> Biology Group. 2021 Feb 27;2(2):091-094. doi: 10.37871/jbres1199.
Beta-Chitosane as a Treatment for Ulcerative Colitis: Therapeutic Effectiveness and Possible Mechanisms of Action
Laribi-Habchi Hassiba1,2*, Laichi Yasmine1, Saadoune Zineb1, Boucherit Ahmed3 and Azine Kenza4
2Loboratory of energy applications of hydrogen, University Saad Dahlab of Blida 1, P.O. Box 270, 09000, Blida, Algeria
3Laboratory of Chemical Engineering, University Saad Dahlab of Blida 1, P.O. Box 270, 09000, Blida, Algeria
4Laboratory of Toxicology, Center of Research and Development of the Pharmaceutical Group SAIDAL, Gue of Constantine, Algeria
- Colitis (RCH
- Intestinal epithelial Cells
- β-Chitosane
- Anti-inflammatory
Abstract
Purpose: Colitis is a widespread inflammatory bowel disease with heterogeneous etiology (genetic and immunological). It is treated with drugs such as steroidal and non-steroidal anti-inflammatory that, in the long term, can cause side effects. For this reason, the exploitation of natural resources to combat this type of disease is the concern of researchers. The purpose of our study was to evaluate the anti-colitis (anti-inflammatory) effect of β-Chitosane induced in albino mice by acetic acid (5%).
Methods: Mices were separated into six groups: the witness (untreated and not ulcerated), negative control group (ulcerated and untreated), the positive control ulcerated and treated with the Dexaméthasone® (1 mg/kg), and test groups ulcerated and treated with different doses of β-Chitosane (0.5 g/ kg; 0.75 g/ kg and 1 g/ kg) for the entire treatment estimated to six-days. β-Chitosane efficacy was evaluated by macroscopic and microscopic scores.
Results: The clinical scores showed that β-Chitosane with a dose of 1 g/kg for the entire treatment significantly reduced the damage caused by acetic acid with a score of (3.41 ± 1.45) compared to those of the positive control which reduced less the inflammation (6.26 ± 1.23). The histological study of the colons was able to validate the effect of β-Chitosane by decreasing neutrophil infiltration and ulceration in the colon as well as by structural recovery of the mucosa.
Conclusion: These results provide evidence that β-Chitosane has a protective effect against ulcerative colitis that may be due to its antioxidant, anti infectious, anti-inflammatory and healing activities.
Introduction
Inflammatory Bowel Disease (IBD) is a group of idiopathic conditions including ulcerous Haemorrhagic Colitis (RCH) and Crohn’s Disease (CD) affecting an increasing number of children and adults [1,2]. Their physiopathology involves an abnormal composition of the intestinal microbiota (called dysbiosis: an imbalance between the protective and deleterious bacteria that forms the intestinal microbiota) [3], a dysfunction of the epithelial barrier [4], a lack of innate immunity and a deregulation of adaptive immunity [5]. RCUH is manifested by rectal bleeding, diarrhea, weakness, abdominal pain, weight loss and fever [6]. Environmental factors also seem to play an important role in the occurrence of these diseases [7] as well as other psychological factors such as oxidative stress [8] and genetic factors [9].
The Intestinal Epithelial Cells (IEC), being the main component of the intestinal epithelial barrier, serves as a first sensor providing an immune defence of the mucous membranes against invasive pathogens. Bacterial products such as Lipopolysaccharides (LPS) are recognised by IEC via the Toll 4 receptor (TLR4), which leads to the stimulation of the production of pro-inflammatory cytokines by the NF-κB. In fact, the activation of NF-κB in the IEC and the increased levels of pro-inflammatory cytokines derived from the IEC (in particular TNF- and IL-6), were detected in intestinal biopsies of IBD’spatients. They secret pro-inflammatory cytokines (e.g. TNF-α) and reactive oxygen species produced by the recruited immune cells (e.g. neutrophils) subsequently induce IEC apoptosis, which cause a loss of integrity of the intestinal epithelial barrier and an exaggerated inflammatory response of the mucosa in IBD. Small molecules or antibodies targeting NF-κB and pro-inflammatory cytokines such as TNF-α, IL-6 and their signalling pathways have been reported to be very effective in mitigating intestinal damage associated with IBD.
β-Chitosane (CS) is a natural cationic polysaccharide, sparse in nature, obtained in the industry by the acetylation of chitin, another biopolymer extracted from the exoskeleton of crustaceans, molluscs, insects and some fungal families [10]. This biopolymer consists of a long chain of disaccharide of N-acetyl-glucosamine and glucosamine bound in β(1 → 4) [11]. It is known for its biodegradability and biocompatibility, also for its antifungal and antibacterial activities [12,13], antioxydant activity and its antitumoral activity [14]. However, its anti-inflammatory activity against IBD is not yet confirmed. Here we assessed the therapeutic efficacy of β-Chitosane at a small molecular weight of 1.25 kDa, and a degree of acetylation of 82.7% in an experimental mouse colitis model (acidocile colitis) and the possible mechanisms by which the CS exerted its action were studied on histopathological sections.
