> Biology Group. 2021 Mar 03;2(3):104-113. doi: 10.37871/jbres1198.
Monitoring of Group A Rotavirus Strains Circulating in the Environment and Among Children with Acute Gastroenteritis
Abderrahim Hatib, Najwa Hassou and Moulay Mustapha Ennaji*
Laboratory of Virology, Microbiology, Quality and Biotechnologies/ Eco Toxicology and Biodiversity
Faculty of Science and Technology Mohammédia University Hassan II of Casablanca Morocco
PB 146 YasminaMohammédia, (20650) Morocco
- Rotavirus
- Gastroenteritis
- Environment
- Circulating strains
- Emergence
Rotavirus A is the causative agent of 90% of acute gastroenteritis in children under 5, which kills 1 to 3 million children per year. Their strong resistance in the environment, their inter-species transmission as well as their power of genetic recombination can give rise to new reasserting that may be harmful to public health.
The simultaneous search for the presence of rotavirus A in different environmental and clinical biotopes and matrices as well as the monitoring of the seasonal evolution of episodes is of major importance. At cost, genetic monitoring of rotaviruses shows a correlation between the presence of different genotypes of RVA in the environment and the rate of morbidity, Hence the need to monitor the emergence of new circulating strains with a view to integrating them into routine immunization programmes appropriate for each region in order to limit the spread of the disease.
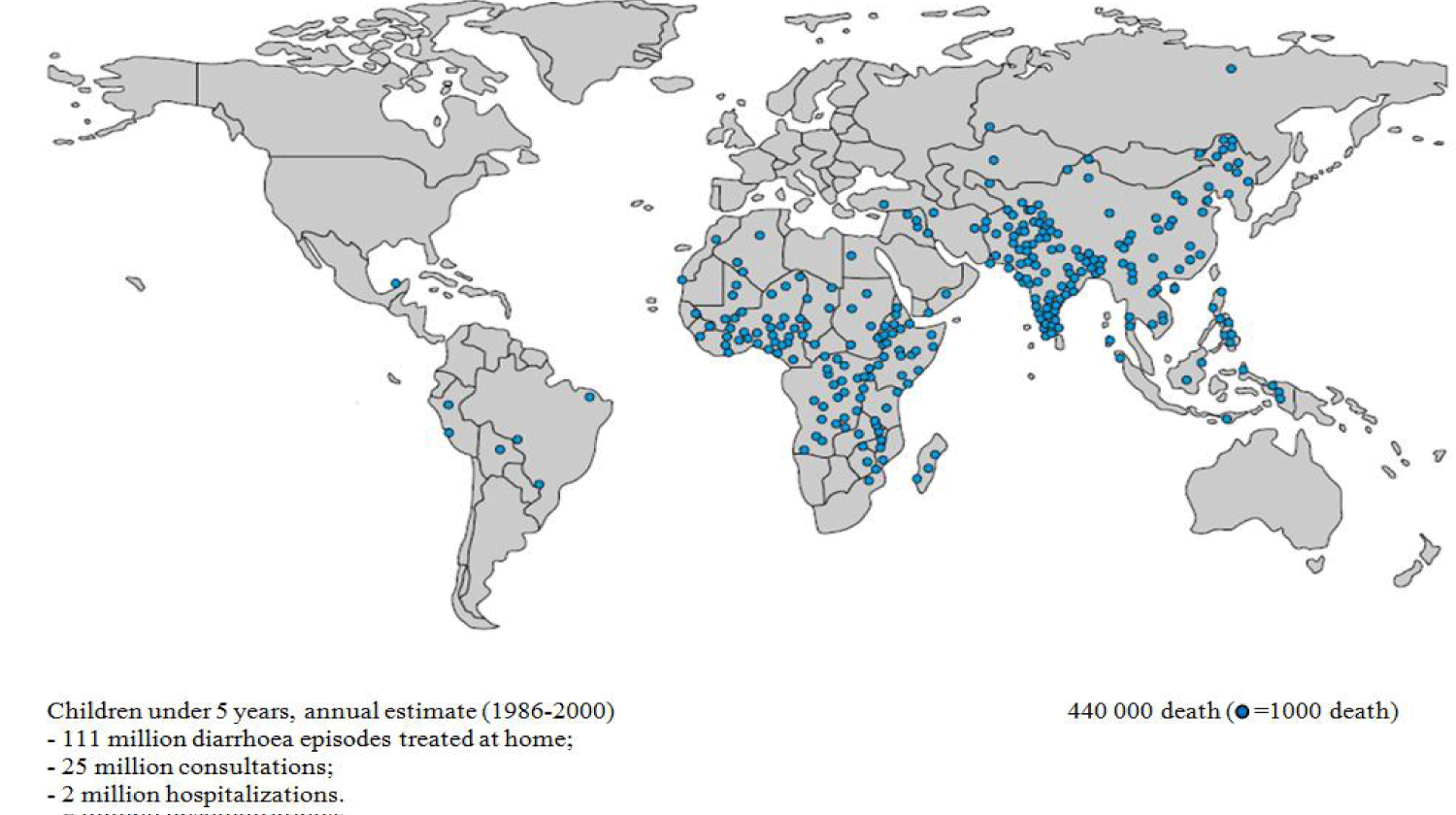
Human enteric viruses pose a major public health risk. This risk is linked to human-to-human, zoonotic, food and water transmission. Infectious agents transported by humans are sometimes asymptomatic or in the incubation phase, those transported by animals, water and food pass as unnoticed also [1]. Epidemics of Acute Gastroenteritis (AGE) are often linked to adenoviruses, noroviruses and rotaviruses [2-5]. Rotaviruses (RVs) are the main causative agents of AGE in children under 5 worldwide [6,7]. They are responsible for high infant mortality, death of 1 to 3 million including more than 800,000 in Africa [8,9] and almost 220,000 hospitalizations per year in industrialized countries, [10]. The prevalence is 35-60% of acute and severe diarrhea, almost every child might went through at least one episode of Rotavirus Gastroenteritis (RVGE) [11], the highest prevalence corresponds to the age range 0-11 months [6,12]. Developing countries [13-15], sub-Saharan Africa and the Indian subcontinent are the most affected [16] (Figure 1). According to surveillance data in 2013 and 2014, there were 215,000 deaths from rotavirus in children under 5 years worldwide, 56% of deaths were reported in sub-Saharan Africa and 22% in India alone [17].
In the same context, faecal contamination of environmental water can play an important role in the transmission and epidemiology of enteric viruses [18]. Indeed, water-borne epidemics pose a significant threat to human health worldwide, despite the relevant advances in water and wastewater treatment technologies, particularly in developing countries where access to drinking water is very limited in favour of untreated surface water and where the appropriate outbreak notification and monitoring system is still deficient.
Epidemiology of rotavirus gastroenteritis
RVs were first described in 1973 by Bishop, et al. [19], although most enteric viruses were discovered in the 1950s and 1960s.They are ubiquitous viruses, very widespread in animal kingdom where viral strains differ from one animal species to another. However, animal-human transmission remains possible and would give new strains in case of co-infection.
RVs can cause diarrhea which is one of the most deadly childhood diseases in Africa [20]. RV infections appear in winter as epidemics on a South-West-North-East gradient in Europe, and on a West-East gradient in the United States [21,22]. In 2013, despite the approval and availability of several RV vaccines on the market, RV caused about 215,000 deaths worldwide [17]. In 2017, New South Wales experienced a major outbreak of rotavirus gastroenteritis [23]. To help control the burden of severe rotavirus diseases, the World Health Organization (WHO) has recommended rotavirus vaccines for routine immunization of all children worldwide [24,25].
