> Medicine. 2020 December 15;1(8):393-403. doi: 10.37871/jbres1171.
-
Subject area(s):
- Metabolism
- Metabolic Syndromes
Metabolic Disturbance in Patients with Muscular Dystrophy and Reflection of Altered Enzyme Activity in Dystrophic Muscle: One Critical View
Niraj Kumar Srivastava1-3*, Somnath Mukherjee2,4 and Vijay Nath Mishra5
2School of Life Sciences, Jawaharlal Nehru University, New Delhi-110067, India
3School of Science (SOS), Indira Gandhi National Open University (IGNOU), New Delhi-110068, India
4Bapu Nature Cure Hospital & Yogashram, Mayur Vihar Phase-1, Delhi-110091, India
5Department of Neurology, Institute of Medical Sciences (IMS), Banaras Hindu University, Varanasi- 221005, India
- Aqueous components
- Enzyme activity
- NMR spectroscopy
- Muscular dystrophy
- Metabonomics
- Myopathy
Muscular dystrophies are inherited myogenic diseases and considered by progressive muscle wasting and weakness with variable distribution and severity. The essential characteristics of muscular dystrophies are selective involvement, significant wasting and weakness of muscles. The most common and frequent types of muscular dystrophies are Duchenne Muscular Dystrophy (DMD), Becker Muscular Dystrophy (BMD), Facioscapulohumeral Dystrophy (FSHD) and Limb Girdle Muscular Dystrophy (LGMD). Metabolic disturbance is observed in muscular dystrophy patients (DMD, BMD, FSHD and LGMD-2B). Alteration in the level of metabolites (BCAA, Glu/ Gln, Ace, alanine, glucose, histidine, propionate, tyrosine and fumarate) in dystrophic muscle reflects the alteration in the activity of enzymes. Collectively, these observations propose that there is alteration in the rate of glycolysis, TCA cycle, fatty acid oxidation, gluconeogenesis pathway and protein metabolism (catabolism & anabolism) in the muscular dystrophy patients. Metabolic disturbance, further provide the explanation about the pathophysiology of muscular dystrophy.
BCA: Branched Chain Amino Acids; Lac: Lactate; Gln/Glu: Glutamine/Glutamate; Ala: Alanine; Ace: Acetate; Suc: Succinate; Cr/Pcr: Creatine/Phosphocreatine; GPC/Car: Glycerophosphocholine/ Carnitine; Fum: Fumarate; His: Histidine; Tyr: Tyrosine; Prop: Propionate; TSP: 3-(trimethylsilyl) propionic-2, 2, 3, 3-d4 acid, sodium salt; DMD: Duchenne Muscular Dystrophy; BMD: Becker Muscular Dystrophy); FSHD: Facioscapulohumeral Dystrophy; LGMD-2B: Limb Girdle Muscular Dystrophy; PCA: Perchloric Acid.
Muscular dystrophies are inherited myogenic diseases and considered by progressive muscle wasting and weakness with variable distribution and severity. The essential characteristics of muscular dystrophies are selective involvement, significant wasting and weakness of muscles. The wasted muscle is replaced by adipose and connective tissue. Numerous types of muscular dystrophies have been described on the basis of the age, progress, site of involvement and the inheritance pattern. The genes and their protein products, which are responsible to produce most of these disorders, have now been well-established and recognized. A broad classification is still founded on clinical signs and symptoms, but immunohistochemical and molecular genetic analysis are useful for sub typing. The most common and frequent types of muscular dystrophies are Duchenne Muscular Dystrophy (DMD), Becker Muscular Dystrophy (BMD), Facioscapulohumeral Dystrophy (FSHD) and Limb Girdle Muscular Dystrophy (LGMD) [1-4].
Duchenne Muscular Dystrophy (DMD)
DMD, the most quickly progressive and deadly form of dystrophy, is also the most common variety, having an incidence of 1 in 3500 live male births and a reported prevalence of about 50-70 x 10-6 total male population in most surveys. The most trustworthy estimates of the DMD range from 18 to 30 per 1, 00000 live born males, and of its prevalence in the population as a whole from 1.9 to 4.8 per 100 000. The mutation rate is about 7-10 x 10-5 per gene per generation [1-5].
Duchenne Muscular Dystrophy (DMD) is distinguished by reducing muscle mass and progressive loss of muscle function in male children. This disease is observed by a mutation in a specific gene within the X chromosome (Gene map locus 12q21, Xp21.2) that affords directions for the production of the dystrophin protein, an essential structural constituent of muscle cell [2-4,6]. The clinical symptoms are characterized by: (a) onset of symptoms usually before the fourth year, rarely as late as the seventh; (b) symmetrical and at first selective involvement of the muscles of the pelvic and pectoral girdles; (c) hypertrophy of the calves and certain other muscles at some stage of the disease in almost every case; (d) relentlessly progressive weakness in every case, leading to inability to walk within 10 years of the onset and later to contracture and thoracic deformity; (e) invariable cardiac involvement; (f) frequent, but not invariable, intellectual impairment; (g) death by second or third decade caused by respiratory or less frequently, cardiac failure, often associated with inanition and respiratory infection; (h) very high activity of certain muscle enzymes, notably CK in serum in the early stages of the disease; and (i) certain characteristics histological features in muscle [1-5,7-9].
Becker Muscular Dystrophy (BMD)
Becker Muscular Dystrophy (BMD) influences approximately 1 in 8000-10,000 males. Becker eponym is similar to Duchenne muscular dystrophy, which is caused by mutation in dystrophin gene and it is a milder form of dystrophinopathies, in the distribution of muscle wasting and weakness, which is mainly proximal, but the course is more benign, with age of onset around 12 years; some patients have no symptoms until much later in life. Loss of ambulation also varies from adolescence onward, with death usually in the fourth or fifth decade. In some cases, as in Duchenne muscular dystrophy, a degree of mental impairment is present [1-4].
Selective muscle involvement in BMD is virtually identical to that in DMD. Most patients present the symptoms between the ages of 5 and 15 years but the onset may not be until the 3rd or the 4th decade of life in some cases. There is selective bilateral and symmetrical wasting & weakness of the costal origin of pectoralis major, latissimus dorsi, brachioradialis, hip flexors and extensors and medial vastus of quadriceps. Later the supinator, biceps, triceps, serratus anterior and neck flexors become weak [1-9].
Facioscapulohumeral Dystrophy (FSHD)
Facioscapulohumeral Muscular Dystrophy (FSHD) is an autosomal dominant disorder and the third most common inherited form of muscular dystrophy. Approximately 1 in 8000 to 22,000 individuals is suffered with FSHD worldwide. FSHD is originated by the anomalous production of the double homeobox protein 4 (DUX4) transcription factor in skeletal muscle. In normal condition, DUX4 is expressed throughout near the beginning of embryonic development, and is then efficiently silenced in all tissues apart from the testis and thymus. Their reactivations in skeletal muscle interrupt several signaling pathways that frequently congregate on cell death [10-13].
FSHD has a characteristic pattern of skeletal muscle weakness and a broad range of disease severity. The clinical symptoms started from infancy to middle age, but the most of patients develop signs and symptoms in their late teens to the early 20s. Muscle weakness and atrophy begin in the face and shoulder muscles, moving ahead to the upper arms, trunk muscles and lower extremities, classically evident first in the anterior leg muscles go behind by the thigh and pelvic girdle muscles. Unlike the majority of other dystrophies, asymmetric association is distinctive and more prominent in FSHD, and contractures are lacking or negligible [10,14,15].
Limb Girdle Muscular Dystrophy (LGMD)
Limb girdle muscular dystrophy or LGMD is an extensive word, which includes numerous entities. In 1954, Walton and Nattrass was first defined this disease. The progression of muscle weakness is exceedingly sluggish. In this disease, weakness affects principally the proximal limb-girdle musculature. In few patients the pelvic girdle is involved early while in others, both the pelvic and shoulder girdles are involved at the same time. Due to defectiveness of 15 genes, fifteen different types have been recognized. These are LGMD1A,1B,1C,1D,1E (autosomal dominant) and LGMD2A,2B,2C,2D,2E,2F,2G,2H,2I,2J (autosomal recessive).There is a huge clinical and genetic diversity appeared in all these types. Autosomal dominant types are extremely exceptional and usually less severe as compared to recessive types. In these LGMD1B, 1C, 2B, 2C, 2D, 2E, 2F are more frequent and common [16-18].