Material and Methods
Animals
In order to evaluate the intestinal anti-inflammatory activity of β-Chitosane, we used 36 male albino mices, of a weight varying between (30-35 g) aged about 8 weeks, obtained from the Pasteur Institute of Algiers (El-Hamma). The mices were transferred to the animal housing of research and development center (RDC-Saidal) of Gue of Constantine where the work was carried out. They were divided into 6 batchs in cages of 6 mices each, subjected to ambient temperature conditions of (23-26°C), and a day and night cycle of 12 hours of lighting/darkness in order to respect their life cycle with free access to food and water.
Preparation of β-Chitosane
In our study we used a β-Chitosane powder with a molecular weight of 1.25 kDa and a 82% degree of acetylation, obtained from squid featheral prepared daily in the laboratory, The squids feathers, once pretreated (washing, grinding and drying), were mixed in aqueous solutions of hydrochloric acid (1.5 M) at a temperature of 65°C overnight, the solid-liquid ratio was 1:10 w/v (dry squid feather weight/volume of diluted HCl solution). Demineralised squids feathers are rinsed thoroughly with distilled water till getting a neutral pH. Then they were mixed in solutions of caustic soda (1 M) in proportion 1:10 w/v at 90°C for 18 hours. The deprotinated squids feathers were again filtered and rinsed thoroughly with distilled water.
After rinsing with distilled water, another rinse with a volume of 20 ml of hydrogen peroxide (H2O2) was carried out to bleach the chitin, then, it was rinsed again abundantly with distilled water until the pH of the wash water reaches neutrality. Chitin has been treated with a 50% sodic solution, with a proportion of (1:10) (m/v), the solution was agitated for 24 hours at 100°C. The solution was then filtered, the retained β-Chitosane was washed continuously in order to remove the residual soda, and until the pH of the wash water reaches neutrality, β-Chitosane was rinsed with distilled water and then dried in a stove at 80°C for 24 hours.
The molecular weight was determined by the viscosity method using the Mark-Houwink formula and the degree of acetylation was determined by the FTIR method.
Characterization of β-Chitosane
Particle size measurement by DLS: In order to determine the size of the β-Chitosane particles in solution, a 35 mg solution of β-Chitosane was dissolved in 15 ml of 2% acetic acid then passed through a 0.45 micrometer filter for a homogeneous distribution of the sample.
A Quartz cell was filled with the solution and placed in the DLS device (SZ-100 (HORIBA–SCIENTIFIC) attached to a computer; the particle size analysis was repeated three times and then the average value was taken.
Measurement of zeta potential: To determine the electrical charge of β-Chitosane’s particles in a solution, a 35 mg solution of β-Chitosane dissolved in 15 ml of 2% acetic acid was injected into a cell (Glod-Plating, 6 mm). Then it pass into the SZ-100 (HORIBA–SCIENTIFIC) to measure the zeta potential.
Evaluation of antioxidant activity: In order to measure the anti-oxidant activity, a DPPH solution of 0.004 g per 100ml of methanol was prepared. 3 ml of the DPPH solution were mixed with 1 ml of β-Chitosane solution at different concentrations (5 mg/ml, 3.75 mg/ml, 2.5 mg/ml, 1 mg/ml and 0.5 mg/ml), after 30 minutes of incubation in the shelter of light, the absorbance of each solution was measured at 517 nm using a UV-visible spectrophotometer of the Schimdzu type. Antioxidant Activity (AA, %) was determined using the following equation
(1)
where:
AA is the percentage of antioxidant activity (%).
A0 is the absorbance of the DPPH solution
AS: is the absorbance of the DPPH solution containing β-Chitosane.
Induction and treatment of disease
The experimental study of this activity was carried out using the method described by Wang, et al. [15] with some modifications, the mices of the 6 batchs were weighed and numbered. Five batchs were deprived of food for 12 hours before inducing colic inflammation by the administration of 100 µL of acetic acide at 5% rectally (Subaigüe Inflammation). The mices were then held in a vertical position for 30 seconds to limit the expulsion of the solution of acetic acid. The sixth batchs (healthy control batch/witness) receive saline solution only.
