> General Science. 2020 October 31;1(6):263-270. doi: 10.37871/jbres1170.
Evaluation of Direct and Indirect Antioxidant Properties of Selected Four Natural Chemical Compounds: Quercetin, Epigallocatechin-3-Gallate, Indole-3-Carbinol and Sulforaphane by DPPH Radical Scavenging Assay
Maha J Hashim* and Jeffrey R Fry
- DPPH assay
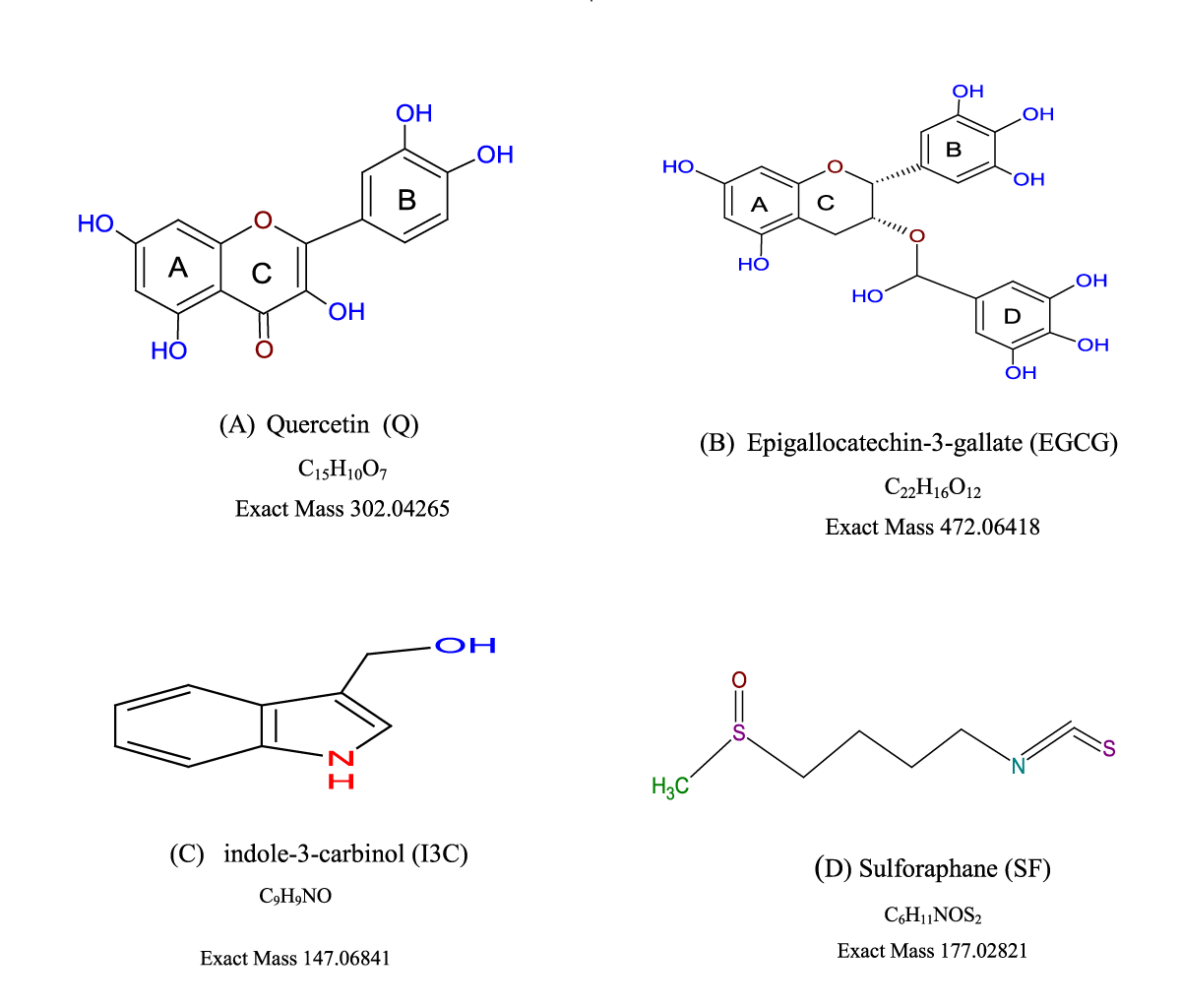
- Quercetin
- Epigallocatechin-3-gallate
- Indole-3-carbinol
- Sulforaphane
- Direct and indirect antioxidants
The main characteristic of antioxidants is the capacity to scavenge free radicals produced during cell metabolism, and thus they prevent oxidative stress, which may reduce the risk of many diseases. In this study, we evaluate the antioxidant properties of selected four compounds Quercetin (Q), Epigallocatechin-3-Gallate, (EGCG), Indole-3-Carbinol (I3C) and Sulforaphane (SF) by DPPH assay. The view is to establish the distinction between direct and indirect antioxidants, which would be the form of the basis for subsequent cellular antioxidant assays in our further studies. For sample assay: 20 μL of antioxidant solutions of Q, EGCG, I3C, and SF was added to 180 of 2,2- Diphenyl-1-Picrylhydrazyl (DPPH) solution. For blank solution, DMSO was used. Leaving the plate for 15 min in a dark place and measure the absorbance at 540 nm. The results demonstrated that Q and EGCG possess direct antioxidant properties, which can be used in further cellular studies. I3C and SFN did not appear to possess any direct antioxidant behaviours during DPPH radical scavenging.
Free radicals are produced in animal cells either deliberately or accidentally. The deliberate production yields profitable entities if they are targeted correctly, such as utilizing free radicals by enzymes at their active sites during the catalysis process. An accidental generation can cause significant production of accumulated reactive oxygen species [1] which consequently result in oxidative stress [2]. This oxidative may be prevented by antioxidants found in citrus fruits, cruciferous and dark-green vegetables [3]. Therefore, increased consumption of these dietary foods has been inversely associated with a wide range of diseases such as cancers [4,5]. The main characteristic of antioxidants is the capacity to scavenge free radicals, and thus they contribute to the lower risks of many diseases such as neurodegenerative