In several studies, more frequent and common type of Limb Girdle Muscular Dystrophy is dysferlinopathy (LGMD-2B). This is an autosomal recessive type and occurred due to deficiency of the sarcolemmal protein dysferlin. This is secondary to DYSF gene mutation on chromosome 2p. Two common clinical entities are described under the label of dysferlinopathy: Miyoshi myopathy (distal onset) and LGMD 2B (proximal onset). Initially, Miyoshi and colleagues described the distal form with burnt on the gastrocnemius muscles and labeled it as Miyoshi distal myopathy. The genetic identification of Miyoshi myopathy coincided with the same genetic defect being identified in a set of proximal myopathies termed LGMD 2B. Hence, both these presentations are clubbed together as dysferlinopathy. While the condition was originally discovered in Japan, it is now recognized to be a common LGMD in most parts of the world [16-20].
Patients present in the second decade of life, earlier presentations are uncommon. The initial weakness is in the gastrocnemius muscles, which is usually discovered while standing on toes for sporting activities or exercise programs. Gradually, patients are unable to stand on toes and calf muscles are wasted. Hamstrings and hip flexors become progressively weakened and patients develop difficulties in climbing stairs and rising from the ground. As people in India customarily squat to defecate, proximal muscle weakness comes to early attention. Upper limbs are affected later and biceps may show a “lump.” This lump is not unique to this condition but is frequently seen. Similarly, the quadriceps muscle is known to show a diamond-like configuration of hypertrophy and atrophy. Ambulation is maintained for many years and the progress is gradual. A proportion of patients begins with proximal weakness and then goes on to have distal involvement. Clinically, the proximodistal weakness is of most frequent occurrence [1,4,18-20].
All the muscular dystrophies are related to the wasting and weakness of skeletal muscle. In this regard, it is necessary to describe the normal skeletal muscle metabolism.
Metabolism is the sum of many interconnected reaction sequences that interconvert cellular metabolites. Metabolites are the end products of cellular regulatory processes, and their levels can be regarded as the ultimate response of biological systems to genetic or environmental changes. The components usually considered as metabolites are molecules with a molecular weight less than 2000 Da. It could be primary metabolites such as sugars, amino acids, organic acids, fatty acids, bioenergetic metabolites (nucleotides) [21-23].
Adenosine Triphosphate (ATP) is the energy currency of the cell and it is directly related to the muscle energy metabolism. The ATP required as the constant energy source for the contraction-relaxation cycle of muscle can be generated (1) by glycolysis, using blood glucose or muscle glycogen, (2) by oxidative phosphorylation, (3) from creatine phosphate, and (4) from two molecules of ADP in a reaction catalyzed by adenylyl kinase .The amount of ATP in skeletal muscle is only sufficient to provide energy for contraction for a few seconds, so that ATP must be constantly renewed from one or more of the above sources, depending upon metabolic conditions [24].
Major features of the skeletal muscle related to its metabolism
Skeletal muscle metabolism is carried out in both aerobic (resting) and anaerobic (eg, sprinting) conditions. In this way, both aerobic and anaerobic glycolysis are operated for performing the muscle functions, which are depending on conditions. There are several specific features related to the skeletal muscle metabolisms, which are listed below:
- Skeletal muscle restrains myoglobin as a pool of oxygen.
- Skeletal muscle encloses diverse types of fibers principally suitable to anaerobic (fast twitch fibers) or aerobic (slow twitch fibers) circumstances.
- Actin, myosin, tropomyosin, troponin complex (TpT, Tpl, and TpC), ATP, and Ca2+ are essential components in the process of contraction.
- The Ca2+ ATPase, the Ca2+ release channel, and calsequestrin are proteins implicated in a variety of phases of Ca2+ metabolism in muscle.
- Insulin performs the stimulation on skeletal muscle to enhance the uptake of glucose.
- In the nourish condition, the major amount of glucose is utilized in glycogen synthesis.
- Epinephrine performs the stimulation of glycogenolysis in skeletal muscle, whereas glucagon does not because of absence of its receptors.
- Skeletal muscle cannot supply in a straight line to blood glucose because it does not have glucose-6-phosphatase.
- Lactate produced by anaerobic metabolism in skeletal muscle and transported to liver via blood for the synthesis of glucose (gluconeogenesis), which can then return to muscle (the Cori cycle).
- Skeletal muscle consists of phosphocreatine, which performs as an energy store for instant (seconds) demands.
- Supply of Free fatty acids in blood is a chief source of energy and specifically underneath marathon circumstances and in long-lasting starvation.
- Skeletal muscle can consumes ketone bodies throughout starvation.
- Skeletal muscle is the major location of metabolism of branched-chain amino acids. These are utilized as an energy source.
- Under the starvation circumstances, proteolysis of skeletal muscle proteins is carried out and releases the amino acids for gluconeogenesis.
- The most important amino acids derives from proteins of skeletal muscle are alanine (intended mainly for gluconeogenesis in liver and forming part of the glucose-alanine cycle) and glutamine (intended mainly for the gut and kidneys) [24-29].
Large storage of glycogen in the skeletal muscle and its role in supply of energy
Glycogen is stored in large amount in the sarcoplasm of skeletal muscle. The liberate of glucose from glycogen is dependent on a specific muscle glycogen phosphorylase, which can be activated by Ca2+, epinephrine and AMP. To produce glucose 6-phosphate for glycolysis in skeletal muscle, glycogen phosphorylase b must be activated to phosphorylase a via phosphorylation by phosphorylase b kinase. Ca2+ encourages the activation of phosphorylase b kinase, also by phosphorylation. Thus, Ca2+ both begins muscle contraction and stimulates a pathway to supply essential energy. The hormone epinephrine also stimulates glycogenolysis in muscle. AMP, created by breakdown of ADP throughout muscular exercise, can also stimulate phosphorylase b without causing phosphorylation [24,27,28].
Muscle generates ATP chiefly by oxidative-phosphorylation (Under aerobic condition)
Oxidative phosphorylation performs the synthesis of ATP through supply of oxygen. Muscles store the myoglobin that have an elevated require for oxygen as a consequence of sustained contraction (eg, to maintain posture). In this way, muscle produced the ATP through oxidative-phosphorylation. Glucose, resulting from the blood glucose or from endogenous glycogen, and fatty acids derived from the triacylglycerols of adipose tissue are the chief substrates used for aerobic metabolism in muscle [24,29].
Creatine phosphate constitutes a major energy reserve in muscle
Creatine phosphate avoids the speedy exhaustion of ATP by providing a readily available high-energy phosphate that can be used to stimulate ATP from ADP [24,25].
Skeletal muscle contains slow (red) and fast (white) twitch fibers
Skeletal muscle contains different types of fibers and subdivides them into type I (slow twitch), type IIA (fast twitch-oxidative) and type IIB (fast twitch-glycolytic). The type I fibers are red and their metabolism is aerobic because they contain myoglobin and mitochondria and they maintain relatively sustained contractions. The type II fibers are white and do not contain myoglobin. These fibers are containing few mitochondria and they derive their energy from anaerobic glycolysis and display relatively short durations of contraction. The proportion of these two types of fibers differs among the muscles of the body, depending on function (eg, whether or not a muscle is involved in sustained contraction, such as maintaining the posture). The proportion also varies with training; for example, the number of type I fibers in certain leg muscles increases in athletes training for marathons, whereas the number of type II fibers increases in sprinters [30,31].