After 2 hours, three tests batches received 0.5 ml of β-Chitosane solution in water orally at doses of 0.5 g/kg, 0.75 g/kg and 1 g/kg for 6 days, equivalent to 0.0833 g/kg, 0.125 g/kg and 0.1666 g/kg per day respectively in a single dose per day. A reference batch was also treated by gavage with an anti-inflammatory used in therapy (Dexamethasone) with a dose of 1 mg/kg per day. The witness and the negative control batch received 0.5 ml of distilled water by gavage each day.
Evaluation of intestinal anti-inflammatory activity of β-Chitosane
The state of the mices in terms of their activity and coat, was followed every day. The effect of different doses of β-Chitosane on mice’s survival was determined by calculating the percentage of viability each day (V, %) according to the following formula:
(2)
Ndx: Number of mices at day of treatment days (day x)
Nd0: Initial number of mices (at day 0)
Index of disease activity
The Index of Disease Activity (IDA) was recorded on a daily basis by observing the evolution of body weight, stool form and bleeding [16]. The activity index of the disease was calculated as the sum of the 3 previous scores as mentioned in table 1.
| Table 1: Score DIA Disease Activity Index. | |||
| Score | Weight loss % | Stool consistency | Hemorrhage |
| 0 | None | Normal | Absence |
| 1 | 1-5 | Loose stools | Blood loss |
| 2 | 5-10 | ||
| 3 | 10-20 | ||
| 4 | >20 | Diarrhea | Significant bleeding |
After 6 days of treatment, the mices were sacrificed by cervical dislocation after anesthesia. Colons were recovered and released from adhering fatty tissue, rinsed gently with saline solution, weighed, measured and observed macroscopically.
Macroscopic score
The macroscopic damage score (ulcerations) was assigned according to Deshmukh and Pawar Deshmukh CD [17]. The scores are presented in table 2.
| Table 2: Ulceration score. | |
| Score | Macroscopic damage |
| 0 | No macroscopic change (no ulceration) |
| 1 and 2 | Mucous erythema, erosion and mild ulceration. |
| 3 and 4 | Severe ulceration, severe erosion, edema |
The effect of different doses of β-Chitosane on the weight-length ratio of colons
The length of the colon was measured in cm between the ileo-caecal junction and the proximal rectum and its weight was measured in gram, the ratio W/L (weight/length) has been calculated for each mouse colon as it is considered as an indicator of the extent of colic inflammation [18], comparing the reduction of inflammation in treated mices with controls not treated with acetic acid. The colons were then stored in a 10% formaldehyde solution for later histological study.
Microscopic observation (histopathological study)
The samples of the colons recovered and preserved in 10% formaldehyde solution were sent to the anatomopathology department of the University Hospital Center (UHC) of Douera (Algiers). This step involves studying the histology of these tissues microscopically and understanding their normal or pathological functioning. The coloration used is that of routine HE (Hematoxylineosine). The nucleus is revealed in blue by Harris’ haematoxylin while the eosin produces a pink cytoplasm.
Statistical analysis
The statistical study was carried out using the Graph Pad Prisme software followed by a one-way Variance Analysis (One way ANOVA) using the Dunnett test to compare the values of the tested groups with the healthy control group. The differences are considered significant at *p < 0.05, highly significant at **p < 0.01, and very highly significant at *** p < 0.001.
Results
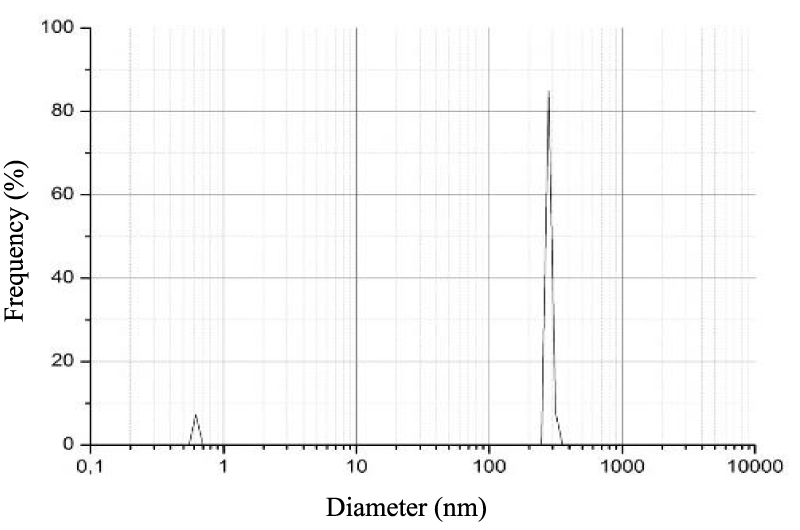
Particle size
The size of the β-Chitosan’s nanoparticles obtained by the DLS analysis appears in the figure 1, it is estimated to 246.0 nm. The results showed that the inhibitory effect of β-Chitosane against E. coli and S. aurus, was influenced by particle size. A greater inhibitory effect was observed as nanoparticles. The smaller particles have a larger surface area that would be in contact with bacteria so it gives better inhibition. As regards the anti inflammatory effect Vllasliu D, et al. [19] showed that β-Chitosane nanoparticles obtained by ionic gelation induce the reversible opening of tight junctions of epithelial cells.