and cardiovascular diseases [6]. Several methods have been used to assess the antioxidants activity to scavenge free radicals. Total Phenolics Content (TCP), 2,2-Diphenyl-1-Picryldrazyl (DPPH), and Ferric Reduction Activity Potential (FRAP) are three assays to determine antioxidant activity. TPC assay is usually considered as a marker for antioxidant capacity and commonly used in conjunction with either or both of the DPPH and FRAP assays to increase the information database on a specific plant extract. It may consider as a screen to evaluate sections further by either the DPPH or FRAP assays. On the other hand, DPPH and FRAP assays give virtually identical results considerably. They are often being used in parallel and following similar mechanism, by transfer of electrons from the antioxidant to reduce an oxidant [7]. Besides, the antioxidant behaviour in both assays may identify by high phenolics content. Clarks, et al. [8] found that two methodological issues with the FRAP assay which, is the interference caused by the colour in some extracts and slow development of colour which, may reduce the usefulness of this assay during testing plant extracts. Therefore, and according to problems of FRAP colour interference, they reported that the DPPH assay is the preferred assay in a preliminary screening of extracts of plants from the Malaysian rainforest. So, the most trusted, reliable, and common method is DPPH assay, which is based on the scavenging of 2,2-Diphenyl-1-Picryldrazyl (DPPH) radicals [8]. The first conception of the DPPH method was illustrated by Blois in 1958 [9] when DPPH free radicals accepted H atom from cysteine molecule:
DPPH ̇ + H → HDPPHH
The principle of the assay is based on the fact that DPPH radical accepts hydrogen atoms from the scavenger such as antioxidants to produce DPPHH that appears yellow colour absorbing at 515 nm. This assay has been adopted in different laboratories with some modifications [10]. In this study, we evaluate the antioxidant properties of selected four compounds Quercetin (Q) (Figure 1A), Epigallocatechin-3-Gallate (EGCG) (Figure 1B), Indole-3-Carbinol (I3C) (Figure 1C) and Sulforaphane (SF) (Figure 1D) by DPPH assay. The view is to establish the distinction between direct and indirect antioxidants, which would be the form of the basis for subsequent cellular antioxidant assays in our further studies.