It is of interest to evaluate their participation in a sprint (eg, 100 meters) and in the marathon (42.2 km). The most important sources of energy in the 100-m sprint are creatine phosphate (first 4–5 seconds) and then anaerobic glycolysis, using muscle glycogen as the source of glucose. The two major locations of metabolic control are at glycogen phosphorylase and at PFK-1 (phosphofructokinase-1). The previous is activated by Ca2+ (released from the sarcoplasmic reticulum throughout contraction), epinephrine and AMP. PFK-1 is activated by AMP, Pi and NH3. Demonstrate to the effectiveness of these processes, the flux through glycolysis can amplify as much as 1000-fold throughout a sprint. In contrast, in the marathon, aerobic metabolism is the major source of ATP. The chief fuel sources are blood glucose and free fatty acids, basically consequent from the breakdown of triacylglycerols in adipose tissue, stimulated by epinephrine. Hepatic glycogen is degraded to sustain the level of blood glucose. Muscle glycogen is also a fuel source, but it is degraded much more progressively as compared in a sprint. It has been calculated that the amounts of glucose in the blood, of glycogen in the liver, of glycogen in muscle, and of triacylglycerol in adipose tissue are sufficient to supply muscle with energy during a marathon for 4 minutes, 18 minutes, 70 minutes, and approximately 4000 minutes, respectively. However, the rate of oxidation of fatty acids by muscle is slower than that of glucose, so that oxidations of glucose and of fatty acids are both major sources of energy in the marathon [27-31].
In the muscular dystrophies, the biochemicals amendments may primarily be restricted and restrained but afterward happen to extensive and connected with the steady deterioration of the muscle tissue. It is rational to assume the straight analysis of affected muscle, its chemical composition and enzyme activities, is the majority probable to lead to understanding of the causes and progression of the muscular dystrophies [31].
All biochemical studies or analysis must acquire into explanation the thoughtful histological transforms happening in the affected muscles, particularly the proliferation of connective tissue and structural changes in the fibers. It is obvious that noticeable variations may happen in the overall chemical and enzymatic composition of the muscle tissue simply as a result of these changes [32,33].
Altered enzyme activities in muscular dystrophies
Several studies or analysis have accounted the biochemical profile in the dystrophic muscle tissue. There are various methods available such as chromatographic, biochemical techniques and NMR (Nuclear Magnetic Resonance) spectroscopy for the estimation of the metabolites [34]. Dreyfus, et al. [35] reported that the rate of glycolysis in dystrophic muscle is much less as compared to normal and the rate is decreases with the progression of the disease. Reduced activity is observed in individual glycolytic enzymes (i.e. α-glucan phosphorylase, phosphoglucomutase and aldolase). In contrast the activity of a number of other enzymes, such as cytochrome oxidase, succinate dehydrogenase, aconitase, fumarase and the aminotransferase is not significantly different as compared to normal. DiMaoro, et al. [36] also mentioned that as compared to other glycolytic enzymes, such as muscle phosphorylase showed an earlier and more marked loss in progressive muscular dystrophies as compared to neurogenic diseases. Research and analysis outcomes of Vignos & Lefkowitz [37] showed that the rate of glycolysis is low in the juvenile forms, but is essentially normal in the adult forms of muscular dystrophy. After this, the researchers also found a low activity of creatine kinase in juvenile muscular dystrophy and neurogenic atrophy of muscle, but solitary marginals' changes in adult muscular dystrophy [38]. Heyck, et al. [39], Hooft, et al. [40] and Kleine, et al. [41] have represented the reduction in the level of adenylate kinase and several other glycolytic enzymes. Great reduction of adenylate kinase 1 is also found in mdx skeletal muscle [42]. The level or activity of enzyme fructose 1, 6-bisphosphatase (which control the glycolytic process) is normal in all types of the muscular dystrophies [43]. There is a marked reduction found in the level or activity of AMP aminohydrolase (AMP deaminase) in both dystrophic mouse muscle and in muscle from patients with Duchenne dystrophy even at an early stage, while in other muscle diseases a low level is seen only in severely affected muscle.Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase are the first two enzymes of the pentose phosphate pathway of glucose utilization and represented the enhanced activity in human dystrophic muscle. Both of these enzymes are NADP-linked. Two other NADP-linked enzymes such as isocitrate dehydrogenase and glutathione reductase also rise in dystrophic muscle [44-46].
Cutillo, et al. [47] showed the higher activity of malate dehydrogenase in the blood of patients with muscular dystrophy. Malate dehydrogenase is a TCA cycle enzyme, which is responsible for the conversion of malate to oxaloacetate [24].
Activities of lysosomal cathepsin enzymes (such as cathepsins D, A, B1, C, and dipeptidyl peptidase II; protein hydrolyzing enzymes) are higher or elevated in muscle tissue of patients with muscular dystrophies [48].
Abnormal regulation of calcium dependent enzymes is also found in mdx muscles, which is further responsible for disturbing intracellular signaling mechanisms [49].
Analysis of muscle PCA (perchloric acid) extracts to detect the alteration in the metabolites in muscular dystrophies
Several methods are available such as chromatographic, biochemical techniques and NMR (Nuclear Magnetic Resonance) spectroscopy for the estimation of the metabolites. One of the major advantages of NMR is that it is not biased towards a particular metabolite, but simultaneously a large number of metabolites can be detected in one pulse proton NMR spectra which are predictable or unpredictable or which are difficult to evaluate using standard biochemical methods [50].
Venkatasubramanian, et al. [51] analyzed the two dimensional proton NMR spectra of human muscle tissue extracts and recognized distinctions between the metabolite composition of normal and diseased muscles. Sharma, et al. [52,53] achieved the analysis on the PCA extract of the muscle tissue of the DMD and LGMD patients and observed the noteworthy differentiation in the glucose, lactate, alanine etc. Description of studies supplied an opinion to investigate NMR spectroscopy for the observation of the biochemical alters in the muscle tissue of the patients with muscular dystrophy. NMR spectroscopy based qualitative and quantitative analyses of metabolites in the Perchloric Acid Extract (PCA) of the muscle tissue of the patients with DMD, BMD, FSHD and LGMD-2B as compared to normal individuals were also performed [54].