In the treatment of IBD, Lamprecht A, et al. [20] demonstrated the benefits of administering PA as nanoparticles. In fact, nanoparticles accumulate specifically at the inflammatory site, systemic passage of the active PA is less and therefore the side effects are less. In the previous studies, the deposition of particles along the digestive mucosa was shown to depend on their size. They observed the same phenomenon in inflammatory areas, but the influence of size in this one was much more pronounced.
The fixation of particulate systems is more important on the active inflammatory zones and on the ulcerated zones than on the healthy zones. The decrease in the diameter of the particles allows very clearly an increase in their fixation on inflammatory areas [21].
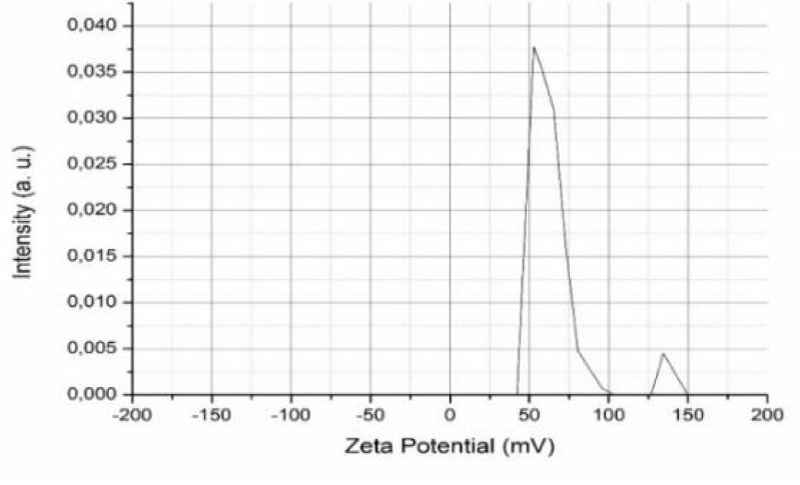
Zeta potential
Zeta potential measurements were analyzed to understand the surface charge effect of β-Chitosane particles. Figure 2 gives a representative peak of the β-Chitosane zeta potential obtained from the zetametric analysis of this polymer. According to this study, the measurement of zeta potential has allowed us to confirm the cationic character of β-Chitosane and the value of its potential was 53.3 mV.
When a charged particle is suspended in a liquid, the ions having an opposite charge to the charge of the particle are attracted to the surface of the suspended particle. A negative charge particle attracts positive ions and conversely, a positive charge particle attracts negative ions [22].
Intestinal inflammation is accompanied by a change in the composition of the intestinal microbiota (dybioses), the major alteration observed is the increase in enterobacteria, a decrease in bifido-bacteria and the presence of a high density of potentially pro-inflammatory bacteria (E. coli enteroadherents and enteroinvasives). This last one is a bacterium with a negatively charged wall [23].
An antibacterial effect of β-Chitosane has been reported due to the high density of the amino groups –NH3+ which confers a positive charge on the polymer, this charge is represented by the zeta potential. It is attributed to the formation of polyelectrolytic complexes with negative peptidoglycans of the bacterial cell wall. This interaction can disrupt the cells walls and inhibit bacterial growth [24].
It has also been shown that a positive charge of particles is desirable to prevent particle aggregation and to promote electrostatic interaction with the overall negative charge of microbial cells [25]. β-Chitosane interacts with the cell membrane to alter its permeability, thus hindering the entry of some nutrients.