Clinical trials of the two RVs vaccines (RotaTeq, Merck and Rotarix, GSK Biologicals) recommended by the WHO for global use since 2009 have successfully demonstrated the effectiveness of these vaccines in a wide range of countries [26-30]. Based on WHO recommendations, the Moroccan Ministry of Health introduced the single-strain rotavirus vaccine, Rotarix, into its national vaccination program in 2010 to reduce the high rate of rotavirus disease in Morocco [31].
Source of rotavirus contamination and transmission
The transmission of RV is faecal-oral, by direct contact during nosocomial infections in health units. Vomits aerosols also ensure human-to-human transmission of the virus. Man is therefore a natural reservoir of human rotavirus that contaminates its environment through wastewater [32]. RVs are excreted in very large numbers in stool, with more than 1011 particles per gram. Faecal contamination of vegetable and fish products such as molluscs and crustaceans by viruses has been reported with a load of between 102 and 104 viral particles per gram of tissue [33-36]. This mode of transmission to healthy people is ensured by the consumption of raw or undercooked seafood products.
Due to the resistance to protein degradation conferred by the three-layer structure of the protein capsid, ribonucleic acid from rotavirus may remain infectious. RVs are highly resistant to physical inactivation, but remain sensitive to chlorinated derivatives (Bleach) and 70% diluted ethanol. Hence, the virus is highly resistant in the environment and can keep its infectivity for weeks outside the human body [37]. And for several days, up to two months in water at 20°C, in groundwater sources for several months and several hours on the hands [38]. In addition, rotaviruses are highly contagious, so even small amounts level (10 to 100 particles) of RVs are capable of infecting humans [37]. Abundant diarrhoea promotes the secretion and spread of new viruses in the environment, thereby amplifying the spread of outbreaks.
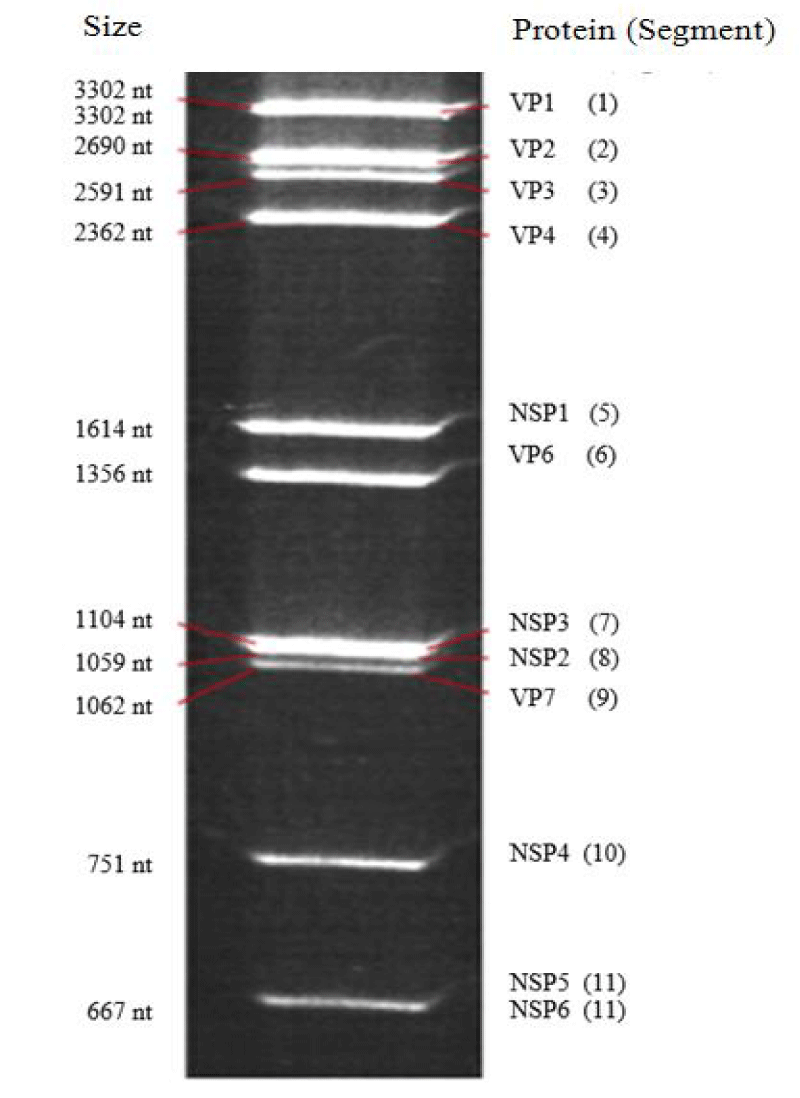
The RVs are non-enveloped viruses, whose mature, wheel-shaped virus particles are about 75 nm in diameter, which can increase up to 100 nm with spicules. Its double-stranded Ribonucleic Acid (RNA) genome contains 11 segments (Figure 2) encoding six structural proteins (VP) and six Non-Structural Proteins (NSP), the size of these segments ranges from approximately 667 (Segment 11) to 3 302 base pairs (Segment 1) [39]. One RNA segment codes two proteins, while the other ten segments code one protein each [40]. The capsid is icosahedral with three concentric layers of a protein nature, the innermost VP2 encoded by segment 2, contains the enzymes needed for the viral replication, the outer layer consists of 780 molecules of glycoprotein VP7 encoded by segment 9. This outer layer is surmounted by spicules of a protein nature VP4 (120 molecules) encoded by segment 4 [41].These last two proteins have antigenic activity in particular VP7 defining serotype G, and VP4 defining serotype P, both inducing neutralizing antibodies in the host, these 2 proteins define the viral serotype and appear to be a major target of vaccination. The intermediate protein layer VP6 encoded by segment 6 whose conserved sequences at the 5’ and 3’ ends of the VP6 gene are specific to each rotavirus group (A - G). The VP1 and VP3 proteins, encoded by segments 1 and 3 respectively, are associated with the genome; their enzymatic activity is responsible for the infectivity of the virion. The virulence and pathogenesis of RV diarrhoea is closely related to non-structural NPS proteins in particular the NPS4 encoded by segment 10, which plays the role of intestinal toxin.
Glycoprotein VP7, and protein VP4 have antigenic properties involved in anti-RV immunity. They are neutralizing antibody targets, which allow the determination of genotypes G and P respectively. VP4 and VP7 proteins in the outer layer of rotavirus particles induce neutralizing antibodies [42]. However, some VP6-oriented antibodies [43,44], and enterotoxin NSP4 [45,46] may also provide effective protection against rotaviruses.
The strong tropism of RVs for mature enterocytes of the small intestine induces infection and lesion of the villi of the intestinal brush border inducing severe diarrhoea responsible for morbidity. Viral replication usually occurs in enterocytes, but in immunocompromised children, the infection can also affect the liver and kidneys. VP7 and VP4 proteins play a specific role in the early stages of the viral cycle by expressing the antigenic properties of the virus [47]. This tissue tropism can be inhibited by neutralizing antibodies that prevent the virus from physically attaching to the cell receptor or stabilize the outer layer, thereby preventing the virus from entering the enterocyte and detaching the capsid [48].
Under the action of proteolytic enzymes, the VP4 protein from the capsid cleaves and the cell membrane becomes permeable which facilitates the penetration of the virus into the cytoplasm, the Rotavirus-specific VP1 polymerase, is activated following partial decapsidation and begins transcription of viral RNA to RNA-. The 11 newly formed RNA+ segments assemble with the VP1, VP2, VP3, and VP6 proteins in cytoplasmic inclusions (Viroplasm) wich bind to the NSP4 protein at the endoplasmic reticulum membrane, which provides them with a transient envelope by budding. Subsequently, the VP4 and VP7 proteins attach to the bud which loses its envelope and the release of the mature viral particle by cell lysis [49] or by destabilization of the brush border [50].