On the basis of all these studies and analysis, metabolic disturbance or alteration in the level of metabolite is observed in patients with muscular dystrophies (DMD, BMD, FSHD and LGMD-2B). These altered metabolites are described below:
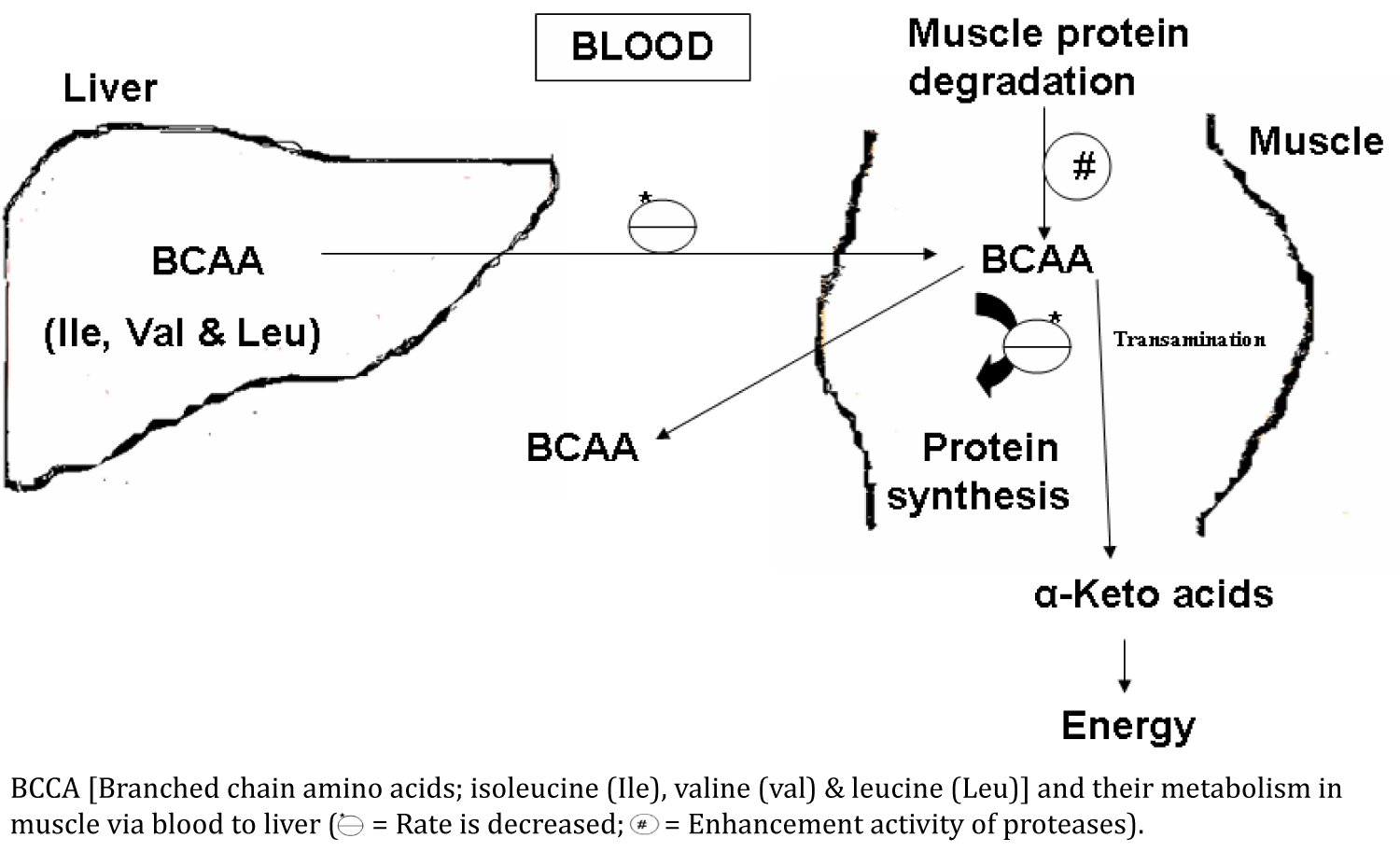
BCAA (Branched Chain Amino Acids): Level of branched chain amino acids (Isoleucine, Leucine and Valine) is reduced in the dystrophic muscle or in patients with the muscular dystrophies. Lower values of branched chain amino acids in the dystrophic muscle could be due to two reasons: (i) Higher activity of cathepsin A, cathepsin B1 and dipeptidyl peptidases in the affected muscle or dystrophic muscle. These enzymes are lysosomal protein hydrolases and responsible for the degradation of protein [54-57]. (ii) Decreased regeneration and increased degeneration of the muscle proteins in dystrophic muscle [2]. Higher activity of proteases also produces these amino acids and these are poured into the blood (Figure 1).
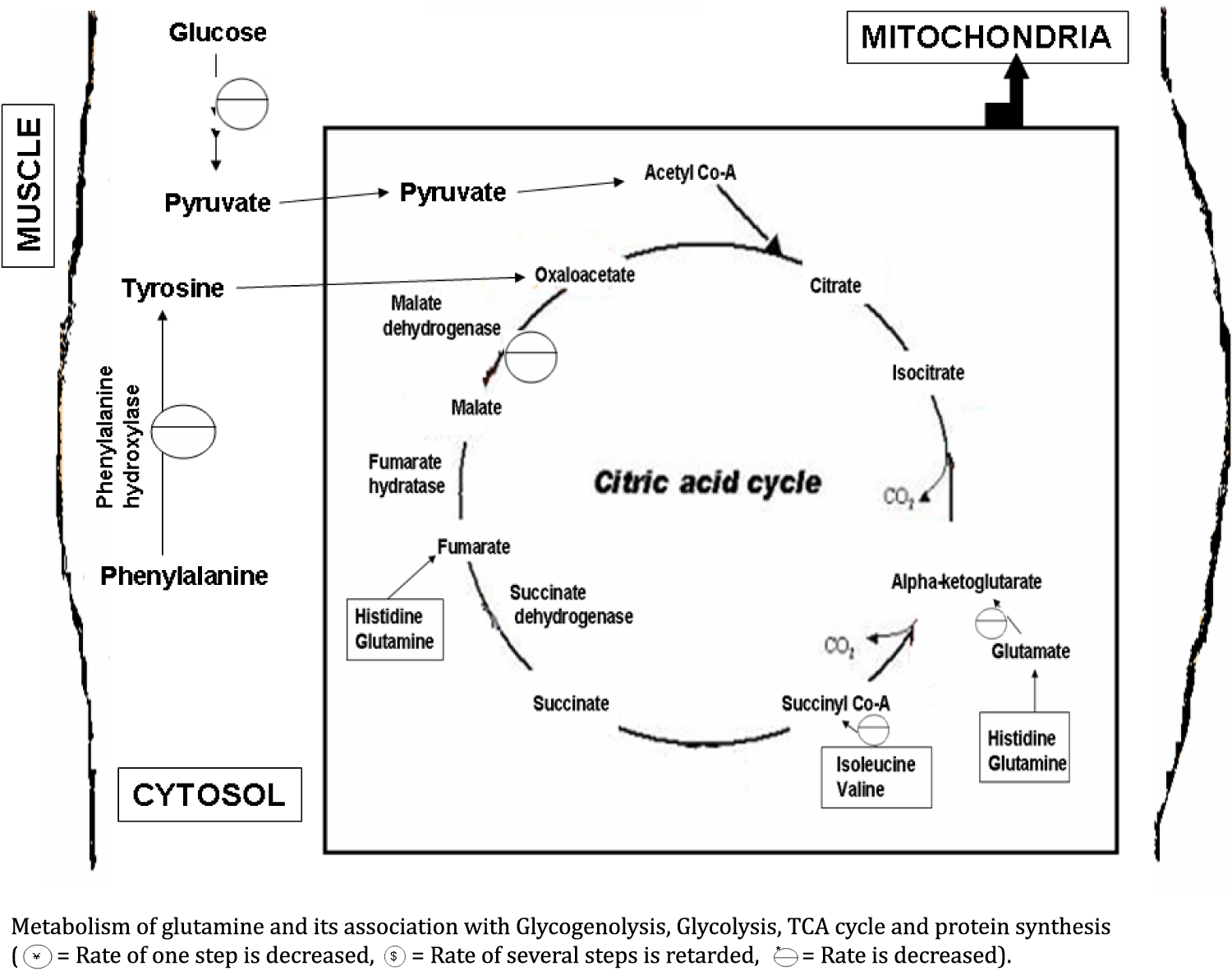
Gln/Glu (Glutamine/Glutamate): Reduced level of Gln/Glu in the dystrophic muscle may occur due to its impaired synthesis [54]. Glutamine is synthesized from alpha-ketoglutarate. Alpha-ketoglutarate is a TCA (Tricarboxylic acid) cycle intermediate. Since, TCA cycle is also linked and depends on the glycolysis [24]. The impaired synthesis is responsible for the slower rate of glycolysis. Glycolytic alteration is occur due to alteration or reduction in the activity of enzymes i.e. aldolase and fructose 1, 6-bisphosphatase. Glycogenolysis in muscle is also impaired due to alteration in the activity of phosphoglucomutase enzyme. So, conversion of glucose-1-phosphate to glucose-6-phosphate is also affected. Glucose-6-phosphate is again linked with glycolysis [35]. Glutamine is a major gluconeogenic precursor, which serves as a fuel for tissues with a high cell turnover rate and plays a essential role in the regulation of protein synthesis [58,59] but in degenerated muscle and the process of lower regeneration required less amount of glutamine which is further responsible for its reduction in dystrophic muscle [54]. Mitochondrial alteration and oxidative stress are responsible for creating a strong impact on muscle degeneration in mouse model of muscular dystrophy [60]. All the above rationales are responsible for lowering the amount of Gln/Glu in dystrophic muscle (Figures 2 & 3).