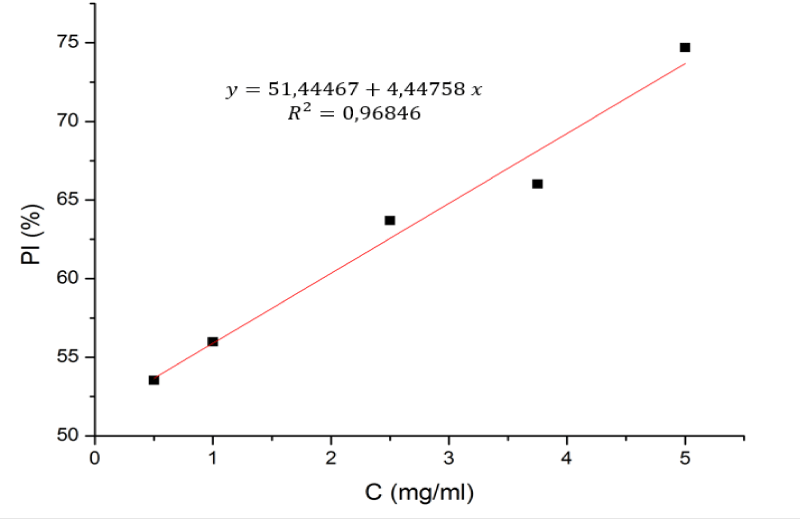
Antioxydant activity
In the current study, the ability to trap β-Chitosane DPPH radicals was studied at different concentrations (0.5 to 5 mg/ml) as shown in the figure 3, β-Chitosane obtained from squids feathers a noticeable antioxidant activity against DPPH, in an independent way in the different concentrations.
The results of the antioxidant power of the β-Chitosane tested show that the percentage of inhibition is more than 70% at a concentration of 5.296 mg/ml for β-Chitosane. These results show that β-Chitosane has an antioxidant activity which explains its antioxidant and antibacterial effects.
Macroscopic observation
The mices that were used in this study developed colic inflammation following to the administration of acetic acid, characterized by loss of body weight, blood in feces, diarrhea, and irritations in the anus and bleeding. Subsequently, we also noticed behavioural changes (immobilization of the mices in the corners of the cages, accompanied by a reduction in their activities, a decrease in the intake of food and a felting of drilling due to dehydration).
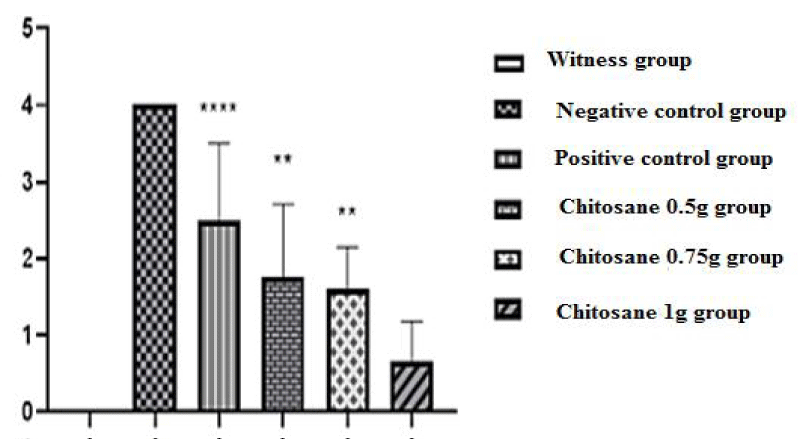
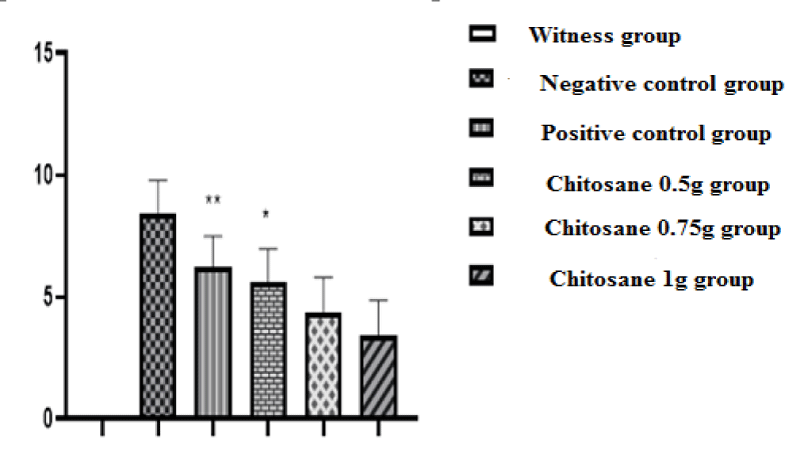
Index of disease activity: In our study we used the score evaluation model, which indicates the damage caused by the induction of acetic acid, the severity of RCH (ulcerative colitis) and the degree of inflammation. The scores obtained were shown in the figure 4.