Human RVs can be grown on a wide variety of cells. MA104 monkey kidney cells represent the most classic line in which culture lasts 10-12 hours at 37°C [51] but this diagnostic technique remains difficult compared to animal RV which makes it not suitable for routine amplification technique.
Diversity of rotaviruses and risk of emergence of new strains
RVs belong to the family Reoviridae, the Rotavirus genus consists of 7 groups (A to G), these groups are identified by the antigenic determinant of the internal Protein of the Capsid (VP6). Rotaviruses infect several species where humans are susceptible to groups A, B and C, while rotaviruses from groups D to G have only been found in animals. Group A is responsible for the majority of AGEs in young children. Although rare, group B rotavirus causes sometimes severe epidemics in China [52], while Rotavirus C remains the cause of sporadic cases of intrafamilial epidemics.
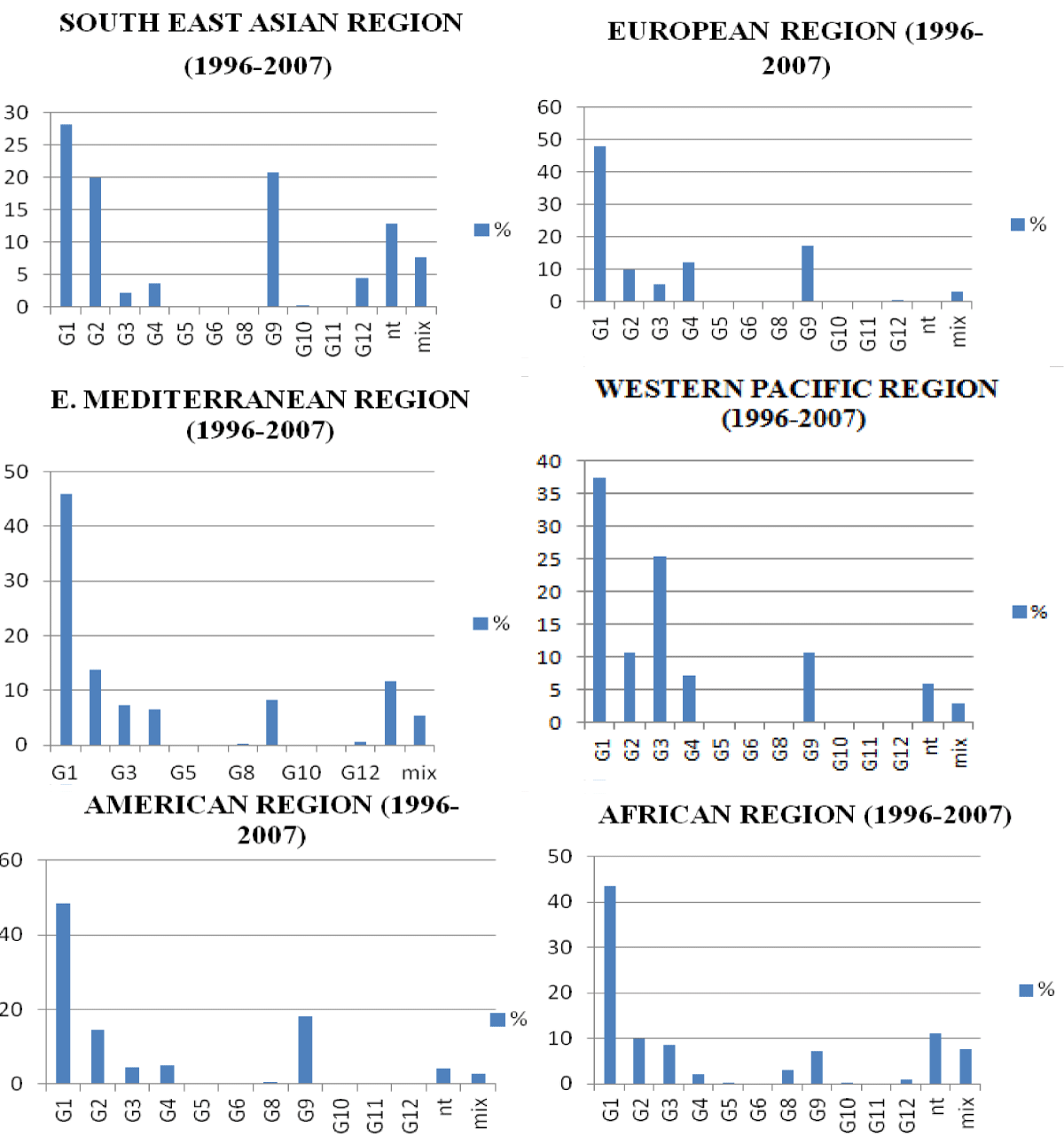
There are currently 32 G and 47 P genotypes in Rotavirus group A [53], of which 12 G and 15 P have been reported to cause GEA in humans [54,55]. However 90% of clinically relevant genotypes characterized globally correspond to genotypes G1, G2, G3, G4 and G9 [56]. Other genotypes occur and rarely emerge in areas such as the G5 in South America, the G8 in sub-Saharan Africa and the G12 in south-eastern Asia. Genotype G5 circulating in Brazil since 1982 has been found to be of porcine origin [57], and is often combined with human genotype P[8]. This same G5 genotype was also characterized in Cameroon in 2000 [58] but with phylogenetic characteristics closer to the porcine strain found in Brazil. The G8 and G10 genotypes are known through human-to-human transmission in Africa and India respectively, while the G6 genotype is common in cattle. The G8 genotype encountered in Malaoui in 1977 and currently in Australia is often associated with genotypes P[4], P[6] or P[8] [59,60].
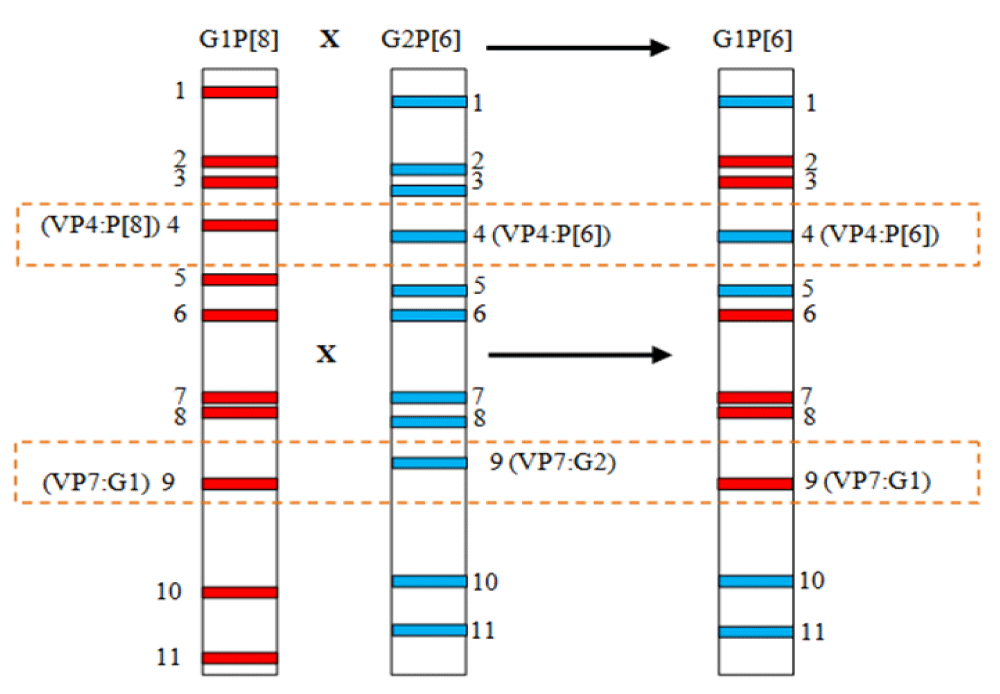
The most common P genotypes worldwide are P[4] and P[8], the least common are [P6] and [P9] and the very few are [P3], [P11], [P12] and [P14]. The latter genotypes are marked by zoonotic or inter-species transmission, which results in human-animal reassortments. The transmission of certain Rotaviruses from animals (Bovine or porcine) to humans or co-infection by several strains could lead to reassortments (Figure 3), by gene exchange and generate unusual genotypes or new emerging strains [61]. Phylogenetic analysis of the recently described G8P[7] and G6P[7] bovine strains in Korea has shown that the genomic segments have bovine but also human and porcine origins [62].