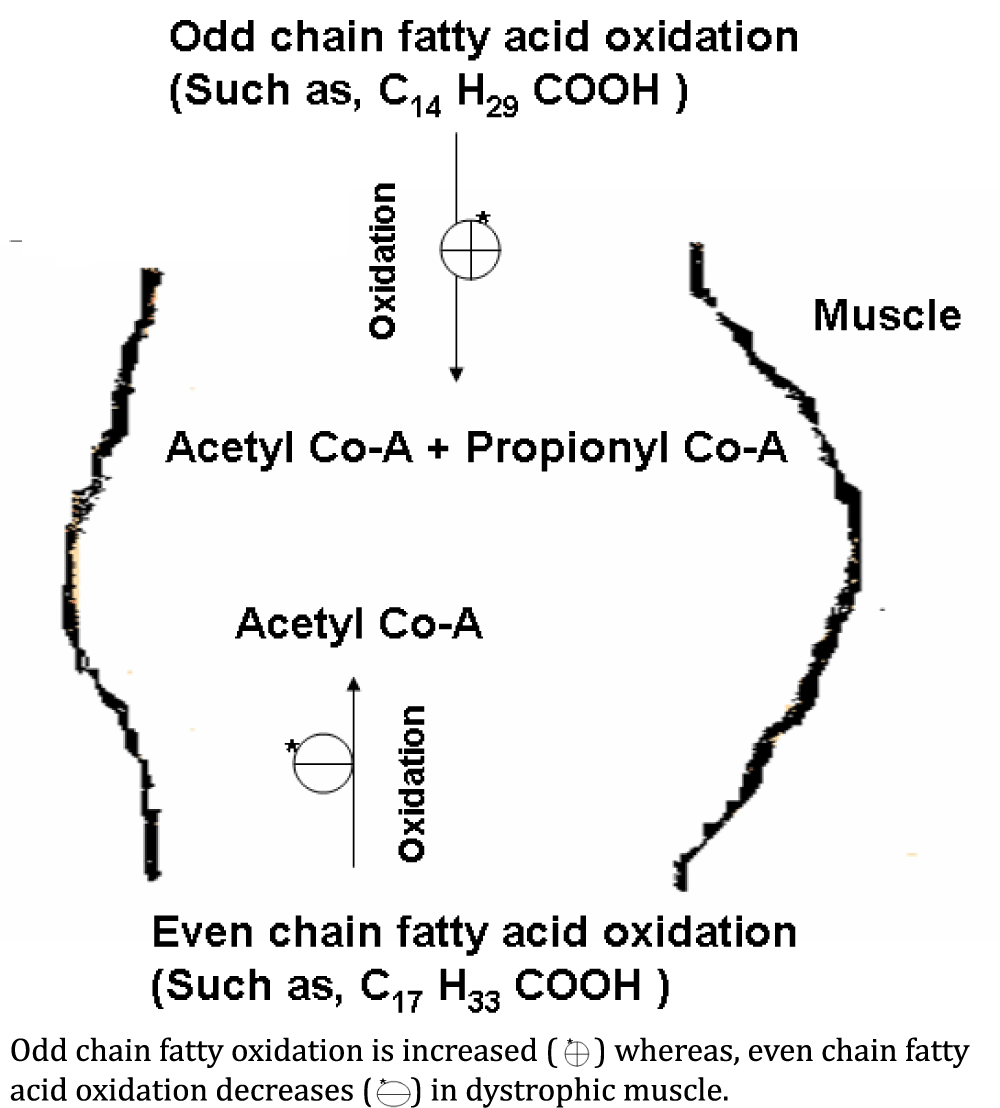
Ace (Acetate): Level of Ace also reduced in the muscle tissue of patients with muscular dystrophies. Fatty acid catabolism is responsible for energy production. The ultimate product of even chain fatty acid oxidation is acetate [24]. Reduced level of acetate in the dystrophic muscle occur due to decrease in the even chain fatty acid oxidation (Figure 4). Carnitine is reduced in dystrophic muscle of DMD patients [61]. Decrease in the quantity of carnitine may affect the transport of fatty acids. Fatty acids are broken down by the repeated pair-wise removal of carbon residues starting at the -COOH end of the fatty acid molecule leading to the formulation of acetate or propionate residue, in even or odd numbered fatty acids [61,62].
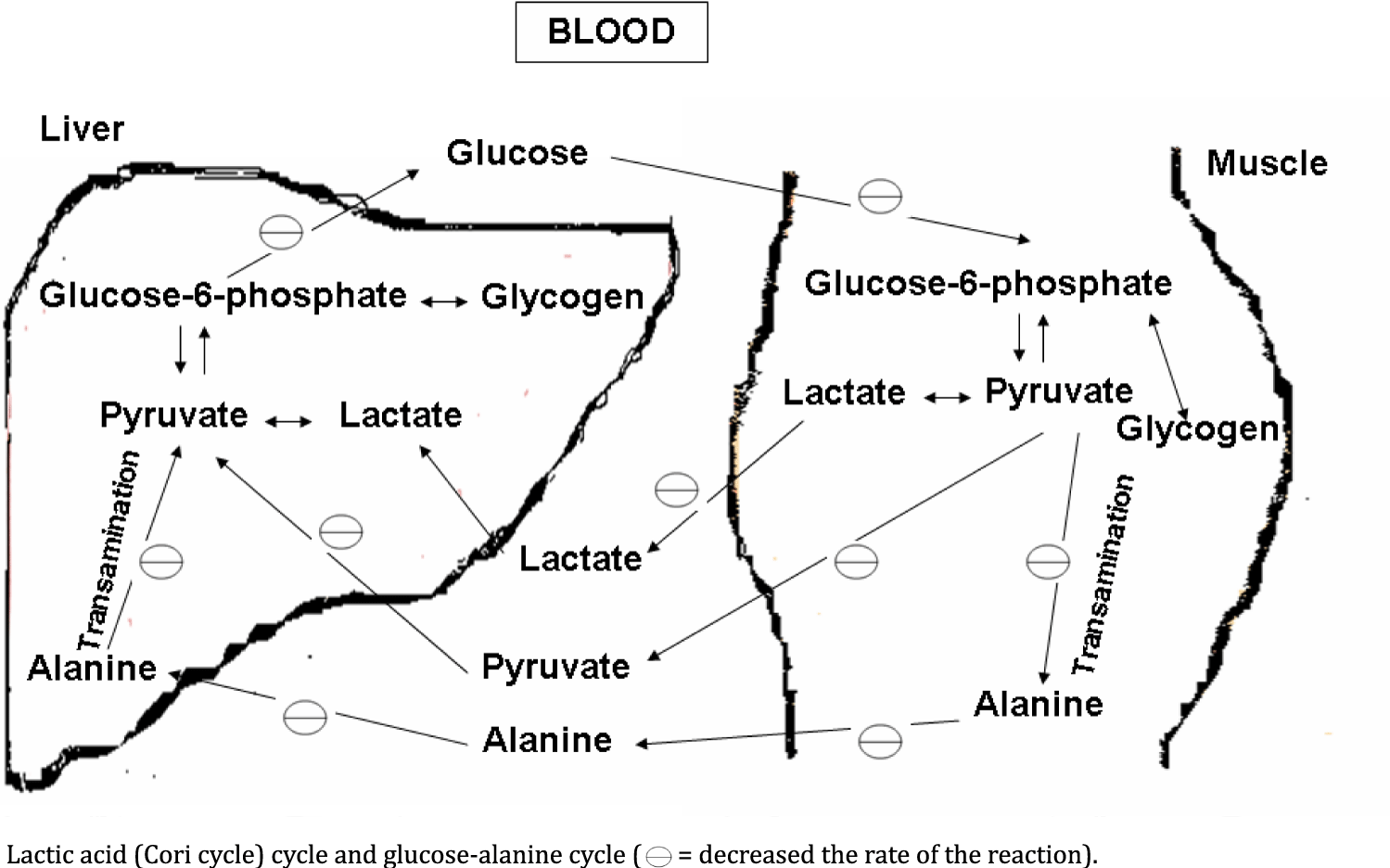
Alanine: Level of alanine is reduced in the muscle tissue of patients with muscular dystrophies, except DMD patients. Alanine, which is usually exported to the liver from muscle, is actually taken up by muscle and used as fuel directly in the muscle without going to the liver first to be converted to glucose which then in turn is shunted to skeletal muscle [24]. Alanine is decreased because the alanine by serving as a direct (oxidized directly in muscle, the alanine is delaminated and decarboxylated to form pyruvate and through the action of pyruvate dehydrogenase enters the Krebs Cycle as acetyl-CoA) and indirect (acting as a substrate for gluconeogenesis, the pyruvate is carboxylated by pyruvate carboxylase and converted into oxaloacete) fuel source for decreasing the need of ketones bodies as fuel [63,64]. In dystrophic muscle, the bioenergetics metabolism (glycolysis, gluconeogenesis and TCA cycle) is impaired due to muscle degeneration and in this way, alanine level is going too reduced (Figures 2 & 3) [54].