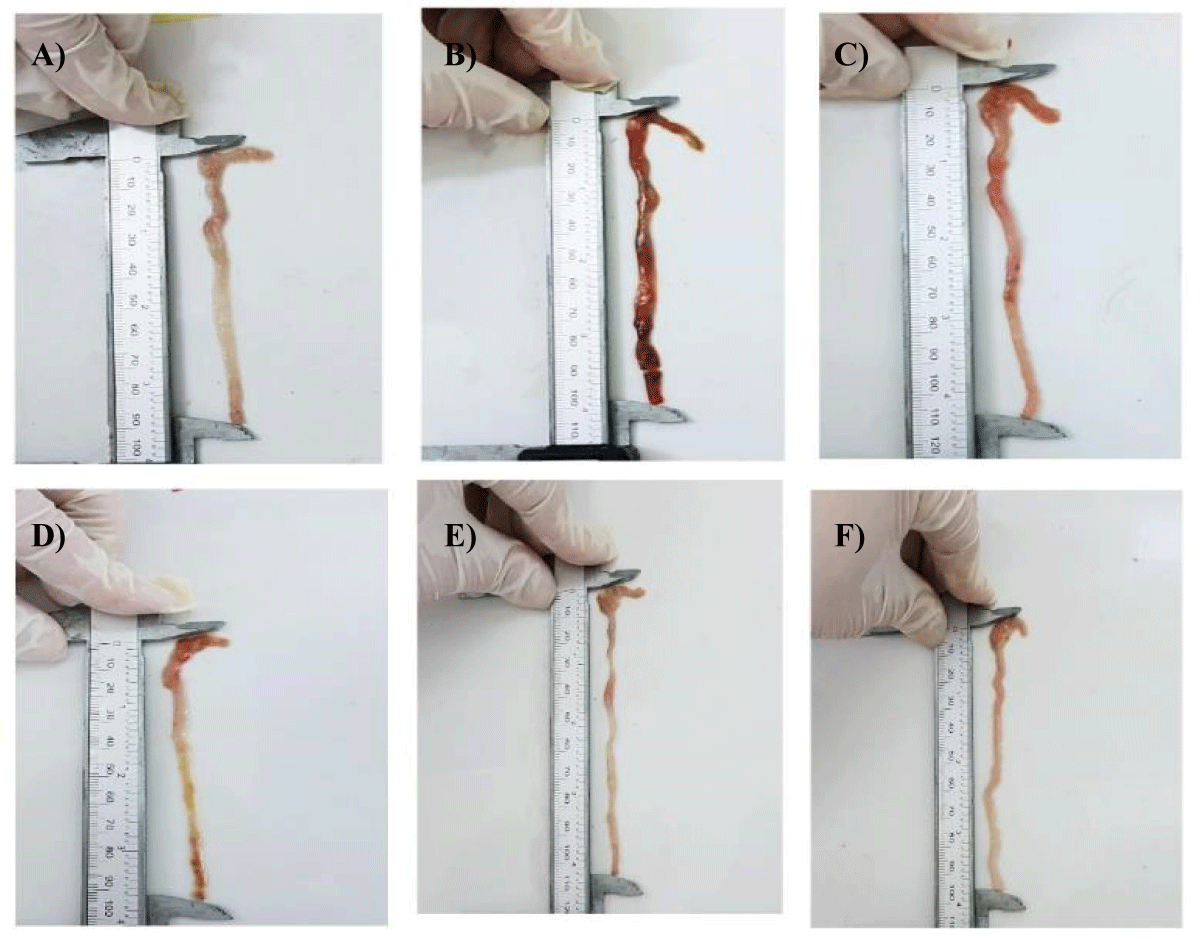
Macroscopic score of ulceration: Figure 5 shows the appearance of ulcers and other macroscopic lesions observed during colon recovery, in comparison with the mices of the non-colic control batch, negative control batch , reference batch and those of the batches treated with the solution of β-Chitosane with different doses. A clinical score of (0.00 ± 0.00) was revealed for mices in the control group, this is justified by a healthy colon, in which no morphological change or inflammation was shown by figure 5A and no ulceration was observed in figure 6.
On the other hand, for mices in the negative control group (acetic acid batch) acute diarrhea was observed in this group, likely resulting from erosion and alteration of the mucosa and apoptosis of the colon epithelial cells whose main role is the reabsorption of water. This physiological function is no longer assured, water and electrolytes are no longer absorbed; which results in permanent removal of stool and change in its consistency (diarrhea) [26]. Scores of (8.45 ± 1.33) were noted, corresponding to acute inflammation, (p < 0.001) compared to the witness group.
Macroscopic observations of the colons collected revealed rectal bleeding due to haemorrhage of the lower digestive tract (recetorragie). This is probably due to damage caused by acetic acid as shown in figure 5B. These lesions triggered an inflammatory reaction initiated by an alarm signal, including the dilation of the blood vessels in the injured area, so as to allow a massive intake of white blood cells necessary for immune defence [27]. Thus leading to an increased blood flow, responsible for the appearance of a huge epithelial damage [28] shown in figure 5B. A maximum ulceration rate was recorded (4 ± 0) as shown in figure 6.