Chemicals and reagents
All chemicals used in this study were obtained from Sigma Chemical Co. Ltd., Poole, Dorset, UK unless otherwise noted. Stock solutions of Q, EGCG, I3C, and SF were prepared in DMSO at μg/mL concentration units and stored at 4°C until use. The four selected chemicals were of >95% purity, as specified by the supplier.
The power of phytochemicals to scavenge free radicals was achieved by using 1,1 Diphenyl-2-Pycrylhydrazl (DPPH) radical. The method was based on that of [11] with some modifications into a 96-well plate in triplicate and for the blank assay, 20 μL of DMSO is added to 180 μL of 0.004% DPPH in methanol working solution. For sample assay 20 μL of antioxidant solution Q, EGCG, I3C, and SFN (320 μg/ mL, 160 μg/ mL, 80 μg/ mL, 40 μg/ mL, 20 μg/ mL and 10 μg/ mL) was added to 180 μL of DPPH solution. The plate had been left standing for 15 minutes in a dark place to avoid dissociation; the absorbance was measured spectrophotometrically at 540 nm after shaking for one minute. The scavenging of DPPH radical percentage was calculated from the difference between the control run with no antioxidant addition and the absorbance in the presence of antioxidants [12].
% Scavenging = 100 x [A0-(A+DPPH - A-DPPH)]/A0
Where A0 is the absorbance of sample solvent (DMSO) plus DPPH, A-DPPH is the absorbance of DMSO in methanol, and A+DPPH is the absorbance of the sample (i.e. phytochemicals) with DPPH.
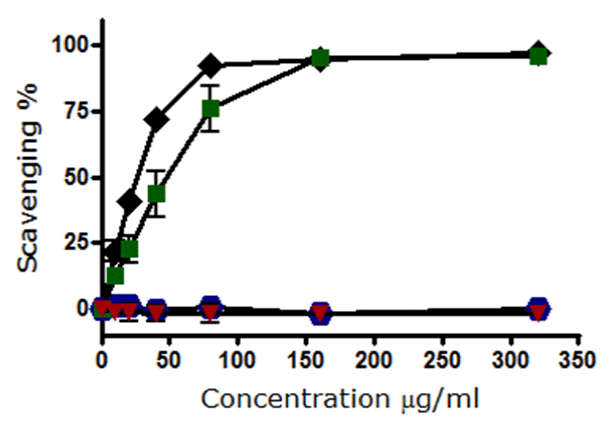
The results for the four selected four compounds demonstrated that Q and EGCG possessed radical-scavenging activity and act as direct antioxidants, with 100% scavenging being achieved at a concentration of 160 μg/mL. While SF and I3C did not display any antioxidant activity in that both failed to scavenge DPPH radicals and remained inactive in the concentrations range 0-320 μg/mL (Figure 2).
The daily consumption of vegetables and fruits rich with antioxidants such as onion, garlic, green tea, citrus fruits, and cruciferous vegetables has a clear impact on improving the health of the individual and disease resistance [5]. Therefore, scientists have interested in compounds that possess antioxidant properties [13]. However, their bioavailability is affected by several factors such as plasma protein, where the hydroxyl group in the B-ring of flavonoids has enhanced the binding affinities to proteins [14]. Moreover, plasma proteins may influence the cytoprotective effect of these compounds such as Q and EGCG during human hepatoma HepG2 cells exposed to oxidative stress elicited by t-BHP [15]. In this study, we have selected four natural chemical compounds Q, EGCG, I3C, and SF to assess their ability in scavenging free radicals when they possess antioxidant properties. The mechanism of direct trapping action of free radicals is based on the structure of the antioxidant and hydroxyl groups in particular. Therefore and relying on our results, Q and EGCG have exhibited a notable action in trapping free radicals confirming that they possess direct antioxidants activity, while I3C and SF are not.