Diversity, the result of possible combinations, is at the origin of the binary antigenic classification linked to two segments 4 and 9 of the Rotavirus genome. This classification should theoretically generate from 32 G and 47 P a number of 1504 combinations. Nevertheless, the majority of epidemiological studies worldwide show that 75% of RVs infections in humans are related to G1[P8], G2[P4], G3[P8], G4[P8], and G9[P8] [22,63,64,56]. The G1[P8] strains represent 40% of the strains characterized worldwide; this predominance could be explained by the emergence of new strains. Strains G2[P4], G3[P8] and G4[P8] each account for approximately 10%.
Sequencing of the whole genome of the different rotavirus strains has become the tool of choice for classifying the 11 genome segments and therefore determining the origin of the strain [65].
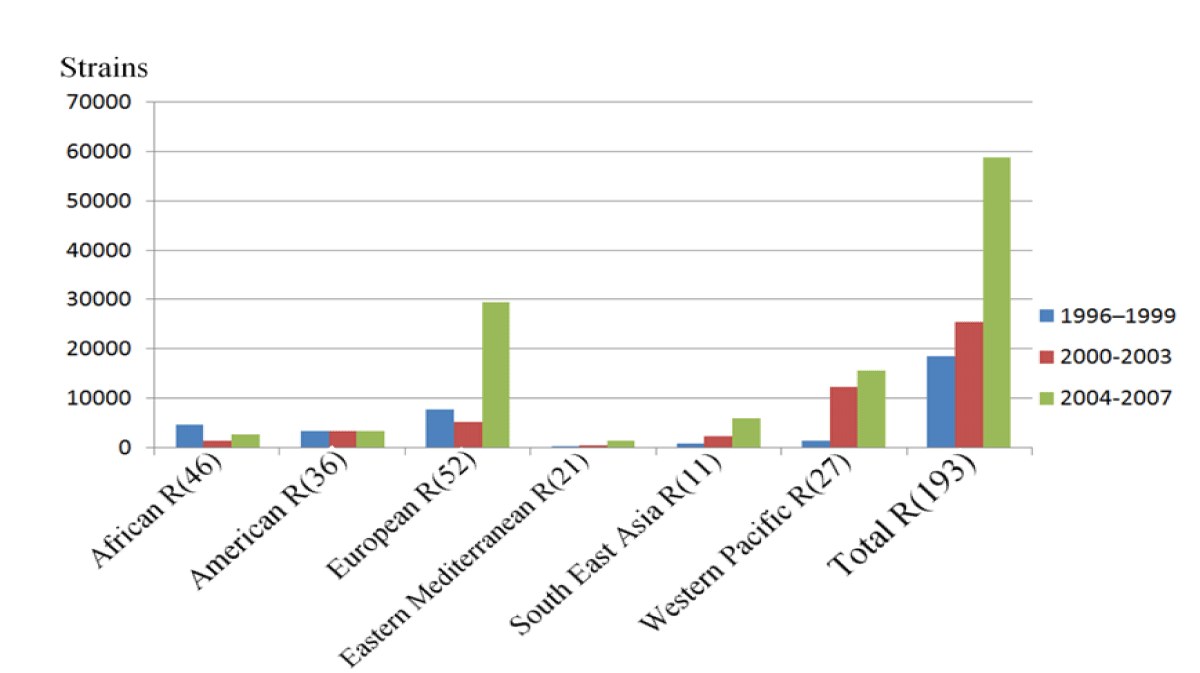
The distribution patterns of rotavirus outbreaks in different geographical regions of the world are closely linked to the socio-economic development of countries [16,66] and climate parameters. Temperate countries experience winter peaks of Rotavirus-related epidemics while these epidemics occur throughout the year in equatorial countries and a less pronounced seasonality in tropical regions. Peaks of infection may vary from province to province and month to month depending on surveillance data [22,56].
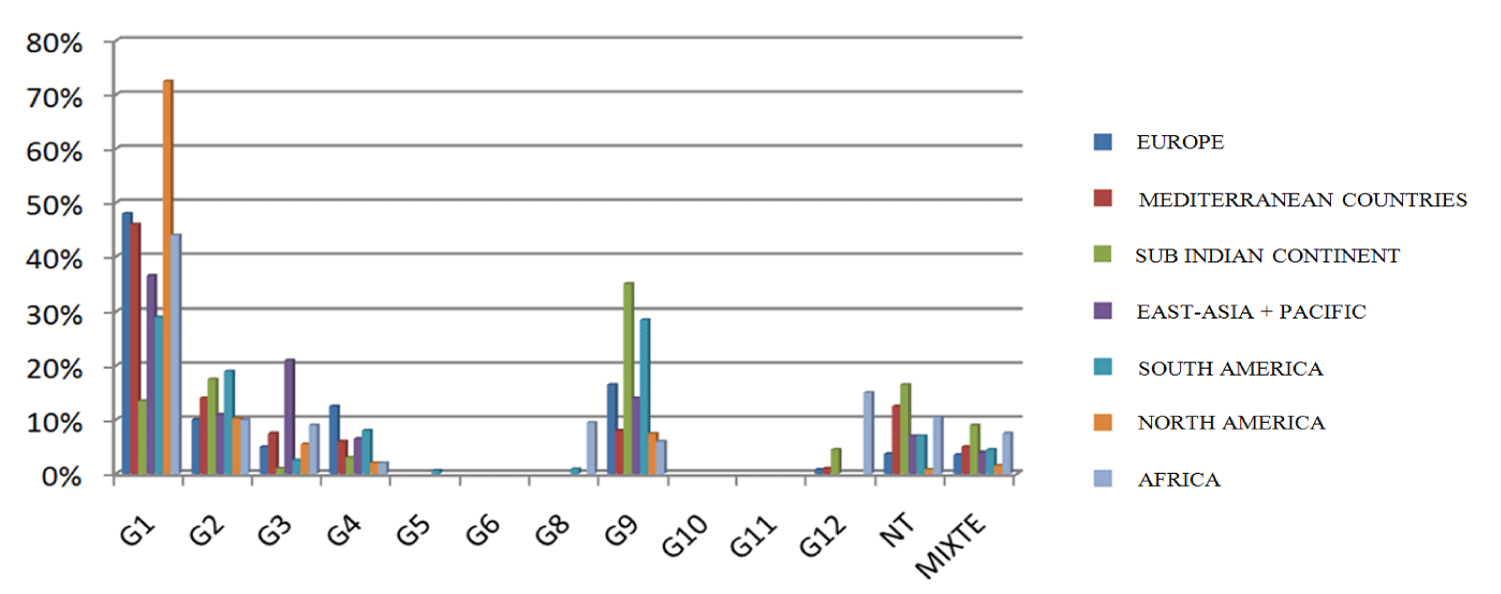
Global data [56] support this geographic and temporal variability in Rotavirus genotypes and provide a prevalence profile of the virus genotypes for each country (Figures 4-6).