Positive control mices, treated with the reference drug « Dexamethasone » at a dose of 1 mg/kg, revealed a macroscopic score of (6.26 ± 1.23) (p > 0.01) compared to the negative control group (8.45 ± 1.33), which shows a decrease in diarrhea and stops bleeding, thus showing a slight improvement in the condition of the colic mucosa, this improvement is reflected to a decrease in the intensity of inflammation including redness (Figure 5C) as well as a slight decrease in the rate of ulceration but it is not significant (Figure 6).
The treatments of mices with the three doses of β-Chitosane, 0.5 g/kg, 0.75g/kg and 1 g/kg, reveal respectively the following clinical scores (5.62 ± 1.33; 4.36 ± 1.44; 3.41 ± 1.45). The mices of the 0.5 g/kg CS group showed a slight improvement illustrated by the disappearance of diarrhea and the appearance of soft stool, stopping bleeding and recovering activity and weight as well as improving coat state. On the macroscopic scale, β-Chitosane at 0.5 g/kg could not completely suppress the symptoms and damages caused by acetic acid in the epithelial mucosa, there is a better improvement than that of the reference, a disappearance of redness and swelling were subsequently observed with a significant decrease in inflammation as shown in figure 5D. Thus a reduced rate of ulceration compared to negative and positive controls lots as shown in figure 6.
By analysing the results obtained, it can be seen that the two remaining doses (CS 0.75 g and CS 1g) revealed very close results with a slight difference between them as well as a very significant improvement compared to the negative and positive controls batchs. This improvement is reflected in a total disappearance of diarrhea and bleeding and return to normal, mices gained back weight as well as their activity, mobility in the cages and their coat state.
Colon observation revealed colons less affected by intestinal lesions. For the batch CS 0.75, total absence of bleeding and significant reduction of redness as shown in figure 5E with a reduced mean ulceration compared to previous batches mentioned in figure 6.
For the batch of CS 1g, healthy colons with no redness and bleeding which means a disappearance of inflammation and it is a signe of healing figure 5F compared to the normal state (healthy witness) figure 5A and the ill state (Negative control) figure 5B also the rate of ulceration was very significantly reduced in the process of elimination as shown in figure 6.
The effect of different doses of β-Chitosane on colon’s weight and length: After the sacrifice, the colons were recovered, weighed and measured to calculate the weight/length (W/L) ratio, which is an indicator of the extent of colic inflammation [18] and the results showed a reduction in the length of the colons and an increase in their weight, compared to the mices of the witness batch (normal), and the other five batchs treated with acetic acid and then with the different molecules. The results are noted in table 3.
| Table 3: Mean of weight, length, and weight/length ratio of recovered colons. | |||
| Batch | Length (cm) | Weight (mg) | W/L |
| Witness | 11.5 ± 0.58 | 514.8 ± 32.3 | 44.89 ± 2.30 |
| Negative control group | 8.52 ± 0.35 | 656.7 ± 39.06 | 76.09 ± 7.5 |
| Positive control group | 9.52 ± 0.57 | 579 ± 51.92 | 61.61 ± 7.82 |
| Chitosane 0.5 g group | 9.59 ± 0.16 | 572.8 ± 77.53 | 57.56 ± 10.02 |
| Chitosane 0.75 g group | 9.74 ± 0.52 | 530.7 ± 41.17 | 54.2 ± 4.10 |
| Chitosane 1 g group | 10.45 ± 0.27 | 523 ± 28.31 | 51.11 ± 3.45 |
The batch treated with acetic acid shows a remarkable reduction in colons’s length (8.52 ± 0.35) as well as an increase in its weight (656.7 ± 39.06) with a W/L ratio of (76.09 ± 7.50) compared to the healthy control not treated with acetic acid (44.89 ± 2.30).
The treatment with the Dexamethasone (DEX 1 mg/kg) showed an improvement in colon’s length and a slight reduction in ratio W/L (61.61 ± 7.82) comparing to the one observed with the acetic acid group (76.09 ± 7.50), this reveals that DEX has a positive impact on colic inflammation but it is not significant.
Administration of the doses (0.5, 0.75 and 1g/kg) of β-Chitosane (for 6 days) by gavage significantly reduces inflammation. The improvement in colon’s length and weight and a significant decrease in W/L is remarkably significant in the batch treated with (1 g/kg) dose of β-Chitosane (51.11 ± 3.45), compared to the batch treated with the dose of (0.75 g/kg) and (0.5 g/kg), (54.20 ± 4.10) and (57.56 ± 10.02) respectively. This reduction in the W/L ratio provided by the dose of (1 g/kg) is significant in comparison with the untreated ill batch. The length of the colon is an another parameter affected during ulcerative colitis. The transition to the chronicity of the disease cause a colon shortening following to the epithelial cells apoptosis and alteration of proliferative function [29]. The weight of colic tissue is considered a reliable and sensitive indicator of the severity and extent of intestinal inflammation [30].