The free radical scavenging action of Q and EGCG may attribute to the hydroxyl groups present in those compounds. Q has 5 while; EGCG possesses 9 groups on their structure. These groups represent the possible attack sites for the free radicals resulting in the radicalization of all hydroxyl groups [17,18]. This reaction includes the transfer of hydrogen atoms from antioxidant to the active radicals to produce oxidized antioxidant radicals [17], which are less reactive than the active free radical attacker. Scientists have confirmed the power trapping of free radicals by Q during the inactivation of lipid peroxide radicals [15-17]. Trouillas, et al. [19] have reported that the hydroxyl groups on ring B of Q are responsible for the antioxidant properties. RiceEvans, et al. [20] have confirmed that when the 3-OH group on ring B is blocked by adding sugar as in rutin, which causing the antioxidant activity decreased significantly.
Our results for the capacity of EGCG to scavenge free radicals were compatible with Salah, et al. [21]. They have attributed this superior action to the contribution of multiple numbers of hydroxyl groups when the ortho-dihydroxyl groups on ring B confer high stability for oxidized EGCG in particular. On the other hand, I3C and SF didn’t display any direct action in scavenging DPPH radicals. I3C has only one hydroxyl group on its structure lead to insufficient attacking sites by free radical atoms. According to this, the radicalization of the hydroxyl group is absent. The story of SF looks different, as its structure has no hydroxyl group, then any donation for the hydrogen atom is missing resulting in that SF is inactive completely and DPPH radicals are accumulated without any trapping.
In conclusion, this investigation indicates that Q and EGCG possess direct antioxidant properties, which can be used in further cellular studies. I3C and SF did not appear to possess any direct antioxidant behaviors during DPPH radical scavenging. Thus, any cytoprotection exerted by either I3C or SF would be due to mechanisms other than direct antioxidant mechanisms.
The authors are thankful to the University of Nottingham and the School of Life Sciences (UK) for all scientific support. Dr Maha J Hashim would like to thank Mr Samir Meissir (Department of cell biology) and Dr Abdul Khader Karakka Kal (Equine Forensic Unit), Central Veterinary Research Laboratory, Dubai\CVRL for their guidance and assistance in publishing this paper.
All words in the world are not enough to thank my father (Jalal Hashim Mohammed Tabana), in Iraq, who has funded my Ph.D. study (including this research) with all living costs in the United Kingdom for five years.
- Reichard P, Ehrenberg A. Ribonucleotide reductase--a radical enzyme. Science. 1983 Aug 5;221(4610):514-9. doi: 10.1126/science.6306767. PMID: 6306767.
- Lima CF, Valentao PC, Andrade PB, Seabra RM, Fernandes-Ferreira M, Pereira-Wilson C. Water and methanolic extracts of Salvia officinalis protect HepG2 cells from t-BHP induced oxidative damage. Chem Biol Interact. 2007 Apr 25;167(2):107-15. doi: 10.1016/j.cbi.2007.01.020. Epub 2007 Feb 12. PMID: 17349617.
- Agudo A, Cabrera L, Amiano P, Ardanaz E, Barricarte A, Berenguer T, Chirlaque MD, Dorronsoro M, Jakszyn P, Larrañaga N, Martínez C, Navarro C, Quirós JR, Sánchez MJ, Tormo MJ, González CA. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Am J Clin Nutr. 2007 Jun;85(6):1634-42. doi: 10.1093/ajcn/85.6.1634. Erratum in: Am J Clin Nutr. 2008 Oct;88(4):1181. PMID: 17556703.