AGE occurs in children under 5 years of age in general and in infants from 3 to 11 months in particular. However, at 3 months of age and younger may not develop symptoms of diarrhea when infected with RV since they are protected in the first months of life by transplacental maternal antibodies [67].
The predominantly oral-faecal mode of transmission is a major factor in the prevention and control of rotavirus AGE. The non-specific prevention of viral AGE is based on compliance with universal hygiene rules and the consumption of sufficiently cooked foods, in particular shellfish dishes. Specific prevention against VRs is, according to WHO recommendations, based on vaccination [68]. Moreover, the implementation of the RV eradication program is only possible thanks to the existence of effective vaccines. The current strategy is based on the use of live and attenuated antiviral vaccines designed to achieve immunity comparable to that induced by natural rotavirus infections by providing homotypic or heterotypic protection against severe diarrhoea caused by major circulating rotavirus serotypes [69].
Two safe and effective RVA vaccines have been approved in approximately 100 countries worldwide since 2006 [70] in particular (RotaTeq, Merck Inc., USA and Rotarix, GlaxoSmithKline Biologicals, Belgium). Another live oral monovalent vaccine (ROTAVAC®) was found to be effective in a large clinical trial in India. This vaccine is given in three doses to infants [71]. Injectable vaccines among other RV vaccines are also being studied [72]. The vaccine against pentavalent rotavirus was effective against severe RVA gastroenteritis in the first 2 years of life in high-mortality African countries in infants under 5 years of age [29]. In 2014, more than 65 countries have already introduced RVA vaccines into their national vaccination programs, including more than 20 countries in Africa [73]. Thus the mortality rate per 1 000 live births among children under the age of 5 has increased from 73 to 57 [74], which shows an improvement in child survival worldwide over the past decade. Other countries, such as South Africa, have seen a remarkable decrease in the rate of hospitalizations due to RVA, from 69% to 54% after two years of the introduction of the vaccine in 2009 [75].
Vaccination of young children has shown high efficacy against severe diarrhea and hospitalization due to RVA in high- and middle-income countries [27]. However, both vaccines showed significantly lower immunogenicity and efficacy in low-income countries in Africa and Asia [76], compared to developed countries in this case Australia where two-dose vaccine efficacy estimates are 88.4%, 83.7% and 78.7% in individuals 6-11 months of age, 1-3 years of age, and 4-9 years of age, respectively [23]. However, in Finland after the introduction of the vaccine in children under 5 years of age, the infection became more frequent in unvaccinated children aged 6 to 16 and people over 70 years of age [77].
Infection control measures begin with isolation of infected children; hygiene and hand cleaning with alcohol-containing agents, to be used after contact with infected children; improved water quality; the disinfection of environmental surfaces with appropriate detergents and finally ensure the widespread access to sanitation [78], [79].
The monitoring of the AGE is of particular interest, in order to comply with the directives of the international bodies advocating the monitoring of the genetic dynamism of the RV in particular in species living close to humans such as cattle, pigs, cats, horses, chickens etc... Second, direct RV vaccine prophylaxis towards the use of a regional product that includes contemporary strains, which could help determine the strains that may not be covered by the vaccine.
In addition, phylogenetic analysis of strains isolated from the world makes it possible to determine their belonging to which phase and which lineage. The installation of a regional or national network of epidemics-surveillance of the viral etiology AGE makes it possible, undoubtedly, to orientate towards the answers and thus offer the researchers a platform for carrying out virological investigations later.
The surveillance of RVs, that are responsible for AGE in young children, known by their ability for adaptation and the emergence of new reasserting strains, must be continuously ensured in parallel with vaccination. In order to assess the risk of contamination, the establishment of a surveillance program to monitor RV infection and the burden of related diseases is needed. Hence, by establishing sentinel sites as well as environmental monitoring, characterization of VR strains circulating in different biotopes, as a retrospective procedure. The persistence of RV and other enteric viruses in the environment must also be addressed by adapting the wastewater treatment method in wastewater treatment plants to manage the reduction of the viral load.
Documentation of all clinical presentations and RV-related environmental analysis results should be maintained in a systematic manner to correlate the seasonal distribution of VR-related hospitalizations, while identification of circulating RV strains, as a platform for epidemiological and environmental studies, and to identify any potential emergence of new strains related, may be, at vaccine-induced selective pressure. However, further strain-specific vaccine efficiency studies are required to follow-up period and accompanying genotypic analysis to assess genotypic variation and to understand the complexities of RV epidemiology.
The intensity of this surveillance should be reduced only when the impact of the vaccine is demonstrated. The adoption of this policy is the most effective way to anticipate any possible intervention when new strains or outbreaks emerge.
We are grateful to the University Hassan II of Casablanca, and to the members of the Laboratory of Virology, Microbiology, Quality and Biotechnology/ Eco Toxicology and Biodiversity. We appreciate the efforts of the staff of the molecular biology laboratory at the Institute Pasteur Morocco of Casablanca.
- Hatib A, Hassou N, Benchekroun MN, Bouseettine R, Hafid J, Bessi H, Ennaji MM. Chapter 44 - The Waterborne and Foodborne Viral Diseases Related to Reemerging of Poliovirus. Emerging and Reemerging Viral Pathogens. 2020:V(1);p. 999‑1015. doi: 10.1016/B978-0-12-819400-3.00044-2.
- Boccia D, Tozzi AE, Cotter B, Rizzo C, Russo T, Buttinelli G, Caprioli A, Marziano ML, Ruggeri FM. Waterborne outbreak of Norwalk-like virus gastroenteritis at a tourist resort, Italy. Emerg Infect Dis. 2002 Jun;8(6):563-8. doi: 10.3201/eid0806.010371. PMID: 12023910; PMCID: PMC2738487.
- Brassard J, Seyer K, Houde A, Simard C, Trottier YL. Concentration and detection of hepatitis A virus and rotavirus in spring water samples by reverse transcription-PCR. J Virol Methods. 2005 Feb;123(2):163-9. doi: 10.1016/j.jviromet.2004.09.018. PMID: 15620398.
- Gutiérrez MF, Alvarado MV, Martínez E, Ajami NJ. Presence of viral proteins in drinkable water--sufficient condition to consider water a vector of viral transmission? Water Res. 2007 Jan;41(2):373-8. doi: 10.1016/j.watres.2006.09.022. Epub 2006 Nov 7. PMID: 17084879.
- Seo DJ, Lee MH, Son NR, Seo S, Lee KB, Wang X, Choi C. Seasonal and regional prevalence of norovirus, hepatitis A virus, hepatitis E virus, and rotavirus in shellfish harvested from South Korea. Food Control. 2014 July;V(41): p. 178‑184. doi: 10.1016/j.foodcont.2014.01.020.
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013 Jul 20;382(9888):209-22. doi: 10.1016/S0140-6736(13)60844-2. Epub 2013 May 14. PMID: 23680352.
- Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque AS, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016 Sep 24;388(10051):1291-301. doi: 10.1016/S0140-6736(16)31529-X. PMID: 27673470; PMCID: PMC5471845.
- Boschi-Pinto C, Velebit L, Shibuya K. Estimating child mortality due to diarrhoea in developing countries. Bull World Health Organ. 2008 Sep;86(9):710-7. doi: 10.2471/blt.07.050054. PMID: 18797647; PMCID: PMC2649491.
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C; Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010 Jun 5;375(9730):1969-87. doi: 10.1016/S0140-6736(10)60549-1. Epub 2010 May 11. PMID: 20466419.
- Grimwood K, Bines JE. Rotavirus vaccines must perform in low-income countries too. Lancet. 2007 Nov 24;370(9601):1739-40. doi: 10.1016/S0140-6736(07)61730-9. PMID: 18037071.