Glucose: Glucose is significantly reduces in muscle tissue of the muscular dystrophies, except LGMD-2B patients. In a detailed biochemical study on DMD patients, Nisho, et al. [65] have documented that the glucose concentration was significantly lowered in the skeletal muscle with low creatine kinase activity. Glucose is also found to reduce in muscle of mouse models of Duchenne muscular dystrophy [66]. Decrease in glucose concentration is possibly due to the reduction of bioenergetics of the degenerated muscles, which cannot provide the sufficient supply of gluconeogenic substrates such as alanine, pyruvate and lactate. Alanine is already reduced in the patients with muscular dystrophies. So, reduction in both, glucose and alanine are mutually depending on one another [54] (Figures 2 & 3). Glutamine is a major gluconeogenic precursor and vehicle for interorgan carbon transport in human [58]. Since, glutamine concentration is also reduced in the dystrophic muscle. In this way, less availability of glutamine in dystrophic muscle is also responsible for reduced concentration of glucose in dystrophic muscle.
Histidine: Level of histidine is higher in the muscle tissue of the patients with muscular dystrophies. This could be possibly due to the fact that during regeneration process of muscle protein and requirement of histidine is enhanced. This happens through higher histidine transportation from blood to the muscle tissue. Histidine is also converted to glutamate [24]. This may be possible that rate of the conversion of histidine to glutamate is decreased and in this way, level of histidine is elevated in muscle. Since, Glu/Gln level is also decreased in the dystrophic muscle [54] (Figure 2).
Propionate: Propio nate is completely absent in normal individuals. Occurrence of propionate in the muscular dystrophies showed the possibility of the enhancement of odd chain fatty acids oxidation rate (Figure 4) [25,29,54].
Tyrosine: Absence of tyrosine in the dystrophic muscle may be due to reduction of phenylalanine hydroxylase activity in degenerated muscle. Due to higher degradation and lower regeneration required less protein synthesis, and further requirement of tyrosine is also reduced. In this way, regulatory feedback mechanism of enzymes, conversion of phenylalanine to tyrosine by phenylalanine hydroxylase is decreases (Figure 2) [24,54].
Fumarate: Fumarate level is reduced in the dystrophic muscle [54]. Fumarate level is also found to decrease in golden retriever muscular dystrophy [67]. Cutillo, et al. [46] reported the higher activity of malate dehydrogenase in the blood of patients with muscular dystrophy. Under consideration of this report, activity of malate dehydrogenase should be lowered in the dystrophic muscle tissue due to higher rate of muscle degeneration. Malate dehydrogenase is a TCA cycle enzyme, which is responsible for the conversion of malate to oxaloacetate. Fumarate is normally converted into malate by the enzyme fumarate hydratase [24]. Lower utilization of malate occurs due to lower activity of the malate dehydrogenase. Non-utilized malate accumulates and inhibits the enzyme fumarate hydratase. Such event is responsible for the slower production of malate from fumarate. Further, fumarate accumulation inhibits the conversion of succinate to fumarate via enzyme succinate dehydrogenase. This mechanism is finally accountable for reduction of fumarate concentration. All these metabolic reactions are regulated by feedback mechanisms of enzymes [24,26]. In degenerated muscle, level of Glu/ Gln and BCAA is decreased. Here, Glu/ Gln are contributed in the production of alpha ketoglutarate and fumarate. Alpha ketoglutarate and fumarate are the TCA cycle intermediates [24]. Levels of these intermediates are decreased due to reduction of Glu/ Gln. BCAA amino acids such as valine and isoleucine are produced the succinyl Co-A (one of the TCA cycle intermediate) [24,54]. Production of succinyl Co-A is also impaired due to lower quantity of BCAA. Next, tyrosine is contributed in the production of oxaloacetate [2,26]. No availability of tyrosine in dystrophic muscle is responsible for reducing the quantity of oxaloacetate. This is further responsible for slower the rate of TCA cycle because oxaloacetate and acetyl Co-A produced the first product of TCA cycle i.e. citrate, which is required for continuation of TCA cycle. Impairment of bioenergetics metabolism (such as, glycolysis) is also responsible for slower the rate of TCA cycle [54]. So, a reduction in the TCA cycle rate is responsible for the reduced quantity of fumarate in dystrophic muscle (Figures 1, 2 & 3).
Metabolic disturbance is observed in muscular dystrophy patients (DMD, BMD, FSHD and LGMD-2B). Alteration in the level of metabolites (BCAA, Glu/ Gln, Ace, alanine, glucose, histidine, propionate, tyrosine and fumarate) in dystrophic muscle reflects the alteration in the activity of enzymes. Collectively, these observations suggest that there is alteration in the rate of glycolysis, TCA cycle, fatty acid oxidation, gluconeogenesis pathway and protein metabolism (catabolism & anabolism) in the muscular dystrophy patients. Metabolic disturbance, further provide the explanation about the pathophysiology of muscular dystrophy.
Authors wish to thank Council of Scientific & Industrial Research [No.13 (8660-A)/2013-Pool] and University Grant Commission [No.F.4-2/2006 (BSR)/ 13-194/2008(BSR)], Government of India, for their generous financial support. Authors sincerely thanks to staff of neurophysiology laboratory for providing help in collecting electromyographical data. Senior residents are acknowledged for providing clinical data and collection of tissue specimens. Professor Rajkumar (Department of neurosurgery, SGPGIMS, Lucknow) is acknowledged for providing the normal muscle specimens.
- Anthony Amato, James A. Russell. Neuromuscular Disorders. Published by McGraw-Hill Professional; 2008.
- Anthony H, Schapira V, Robert C. Griggs. Muscle Diseases. Published by Butterworth-Heinemann. 1999.
- Alan Emery. Muscular Dystrophy: The Facts. Published by Oxford University Press. 2000.
- Alan EH Emery. Neuromuscular Disorders: Clinical and Molecular Genetics. Wiley. 1998.
- Centers for Disease Control and Prevention (CDC). Prevalence of Duchenne/Becker muscular dystrophy among males aged 5-24 years - four states, 2007. MMWR Morb Mortal Wkly Rep. 2009 Oct 16;58(40):1119-22. PMID: 19834452.
- Nowak KJ, Davies KE. Duchenne muscular dystrophy and dystrophin: Pathogenesis and opportunities for treatment. EMBO Rep. 2004 Sep;5(9):872-6. doi: 10.1038/sj.embor.7400221. PMID: 15470384; PMCID: PMC1299132.
- Jones H, De Vivo DC, Darras BT. Neuromuscular disorders of infancy, childhood and adolescence. A clinician's approach. Oxford: Butterworth-Heinemann; 2003.
- Chenard AA, Becane HM, Tertrain F, de Kermadec JM, Weiss YA. Ventricular arrhythmia in Duchenne muscular dystrophy: prevalence, significance and prognosis. Neuromuscul Disord. 1993 May;3(3):201-6. doi: 10.1016/0960-8966(93)90060-w. PMID: 7691292.
- Yiu EM, Kornberg AJ. Duchenne muscular dystrophy. Neurol India. 2008 Jul-Sep;56(3):236-47. doi: 10.4103/0028-3886.43441. PMID: 18974549.
