chauhan NM, Erniso F. Prevalence of Intestinal Parasite Infections and Their Associated Factors among Food Handlers Working in Selected Catering Establishments from Bule Hora, Ethiopia. BioMed Research International. 2021;2021:1-15. doi:10.1155/2021/6669742. PMID: 34458370; PMCID: PMC8397551.
- Todd E. Food-Borne disease prevention and risk assessment. International Journal of Environmental Research and Public Health/International Journal of Environmental Research and Public Health. 2020;17(14):5129. doi:10.3390/ijerph17145129. PMID: 32708573; PMCID: PMC7399861.
- Slany M, Dziedzinska R, Babak V, Kralik P, Moravkova M, Slana I. Toxoplasma gondii in vegetables from fields and farm storage facilities in the Czech Republic. FEMS Microbiology Letters. 2019;366(14). doi:10.1093/femsle/fnz170. PMID: 31365074.
- Nooraldeen K. Contamination of public squares and parks with parasites in Erbil city, Iraq. AAEM Annals of Agricultural and Environmental Medicine/Annals of Agricultural and Environmental Medicine. 2015;22(3):418-420. doi:10.5604/12321966.1167705. PMID: 26403106.
- Wang M, Bai L, Gong S, Huang L. Determinants of consumer food safety self-protection behavior- an analysis using grounded theory. Food Control. 2020;113:107198. doi: 10.1016/j.foodcont.2020.107198.
- Ziarati M, Zorriehzahra MJ, Hassantabar F, et al. Zoonotic diseases of fish and their prevention and control. ˜the œVeterinary Quarterly. 2022;42(1):95-118. doi:10.1080/01652176.2022.2080298. PMID: 35635057; PMCID: PMC9397527.
- Smith NC, Goulart C, Hayward JA, Kupz A, Miller CM, Van Dooren GG. Control of human toxoplasmosis. International Journal for Parasitology/International Journal for Parasitology. 2021;51(2-3):95-121. doi:10.1016/j.ijpara.2020.11.001. PMID: 33347832.
- Kelly A. Using novel tools for risk reduction in pre- and Post-Harvest food safety. IFIS Publishing. 2024.
- Fitzpatrick JL. Global food security: The impact of veterinary parasites and parasitologists. Veterinary Parasitology. 2013;195(3-4):233-248. doi:10.1016/j.vetpar.2013.04.005. PMID: 23622818.
- Lightowlers MW, Gasser RB, Hemphill A, et al. Advances in the treatment, diagnosis, control and scientific understanding of taeniid cestode parasite infections over the past 50 years. International Journal for Parasitology/International Journal for Parasitology. 2021;51(13-14):1167-1192. doi:10.1016/j.ijpara.2021.10.003. PMID: 34757089.
- Antczak M, Dzitko K, Długońska H. Human toxoplasmosis–Searching for novel chemotherapeutics. Biomedicine & Pharmacotherapy. 2016;82:677-684. doi:10.1016/j.biopha.2016.05.041. PMID: 27470411.
- El-Rahman NIHISMMN Marwa Ahmed Mohammed Salama, Eman Mostafa Abd. Treatment modalities of toxoplasmosis. Tobacco Regulatory Science (TRS). 2023. doi: 10.18001/TRS.9.1.68.
- Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as Drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers. 2023;15(7):1596. doi:10.3390/polym15071596. PMID: 37050210; PMCID: PMC10096782.
- Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules/Molecules Online/Molecules Annual. 2020;25(9):2193. doi:10.3390/molecules25092193. PMID: 32397080; PMCID: PMC7248934.
- Bamarni SSI. Role of nanotechnology in treating of toxoplasma gondii. International Journal of Agriculture and Biosciences. 2023;2:202-212. doi: 10.47278/book.zoon/2023.64.
- Arafa FM, Mogahed NMFH, Eltarahony MM, Diab RG. Biogenic selenium nanoparticles: trace element with promising anti-toxoplasma effect. Pathogens and Global Health. 2023;117(7):639-654. doi:10.1080/20477724.2023.2186079. PMID: 36871204; PMCID: PMC10498805.
- Maeta M, Miura N, Tanaka H, et al. Vitamin E scaffolds of PH-Responsive lipid nanoparticles as DNA vaccines in cancer and protozoan infection. Molecular Pharmaceutics. 2020;17(4):1237-1247. doi:10.1021/acs.molpharmaceut.9b01262. PMID: 32129629
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.