- Hoffmann T, Iturriza M, Faaborg-Andersen J, Kraaer C, Nielsen CP, Gray J, Hogh B. Prospective study of the burden of rotavirus gastroenteritis in Danish children and their families. Eur J Pediatr. 2011 Dec;170(12):1535-9. doi: 10.1007/s00431-011-1465-y. Epub 2011 Apr 16. PMID: 21499690.
- Mwenda JM, Ntoto KM, Abebe A, Enweronu-Laryea C, Amina I, Mchomvu J, Kisakye A, Mpabalwani EM, Pazvakavambwa I, Armah GE, Seheri LM, Kiulia NM, Page N, Widdowson MA, Steele AD. Burden and epidemiology of rotavirus diarrhea in selected African countries: preliminary results from the African Rotavirus Surveillance Network. J Infect Dis. 2010 Sep 1;202 Suppl:S5-S11. doi: 10.1086/653557. PMID: 20684718.
- Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81(3):197-204. Epub 2003 May 16. PMID: 12764516; PMCID: PMC2572419.
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003 May;9(5):565-72. doi: 10.3201/eid0905.020562. PMID: 12737740; PMCID: PMC2972763.
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE; WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet. 2005 Mar 26-Apr 1;365(9465):1147-52. doi: 10.1016/S0140-6736(05)71877-8. PMID: 15794969.
- Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, Jiang B, Gentsch JR. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006 Jul 22;368(9532):323-32. doi: 10.1016/S0140-6736(06)68815-6. PMID: 16860702.
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD; World Health Organization–Coordinated Global Rotavirus Surveillance Network. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000-2013. Clin Infect Dis. 2016 May 1;62 Suppl 2:S96-S105. doi: 10.1093/cid/civ1013. PMID: 27059362.
- Sdiri-Loulizi K, Hassine M, Aouni Z, Gharbi-Khelifi H, Chouchane S, Sakly N, Neji-Guédiche M, Pothier P, Aouni M, Ambert-Balay K. Detection and molecular characterization of enteric viruses in environmental samples in Monastir, Tunisia between January 2003 and April 2007. J Appl Microbiol. 2010 Sep;109(3):1093-104. doi: 10.1111/j.1365-2672.2010.04772.x. PMID: 20553345.
- Bishop RF, Davidson GP, Holmes IH, Ruck BJ. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973 Dec 8;2(7841):1281-3. doi: 10.1016/s0140-6736(73)92867-5. PMID: 4127639.
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE; Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012 Jun 9;379(9832):2151-61. doi: 10.1016/S0140-6736(12)60560-1. Epub 2012 May 11. Erratum in: Lancet. 2012 Oct 13;380(9850):1308. PMID: 22579125.
- Turcios RM, Curns AT, Holman RC, Pandya-Smith I, LaMonte A, Bresee JS, Glass RI; National Respiratory and Enteric Virus Surveillance System Collaborating Laboratories. Temporal and geographic trends of rotavirus activity in the United States, 1997-2004. Pediatr Infect Dis J. 2006 May;25(5):451-4. doi: 10.1097/01.inf.0000214987.67522.78. PMID: 16645512.
- Iturriza-Gómara M, Dallman T, Bányai K, Böttiger B, Buesa J, Diedrich S, Fiore L, Johansen K, Koopmans M, Korsun N, Koukou D, Kroneman A, László B, Lappalainen M, Maunula L, Marques AM, Matthijnssens J, Midgley S, Mladenova Z, Nawaz S, Poljsak-Prijatelj M, Pothier P, Ruggeri FM, Sanchez-Fauquier A, Steyer A, Sidaraviciute-Ivaskeviciene I, Syriopoulou V, Tran AN, Usonis V, VAN Ranst M, DE Rougemont A, Gray J. Rotavirus genotypes co-circulating in Europe between 2006 and 2009 as determined by EuroRotaNet, a pan-European collaborative strain surveillance network. Epidemiol Infect. 2011 Jun;139(6):895-909. doi: 10.1017/S0950268810001810. Epub 2010 Aug 16. PMID: 20707941.
- Maguire JE, Glasgow K, Glass K, Roczo-Farkas S, Bines JE, Sheppeard V, Macartney K, Quinn HE. Rotavirus Epidemiology and Monovalent Rotavirus Vaccine Effectiveness in Australia: 2010-2017. Pediatrics. 2019 Oct;144(4):e20191024. doi: 10.1542/peds.2019-1024. Epub 2019 Sep 17. PMID: 31530719.
- Meeting of the immunization Strategic Advisory Group of Experts, April 2009--conclusions and recommendations. Wkly Epidemiol Rec. 2009 Jun 5;84(23):220-36. English, French. PMID: 19499606.
- Benhafid M, Rguig A, Trivedi T, Elqazoui M, Teleb N, Mouane N, Maltouf AF, Parashar U, Patel M, Aouad RE. Monitoring of rotavirus vaccination in Morocco: establishing the baseline burden of rotavirus disease. Vaccine. 2012 Oct 12;30(46):6515-20. doi: 10.1016/j.vaccine.2012.08.058. Epub 2012 Sep 7. PMID: 22959990.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CD, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O’Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA, Heaton PM; Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006 Jan 5;354(1):23-33. doi: 10.1056/NEJMoa052664. PMID: 16394299.
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, López P, Macías-Parra M, Ortega-Barría E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavía-Ruz N, Salmerón J, Rüttimann R, Tinoco JC, Rubio P, Nuñez E, Guerrero ML, Yarzábal JP, Damaso S, Tornieporth N, Sáez-Llorens X, Vergara RF, Vesikari T, Bouckenooghe A, Clemens R, De Vos B, O’Ryan M; Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006 Jan 5;354(1):11-22. doi: 10.1056/NEJMoa052434. PMID: 16394298.
- Jiang V, Jiang B, Tate J, Parashar UD, Patel MM. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin. 2010 Jul;6(7):532-42. doi: 10.4161/hv.6.7.11278. PMID: 20622508; PMCID: PMC3322519.
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, Levine MM, Lewis K, Coia ML, Attah-Poku M, Ojwando J, Rivers SB, Victor JC, Nyambane G, Hodgson A, Schödel F, Ciarlet M, Neuzil KM. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010 Aug 21;376(9741):606-14. doi: 10.1016/S0140-6736(10)60889-6. Epub 2010 Aug 6. PMID: 20692030.
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, Le HT, Coia ML, Lewis K, Rivers SB, Sack DA, Schödel F, Steele AD, Neuzil KM, Ciarlet M. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010 Aug 21;376(9741):615-23. doi: 10.1016/S0140-6736(10)60755-6. Epub 2010 Aug 6. PMID: 20692031.
- Benhafid M, Youbi M, Klena JD, Gentsch JR, Teleb N, Widdowson MA, Elaouad R. Epidemiology of rotavirus gastroenteritis among children <5 years of age in Morocco during 1 year of sentinel hospital surveillance, June 2006-May 2007. J Infect Dis. 2009 Nov 1;200 Suppl 1:S70-5. doi: 10.1086/605048. PMID: 19817617.
- Dubois E, Le Guyader F, Haugarreau L, Kopecka H, Cormier M, Pommepuy M. Molecular epidemiological survey of rotaviruses in sewage by reverse transcriptase seminested PCR and restriction fragment length polymorphism assay. Appl Environ Microbiol. 1997 May;63(5):1794-800. doi: 10.1128/AEM.63.5.1794-1800.1997. PMID: 9143113; PMCID: PMC168473.
- Costafreda MI, Bosch A, Pintó RM. Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Appl Environ Microbiol. 2006 Jun;72(6):3846-55. doi: 10.1128/AEM.02660-05. PMID: 16751488; PMCID: PMC1489592.