- Lim KRQ, Nguyen Q, Yokota T. DUX4 Signalling in the Pathogenesis of Facioscapulohumeral Muscular Dystrophy. Int J Mol Sci. 2020 Jan 22;21(3):729. doi: 10.3390/ijms21030729. PMID: 31979100; PMCID: PMC7037115.
- Wang LH, Tawil R. Facioscapulohumeral Dystrophy. Curr Neurol Neurosci Rep. 2016 Jul;16(7):66. doi: 10.1007/s11910-016-0667-0. PMID: 27215221.
- Tawil R, van der Maarel SM, Tapscott SJ. Facioscapulohumeral dystrophy: the path to consensus on pathophysiology. Skelet Muscle. 2014 Jun 10;4:12. doi: 10.1186/2044-5040-4-12. PMID: 24940479; PMCID: PMC4060068.
- Deenen JC, Arnts H, van der Maarel SM, Padberg GW, Verschuuren JJ, Bakker E, Weinreich SS, Verbeek AL, van Engelen BG. Population-based incidence and prevalence of facioscapulohumeral dystrophy. Neurology. 2014 Sep 16;83(12):1056-9. doi: 10.1212/WNL.0000000000000797. Epub 2014 Aug 13. PMID: 25122204; PMCID: PMC4166358.
- Tawil R. Facioscapulohumeral muscular dystrophy. Handb Clin Neurol. 2018;148:541-548. doi: 10.1016/B978-0-444-64076-5.00035-1. PMID: 29478599.
- Tawil R, Van Der Maarel SM. Facioscapulohumeral muscular dystrophy. Muscle Nerve. 2006 Jul;34(1):1-15. doi: 10.1002/mus.20522. PMID: 16508966.
- Handa V, Mital A, Gupta M, Goyle S. Deficiency of the 50 kDa dystrophin-associated-glycoprotein (adhalin) in an Indian autosomal recessive limb girdle muscular dystrophy patient: Immunochemical analysis and clinical aspects. Neurol India. 2001 Mar;49(1):19-24. PMID: 11303236.
- Khadilkar SV, Singh RK. Current concepts in Limb girdle muscular dystrophy. Does hip adductor weakness mark the Indian phenotype? Reviews in Neurology. 2000:34-43.
- Khadilkar SV, Faldu HD, Patil SB, Singh R. Limb-girdle Muscular Dystrophies in India: A Review. Ann Indian Acad Neurol. 2017 Apr-Jun;20(2):87-95. doi: 10.4103/aian.AIAN_81_17. PMID: 28615891; PMCID: PMC5470147.
- Murphy AP, Straub V. The Classification, Natural History and Treatment of the Limb Girdle Muscular Dystrophies. J Neuromuscul Dis. 2015 Jul 22;2(s2):S7-S19. doi: 10.3233/JND-150105. PMID: 27858764; PMCID: PMC5271430.
- Patel NJ, Van Dyke KW, Espinoza LR. Limb-Girdle Muscular Dystrophy 2B and Miyoshi Presentations of Dysferlinopathy. Am J Med Sci. 2017 May;353(5):484-491. doi: 10.1016/j.amjms.2016.05.024. Epub 2016 May 30. PMID: 28502335.
- Lehninger AL, Nelson DL, Cox MM. Lehninger Principles of Biochemistry. Fourth ed. New York: W.H. Freeman; 2005.
- Beckonert O, Keun HC, Ebbels T M D, Bundy JG, Holmes E, Lindon, J C, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMRspectroscopy of urine, plasma, serum and tissue extracts. Nature Protocols. 2007;2:2692–2703. https://go.nature.com/385fmfw
- Fillet M, Frédérich M. The emergence of metabolomics as a key discipline in the drug discovery process. Drug Discov Today Technol. 2015 Jun;13:19-24. doi: 10.1016/j.ddtec.2015.01.006. Epub 2015 Feb 28. PMID: 26190679.
- Murray KR, Granner DK, Mayes PA, Rodwell VW. Harper's illustrated biochemistry. Lange Medical Books/McGraw-Hill. Twenty-sixth edition. 2000.
- Poortmans JR. Principles of exercise biochemistry. Third edition. Karger publisher. 2000.
- Gilbert HF.Basic concept in Biochemistry. A Student survival guide. Second Edition. McGraw-Hill, Health Professions Division. New York. 2000.
- Harold FM. The vital force: A study in bioenergetics. H. Freeman and Company, NewYork. 1986. https://bit.ly/3gMPucg
- Randle PJ. Metabolic fuel selection: general integration at the whole-body level. Proc Nutr Soc. 1995 Mar;54(1):317-27. doi: 10.1079/pns19950057. PMID: 7568263.
- Montoya HJ, Kemper HCG, Saris WHM and Washburn RA, eds. Measuring physical activity and energy expenditure. Champaign, IL: Human kinetics, 1996.
- Cerny FJ & Burton HW. Exercise physiology for health care professionals. Champaign, IL: Human kinetics, 2003.
- Nagy B & Samaha FJ.Physiology of normal and disease muscle.In Frolich, ED, editor: Pathophysiology, Philadelphia, JB Lippincott, 1983.
- Raymond Delacy Adams, Derek Denny-Brown, Carl M. Pearson. Diseases of Muscle: A Study in Pathology. Published by Harper & Row. 1962.
- Emery AE. The muscular dystrophies. Lancet. 2002 Feb 23;359(9307):687-695. doi: 10.1016/S0140-6736(02)07815-7. PMID: 11879882.
- Lindsay A, Chamberlain CM, Witthuhn BA, Lowe DA, Ervasti JM. Dystrophinopathy-associated dysfunction of Krebs cycle metabolism. Hum Mol Genet. 2019 Mar 15;28(6):942-951. doi: 10.1093/hmg/ddy404. PMID: 30476171; PMCID: PMC6400043.
- Demos J, Dreyfus Jc, Schapira F, Schapira G. Activités enzymatiques du muscle humain; recherches sur la biochimie comparée de l'homme normal et myopathique, et du rat [Enzyme activity in human muscles; research on comparative biochemistry in normal and myopathic men, and in rats]. Clin Chim Acta. 1956 Sep-Oct;1(5):434-49. French. doi: 10.1016/0009-8981(56)90016-x. PMID: 13396986.
- Di Mauro S, Angelini C, Catani C. Enzymes of the glycogen cycle and glycolysis in various human neuromuscular disorders. J Neurol Neurosurg Psychiatry. 1967 Oct;30(5):411-5. doi: 10.1136/jnnp.30.5.411. PMID: 4228900; PMCID: PMC496216.
- VIGNOS PJ Jr, LEFKOWITZ M. A biochemical study of certain skeletal muscle constituents in human progressive muscular dystrophy. J Clin Invest. 1959 Jun;38(6):873-81. doi: 10.1172/JCI103869. PMID: 13654523; PMCID: PMC293236.
- Vretou-Jockers E, Vassilopoulos D. Skeletal muscle CK-B activity in neurogenic muscular atrophies. Journal of Neurology. 1989;236:184-287. https://bit.ly/3qUHpHm
- Heyck H, Laudahn G, Luders CJ. Fermentaktivitatsbestimmungen in der gesunden, menschlichen Muskulatur und bei Myopathien. II Mitteilung. Enzymaktivitatsveranderungen im Musket bei Dystrophia musculorum progressiva. Klinische Wochenschrift. 1963;41: 500.
- Hooft G, de Laey P, Lambert Y. Etude comparative de I' activite enzymatique du tissu musculaire de I'enfant normal et d'enfants atteints de dystrophie musculaire progressive aux différent stades de la maladie. Revue Française d ‘Etudes Cliniques et Biologiques. 1966;11: 510.
- Kleine TO, Chlond H. Enzymmuster gesunder skelettherz und glatter Muskelatur des Menschen sowie ihrer pathologischen Veranderungen. Mit besonderer Berucksichtigung der progressiven muskeldystrophie (Erb). Clin Chim Acta. 1967;15: 19.