- Barrera LN, Cassidy A, Johnson IT, Bao Y, Belshaw NJ. Epigenetic and antioxidant effects of dietary isothiocyanates and selenium: potential implications for cancer chemoprevention. Proc Nutr Soc. 2012 May;71(2):237-45. doi: 10.1017/S002966511200016X. Epub 2012 Mar 6. PMID: 22391025.
- Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003 Sep;78(3 Suppl):559S-569S. doi: 10.1093/ajcn/78.3.559S. PMID: 12936950.
- Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007 Jun;51(6):675-83. doi: 10.1002/mnfr.200700002. PMID: 17533652.
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005 Mar 23;53(6):1841-56. doi: 10.1021/jf030723c. PMID: 15769103.
- Clarke G, Ting KN, Wiart C, Fry J. High Correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging, Ferric Reducing Activity Potential and Total Phenolics Content Indicates Redundancy in Use of All Three Assays to Screen for Antioxidant Activity of Extracts of Plants from the Malaysian Rainforest. Antioxidants (Basel). 2013 Jan 4;2(1):1-10. doi: 10.3390/antiox2010001. PMID: 26787618; PMCID: PMC4665400.
- BLOIS M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature. 1958 April 181;1199-1200.
- https://go.nature.com/3ngcBi8
- Mishra K, Ojha H, Chaudhury NK. Estimation of antiradical properties of antioxidants using DPPH center dot assay: A critical review and results. Food Chemistry. 2012;130(4):1036-1043 doi: 10.1016/j.foodchem.2011.07.127
- Nara K, Miyoshi T, Honma T, Koga H. Antioxidative activity of bound-form phenolics in potato peel. Biosci Biotechnol Biochem. 2006 Jun;70(6):1489-91. doi: 10.1271/bbb.50552. PMID: 16794331.
- Stanner SA, Hughes J, Kelly CN, Buttriss J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Public Health Nutr. 2004 May;7(3):407-22. doi: 10.1079/phn2003543. PMID: 15153272.
- Xiao J, Kai G. A review of dietary polyphenol-plasma protein interactions: characterization, influence on the bioactivity, and structure-affinity relationship. Crit Rev Food Sci Nutr. 2012;52(1):85-101. doi: 10.1080/10408398.2010.499017. PMID: 21991992.
- Maha J Hashim, Jeffrey R Fry. Influence of Extracellular Protein on the Cytoprotective Effects of Two Model Phytochemicals. Mol Biol. 2019;8(1). doi: 10.4172/2168-9547.1000227
- Wagner C, Fachinetto R, Dalla Corte CL, Brito VB, Severo D, de Oliveira Costa Dias G, Morel AF, Nogueira CW, Rocha JB. Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res. 2006 Aug 30;1107(1):192-8. doi: 10.1016/j.brainres.2006.05.084. Epub 2006 Jul 7. PMID: 16828712.
- Chiodo SG, Leopoldini M, Russo N, Toscano M. The inactivation of lipid peroxide radical by quercetin. A theoretical insight. Phys Chem Chem Phys. 2010 Jul 21;12(27):7662-70. doi: 10.1039/b924521a. Epub 2010 Mar 29. PMID: 20596589.
- RiceEvans C, Miller N. Measurement of the antioxidant status of dietary constituents, low density lipoproteins and plasma. Prostaglandins Leukotrienes and Essential Fatty Acids. 1997;57:499-505. https://bit.ly/3mhFTLG
- Trouillas P, Marsal P, Siri D, Lazzaroni R, Duroux JL. A DFT study of the reactivity of OH groups in quercetin and taxifolin antioxidants: The specificity of the 3-OH site. Food Chemistry. 2006;97:679-688. https://bit.ly/2JT3DJi
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20(7):933-56. doi: 10.1016/0891-5849(95)02227-9. Erratum in: Free Radic Biol Med 1996;21(3):417. PMID: 8743980.
- Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, Rice-Evans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch Biochem Biophys. 1995 Oct 1;322(2):339-46. doi: 10.1006/abbi.1995.1473. PMID: 7574706.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.