- Le Guyader FS, Bon F, DeMedici D, Parnaudeau S, Bertone A, Crudeli S, Doyle A, Zidane M, Suffredini E, Kohli E, Maddalo F, Monini M, Gallay A, Pommepuy M, Pothier P, Ruggeri FM. Detection of multiple noroviruses associated with an international gastroenteritis outbreak linked to oyster consumption. J Clin Microbiol. 2006 Nov;44(11):3878-82. doi: 10.1128/JCM.01327-06. PMID: 17088365; PMCID: PMC1698296.
- Nishida T, Nishio O, Kato M, Chuma T, Kato H, Iwata H, Kimura H. Genotyping and quantitation of noroviruses in oysters from two distinct sea areas in Japan. Microbiol Immunol. 2007;51(2):177-84. doi: 10.1111/j.1348-0421.2007.tb03899.x. PMID: 17310085.
- Le Guyader FS, Parnaudeau S, Schaeffer J, Bosch A, Loisy F, Pommepuy M, Atmar RL. Detection and quantification of noroviruses in shellfish. Appl Environ Microbiol. 2009 Feb;75(3):618-24. doi: 10.1128/AEM.01507-08. Epub 2008 Dec 1. PMID: 19047383; PMCID: PMC2632116.
- Carter MJ. Enterically infecting viruses: pathogenicity, transmission and significance for food and waterborne infection. J Appl Microbiol. 2005;98(6):1354-80. doi: 10.1111/j.1365-2672.2005.02635.x. PMID: 15916649.
- Espinosa AC, Mazari-Hiriart M, Espinosa R, Maruri-Avidal L, Méndez E, Arias CF. Infectivity and genome persistence of rotavirus and astrovirus in groundwater and surface water. Water Res. 2008 May;42(10-11):2618-28. doi: 10.1016/j.watres.2008.01.018. Epub 2008 Jan 29. PMID: 18291437.
- Estes MK, Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410-49. PMID: 2556635; PMCID: PMC372748.
- Fujii Y, Shimoike T, Takagi H, Murakami K, Todaka-Takai R, Park Y, Katayama K. Amplification of all 11 RNA segments of group A rotaviruses based on reverse transcription polymerase chain reaction. Microbiol Immunol. 2012 Sep;56(9):630-8. doi: 10.1111/j.1348-0421.2012.00479.x. PMID: 22708835.
- Desselberger U. Rotaviruses. Virus Res. 2014 Sep 22;190:75-96. doi: 10.1016/j.virusres.2014.06.016. Epub 2014 Jul 9. PMID: 25016036.
- Ruggeri FM, Greenberg HB. Antibodies to the trypsin cleavage peptide VP8 neutralize rotavirus by inhibiting binding of virions to target cells in culture. J Virol. 1991 May;65(5):2211-9. doi: 10.1128/JVI.65.5.2211-2219.1991. PMID: 1850007; PMCID: PMC240568.
- Burns JW, Siadat-Pajouh M, Krishnaney AA, Greenberg HB. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996 Apr 5;272(5258):104-7. doi: 10.1126/science.272.5258.104. PMID: 8600516.
- Aladin F, Einerhand AW, Bouma J, Bezemer S, Hermans P, Wolvers D, Bellamy K, Frenken LG, Gray J, Iturriza-Gómara M. In vitro neutralisation of rotavirus infection by two broadly specific recombinant monovalent llama-derived antibody fragments. PLoS One. 2012;7(3):e32949. doi: 10.1371/journal.pone.0032949. Epub 2012 Mar 5. PMID: 22403728; PMCID: PMC3293919.
- Hou Z, Huang Y, Huan Y, Pang W, Meng M, Wang P, Yang M, Jiang L, Cao X, Wu KK. Anti-NSP4 antibody can block rotavirus-induced diarrhea in mice. J Pediatr Gastroenterol Nutr. 2008 Apr;46(4):376-85. doi: 10.1097/MPG.0b013e3181661ae4. PMID: 18367948.
- Desselberger U, Huppertz HI. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis. 2011 Jan 15;203(2):188-95. doi: 10.1093/infdis/jiq031. PMID: 21288818; PMCID: PMC3071058.
- Trask SD, McDonald SM, Patton JT. Structural insights into the coupling of virion assembly and rotavirus replication. Nat Rev Microbiol. 2012 Jan 23;10(3):165-77. doi: 10.1038/nrmicro2673. Erratum in: Nat Rev Microbiol. 2014 Jan;12(1):70. PMID: 22266782; PMCID: PMC3771686.
- Aoki ST, Settembre EC, Trask SD, Greenberg HB, Harrison SC, Dormitzer PR. Structure of rotavirus outer-layer protein VP7 bound with a neutralizing Fab. Science. 2009 Jun 12;324(5933):1444-7. doi: 10.1126/science.1170481. PMID: 19520960; PMCID: PMC2995306.
- Altenburg BC, Graham DY, Estes MK. Ultrastructural study of rotavirus replication in cultured cells. J Gen Virol. 1980 Jan;46(1):75-85. doi: 10.1099/0022-1317-46-1-75. PMID: 6243348.
- Gardet A, Breton M, Fontanges P, Trugnan G, Chwetzoff S. Rotavirus spike protein VP4 binds to and remodels actin bundles of the epithelial brush border into actin bodies. J Virol. 2006 Apr;80(8):3947-56. doi: 10.1128/JVI.80.8.3947-3956.2006. PMID: 16571811; PMCID: PMC1440440.
- McCrae MA, Faulkner-Valle GP. Molecular biology of rotaviruses. I. Characterization of basic growth parameters and pattern of macromolecular synthesis. J Virol. 1981 Aug;39(2):490-6. doi: 10.1128/JVI.39.2.490-496.1981. PMID: 6268838; PMCID: PMC171359.
- Hung T, Chen GM, Wang CG, Chou ZY, Chao TX, Ye WW, Yao HL, Meng KH. Rotavirus-like agent in adult non-bacterial diarrhoea in China. Lancet. 1983 Nov 5;2(8358):1078-9. doi: 10.1016/S0140-6736(83)91058-9. PMID: 6138617.
- Leuven KU. Rotavirus Classification Working Group: RCWG. 2017. https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (consulté le févr. 16, 2021).
- Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Bányai K, Brister JR, Buesa J, Esona MD, Estes MK, Gentsch JR, Iturriza-Gómara M, Johne R, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Parreño V, Rahman M, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Patton JT, Desselberger U, Van Ranst M. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch Virol. 2011 Aug;156(8):1397-413. doi: 10.1007/s00705-011-1006-z. Epub 2011 May 20. PMID: 21597953; PMCID: PMC3398998.
- Trojnar E, Sachsenröder J, Twardziok S, Reetz J, Otto PH, Johne R. Identification of an avian group A rotavirus containing a novel VP4 gene with a close relationship to those of mammalian rotaviruses. J Gen Virol. 2013 Jan;94(Pt 1):136-142. doi: 10.1099/vir.0.047381-0. Epub 2012 Oct 10. PMID: 23052396.
- Bányai K, László B, Duque J, Steele AD, Nelson EA, Gentsch JR, Parashar UD. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine. 2012 Apr 27;30 Suppl 1:A122-30. doi: 10.1016/j.vaccine.2011.09.111. PMID: 22520121.
- Gouvea V, de Castro L, Timenetsky MC, Greenberg H, Santos N. Rotavirus serotype G5 associated with diarrhea in Brazilian children. J Clin Microbiol. 1994 May;32(5):1408-9. doi: 10.1128/JCM.32.5.1408-1409.1994. Erratum in: J Clin Microbiol 1994 Jul;32(7):1834. PMID: 8051281; PMCID: PMC263717.
- Esona MD, Armah GE, Geyer A, Steele AD. Detection of an unusual human rotavirus strain with G5P[8] specificity in a Cameroonian child with diarrhea. J Clin Microbiol. 2004 Jan;42(1):441-4. doi: 10.1128/jcm.42.1.441-444.2004. PMID: 14715801; PMCID: PMC321728.