- Ge Y, Molloy MP, Chamberlain JS, Andrews PC. Proteomic analysis of mdx skeletal muscle: Great reduction of adenylate kinase 1 expression and enzymatic activity. Proteomics. 2003 Oct;3(10):1895-903. doi: 10.1002/pmic.200300561. PMID: 14625851.
- Kar NC, Pearson CM, Verity MA. Muscle fructose 1,6-diphosphatase deficiency associated with an atypical central core disease. J Neurol Sci. 1980 Nov;48(2):243-56. doi: 10.1016/0022-510x(80)90204-x. PMID: 6253603.
- Kar NC, Pearson CM. Muscle adenylic acid deaminase activity. Selective decrease in early-onset Duchenne muscular dystrophy. Neurology. 1973 May;23(5):478-82. doi: 10.1212/wnl.23.5.478. PMID: 4735464.
- Wagner KR, Kauffman FC, Max SR. The pentose phosphate pathway in regenerating skeletal muscle. Biochem J. 1978 Jan 15;170(1):17-22. doi: 10.1042/bj1700017. PMID: 629775; PMCID: PMC1183856.
- McCaman MW. Dehydrogenase Activities in Dystrophic Mice. Science. 1960 Sep 2;132(3427):621-622. doi: 10.1126/science.132.3427.621. PMID: 17842210.
- Cutillo S, Colletta A, Lupi L, Canani MB. The relationship between lactic and α-hydroxybutyric dehydrogenase of the serum in children with progressive muscular dystrophy. Bollettino della Societa Italiana di Biologia Sperimentale. 1962;38: 691.
- Pearson CM, Kar NC. Muscle breakdown and lysosomal activation (biochemistry). Ann N Y Acad Sci. 1979;317:465-77. doi: 10.1111/j.1749-6632.1979.tb56562.x. PMID: 289325.
- Doran P, Dowling P, Donoghue P, Buffini M, Ohlendieck K. Reduced expression of regucalcin in young and aged mdx diaphragm indicates abnormal cytosolic calcium handling in dystrophin-deficient muscle. Biochim Biophys Acta. 2006 Apr;1764(4):773-85. doi: 10.1016/j.bbapap.2006.01.007. Epub 2006 Jan 31. PMID: 16483859.
- Emwas AH, Roy R, McKay RT, Tenori L, Saccenti E, Gowda GAN, Raftery D, Alahmari F, Jaremko L, Jaremko M, Wishart DS. NMR Spectroscopy for Metabolomics Research. Metabolites. 2019 Jun 27;9(7):123. doi: 10.3390/metabo9070123. PMID: 31252628; PMCID: PMC6680826.
- Venkatasubramanian PN, Arús C, Bárány M. Two-dimensional proton magnetic resonance of human muscle extracts. Clin Physiol Biochem. 1986;4(5):285-92. PMID: 3022979.
- Sharma U, Atri S, Sharma MC, Sarkar C, Jagannathan NR. Biochemical characterization of muscle tissue of limb girdle muscular dystrophy: An 1H and 13C NMR study. NMR Biomed. 2003 Jun;16(4):213-23. doi: 10.1002/nbm.832. PMID: 14558119.
- Sharma U, Atri S, Sharma MC, Sarkar C, Jagannathan NR. Skeletal muscle metabolism in Duchenne Muscular Dystrophy (DMD): An In vitro proton NMR spectroscopy study. Magn Reson Imaging. 2003 Feb;21(2):145-53. doi: 10.1016/s0730-725x(02)00646-x. PMID: 12670601.
- Srivastava NK, Yadav R, Mukherjee S, Sinha N. Perturbation of muscle metabolism in patients with muscular dystrophy in early or acute phase of disease: In vitro, high resolution NMR spectroscopy based analysis. Clin Chim Acta. 2018 Mar;478:171-181. doi: 10.1016/j.cca.2017.12.036. Epub 2017 Dec 24. PMID: 29278724.
- Kar NC, Pearson CM. Arylamidase and cathepsin-A activity of normal and dystrophic human muscle. Proc Soc Exp Biol Med. 1976 Mar;151(3):583-6. doi: 10.3181/00379727-151-39264. PMID: 3799.
- Kar NC, Pearson CM. Early elevation of cathepsin B1 in human muscle disease. Biochem Med. 1977 Aug;18(1):126-9. doi: 10.1016/0006-2944(77)90059-x. PMID: 901428.
- Kar NC, Pearson CM. Dipeptidyl peptidases in human muscle disease. Clin Chim Acta. 1978 Jan 2;82(1-2):185-92. doi: 10.1016/0009-8981(78)90042-6. PMID: 618680.
- Nurjhan N, Bucci A, Perriello G, Stumvoll M, Dailey G, Bier DM, Toft I, Jenssen TG, Gerich JE. Glutamine: A major gluconeogenic precursor and vehicle for interorgan carbon transport in man. J Clin Invest. 1995 Jan;95(1):272-277. doi: 10.1172/JCI117651. PMID: 7814625; PMCID: PMC295425.
- Biolo G, Zorat F, Antonione R, Ciocchi B. Muscle glutamine depletion in the intensive care unit. Int J Biochem Cell Biol. 2005 Oct;37(10):2169-2179. doi: 10.1016/j.biocel.2005.05.001. PMID: 16084750.
- Ramadasan-Nair R, Gayathri N, Mishra S, Sunitha B, Mythri RB, Nalini A, Subbannayya Y, Harsha HC, Kolthur-Seetharam U, Srinivas Bharath MM. Mitochondrial alterations and oxidative stress in an acute transient mouse model of muscle degeneration: implications for muscular dystrophy and related muscle pathologies. J Biol Chem. 2014 Jan 3;289(1):485-509. doi: 10.1074/jbc.M113.493270. Epub 2013 Nov 12. PMID: 24220031; PMCID: PMC3879571.
- Green DE. Fatty acid oxidation in soluble systems of animal tissues. Biol Rev. 1954;29:330-366. doi: 10.1111/j.1469-185X.1954.tb00599.x
- LYNEN F, OCHOA S. Enzymes of fatty acid metabolism. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):299-314. doi: 10.1016/0006-3002(53)90149-8. PMID: 13115439.
- Koeslag JH, Levinrad LI, Lochner JD, Sive AA. Post-exercise ketosis in post-prandial exercise: effect of glucose and alanine ingestion in humans. J Physiol. 1985 Jan;358:395-403. doi: 10.1113/jphysiol.1985.sp015557. PMID: 3884775; PMCID: PMC1193348.
- Koeslag JH, Noakes TD, Sloan AW. The effects of alanine, glucose and starch ingestion on the ketosis produced by exercise and by starvation. J Physiol. 1982 Apr;325:363-376. doi: 10.1113/jphysiol.1982.sp014155. PMID: 7050344; PMCID: PMC1251399.
- Nishio H, Wada H, Matsuo T, Horikawa H, Takahashi K, Nakajima T, Matsuo M, Nakamura H. Glucose, free fatty acid and ketone body metabolism in Duchenne muscular dystrophy. Brain Dev. 1990;12(4):390-402. doi: 10.1016/s0387-7604(12)80071-4. PMID: 2240459.
- Griffin JL, Sang E, Evens T, Davies K, Clarke K. Metabolic profiles of dystrophin and utrophin expression in mouse models of Duchenne muscular dystrophy. FEBS Lett. 2002 Oct 23;530(1-3):109-16. doi: 10.1016/s0014-5793(02)03437-3. PMID: 12387876.
- Abdullah M, Kornegay JN, Honcoop A, Parry TL, Balog-Alvarez CJ, O'Neal SK, Bain JR, Muehlbauer MJ, Newgard CB, Patterson C, Willis MS. Non-Targeted Metabolomics Analysis of Golden Retriever Muscular Dystrophy-Affected Muscles Reveals Alterations in Arginine and Proline Metabolism, and Elevations in Glutamic and Oleic Acid In vivo. Metabolites. 2017 Jul 29;7(3):38. doi: 10.3390/metabo7030038. PMID: 28758940; PMCID: PMC5618323.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.