- Cunliffe NA, Gondwe JS, Broadhead RL, Molyneux ME, Woods PA, Bresee JS, Glass RI, Gentsch JR, Hart CA. Rotavirus G and P types in children with acute diarrhea in Blantyre, Malawi, from 1997 to 1998: predominance of novel P[6]G8 strains. J Med Virol. 1999 Mar;57(3):308-12. PMID: 10022804.
- Roczo-Farkas S, Kirkwood CD, Cowley D, Barnes GL, Bishop RF, Bogdanovic-Sakran N, Boniface K, Donato CM, Bines JE. The Impact of Rotavirus Vaccines on Genotype Diversity: A Comprehensive Analysis of 2 Decades of Australian Surveillance Data. J Infect Dis. 2018 Jul 13;218(4):546-554. doi: 10.1093/infdis/jiy197. Erratum in: J Infect Dis. 2019 Mar 15;219(7):1172. PMID: 29790933.
- Martella V, Bányai K, Matthijnssens J, Buonavoglia C, Ciarlet M. Zoonotic aspects of rotaviruses. Vet Microbiol. 2010 Jan 27;140(3-4):246-55. doi: 10.1016/j.vetmic.2009.08.028. Epub 2009 Aug 28. PMID: 19781872.
- Park SI, Matthijnssens J, Saif LJ, Kim HJ, Park JG, Alfajaro MM, Kim DS, Son KY, Yang DK, Hyun BH, Kang MI, Cho KO. Reassortment among bovine, porcine and human rotavirus strains results in G8P[7] and G6P[7] strains isolated from cattle in South Korea. Vet Microbiol. 2011 Aug 26;152(1-2):55-66. doi: 10.1016/j.vetmic.2011.04.015. Epub 2011 Apr 22. PMID: 21592683.
- de Rougemont A, Kaplon J, Pillet S, Mory O, Gagneur A, Minoui-Tran A, Meritet JF, Mollat C, Lorrot M, Foulongne V, Gillet Y, Nguyen-Bourgain C, Alain S, Agius G, Lazrek M, Colimon R, Fontana C, Gendrel D, Pothier P; French Rotavirus Network. Molecular and clinical characterization of rotavirus from diarrheal infants admitted to pediatric emergency units in france. Pediatr Infect Dis J. 2011 Feb;30(2):118-24. doi: 10.1097/INF.0b013e3181ef034e. PMID: 20686439.
- Hull JJ, Teel EN, Kerin TK, Freeman MM, Esona MD, Gentsch JR, Cortese MM, Parashar UD, Glass RI, Bowen MD; National Rotavirus Strain Surveillance System. United States rotavirus strain surveillance from 2005 to 2008: genotype prevalence before and after vaccine introduction. Pediatr Infect Dis J. 2011 Jan;30(1 Suppl):S42-7. doi: 10.1097/INF.0b013e3181fefd78. PMID: 21183839.
- Matthijnssens J, Ciarlet M, Rahman M, Attoui H, Bányai K, Estes MK, Gentsch JR, Iturriza-Gómara M, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Patton JT, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Desselberger U, Van Ranst M. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch Virol. 2008;153(8):1621-9. doi: 10.1007/s00705-008-0155-1. Epub 2008 Jul 5. PMID: 18604469; PMCID: PMC2556306.
- Banerjee I, Ramani S, Primrose B, Iturriza-Gomara M, Gray JJ, Brown DW, Kang G. Modification of rotavirus multiplex RT-PCR for the detection of G12 strains based on characterization of emerging G12 rotavirus strains from South India. J Med Virol. 2007 Sep;79(9):1413-21. doi: 10.1002/jmv.20872. PMID: 17607780; PMCID: PMC2465801.
- Ray PG, Kelkar SD, Walimbe AM, Biniwale V, Mehendale S. Rotavirus immunoglobulin levels among Indian mothers of two socio-economic groups and occurrence of rotavirus infections among their infants up to six months. J Med Virol. 2007 Mar;79(3):341-9. doi: 10.1002/jmv.20804. PMID: 17245723.
- Rotavirus vaccines:an update. Wkly Epidemiol Rec. 2009 Dec 18;84(50):533-40. English, French. PMID: 20034143.
- Kapikian AZ, Hoshino Y, Chanock RM. Fields virology. Philadelphia; Lippincoot Williams & Wilkins. 2001.
- Dennehy PH. Rotavirus vaccines: an overview. Clin Microbiol Rev. 2008 Jan;21(1):198-208. doi: 10.1128/CMR.00029-07. PMID: 18202442; PMCID: PMC2223838.
- Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, Juvekar S, Muliyil J, Arya A, Shaikh H, Abraham V, Vrati S, Proschan M, Kohberger R, Thiry G, Glass R, Greenberg HB, Curlin G, Mohan K, Harshavardhan GV, Prasad S, Rao TS, Boslego J, Bhan MK; India Rotavirus Vaccine Group. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014 Jun 21;383(9935):2136-43. doi: 10.1016/S0140-6736(13)62630-6. Epub 2014 Mar 12. PMID: 24629994; PMCID: PMC4532697.
- Kirkwood CD, Ma LF, Carey ME, Steele AD. The rotavirus vaccine development pipeline. Vaccine. 2019 Nov 28;37(50):7328-7335. doi: 10.1016/j.vaccine.2017.03.076. Epub 2017 Apr 7. PMID: 28396207; PMCID: PMC6892263.
- Anonyme. Rotavirus Disease and Vaccines in Asia. 2014 Aug; p. 2.
- Alkema L, You D. Child mortality estimation: a comparison of UN IGME and IHME estimates of levels and trends in under-five mortality rates and deaths. PLoS Med. 2012;9(8):e1001288. doi: 10.1371/journal.pmed.1001288. Epub 2012 Aug 28. PMID: 22952434; PMCID: PMC3429386.
- Msimang VM, Page N, Groome MJ, Moyes J, Cortese MM, Seheri M, Kahn K, Chagan M, Madhi SA, Cohen C. Impact of rotavirus vaccine on childhood diarrheal hospitalization after introduction into the South African public immunization program. Pediatr Infect Dis J. 2013 Dec;32(12):1359-64. doi: 10.1097/INF.0b013e3182a72fc0. PMID: 24569308.
- Moon SS, Wang Y, Shane AL, Nguyen T, Ray P, Dennehy P, Baek LJ, Parashar U, Glass RI, Jiang B. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr Infect Dis J. 2010 Oct;29(10):919-23. doi: 10.1097/INF.0b013e3181e232ea. PMID: 20442687; PMCID: PMC3704726.
- Markkula J, Hemming-Harlo M, Salminen MT, Savolainen-Kopra C, Pirhonen J, Al-Hello H, Vesikari T. Rotavirus epidemiology 5-6 years after universal rotavirus vaccination: persistent rotavirus activity in older children and elderly. Infect Dis (Lond). 2017 May;49(5):388-395. doi: 10.1080/23744235.2016.1275773. Epub 2017 Jan 9. PMID: 28067093.
- Dennehy PH. Transmission of rotavirus and other enteric pathogens in the home. Pediatr Infect Dis J. 2000 Oct;19(10 Suppl):S103-5. doi: 10.1097/00006454-200010001-00003. PMID: 11052397.
- Ward RL, Bernstein DI, Knowlton DR, Sherwood JR, Young EC, Cusack TM, Rubino JR, Schiff GM. Prevention of surface-to-human transmission of rotaviruses by treatment with disinfectant spray. J Clin Microbiol. 1991 Sep;29(9):1991-6. doi: 10.1128/JCM.29.9.1991-1996.1991. PMID: 1663519; PMCID: PMC270247.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.