Results obtained with the diseased mices group (Colitis) treated with acetic acid revealed the presence of acute inflammation due to the inverse relationship between colon length and weight comparing to the witness batch. This is due to the invasion of the site of inflammation by cells and mediators such as pro-inflammatory cytokines IL-1, IL-6, IL-8, and TNFα, which increased the colon’s mass and it’s shortening due to necrosis, lesions and edema. As a result, the weight/length ratio is higher in the control colic group compared to the non-colic group. The treatment of colic mices with doses of 0.5; 0.75; 1 g/kg of β-Chitosane caused a decrease in the W/L ratio close to the one obtained with the batch treated with the reference drug, this allowed us to note its anti-inflammatory effect which reduced the clinical and the macroscopic damages induced by acetic acid. But the results of the W/L ratio and the macroscopic observations reveal a better effect of the dose 1g/kg than the other two doses and the reference drug. This allows us to suggest that β-Chitosane has an intestinal anti-inflammatory property and that the dose of 1 g/kg was better, more effective, and showed a significant reduction in IDA, in general we can say an improvement in the clinical conditions of mices.
Microscopic observations (histological study)
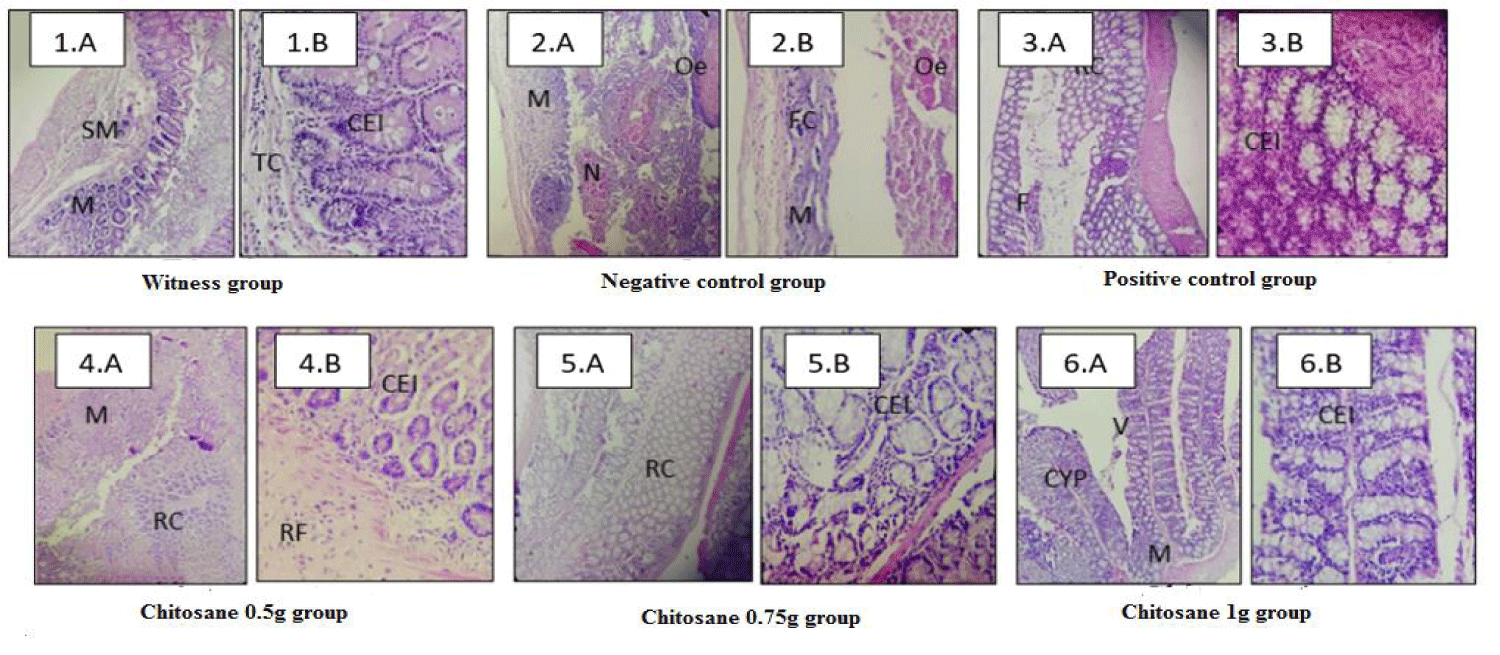
The figure 7.1 shows no histological damages in the witness mices groups. The colic wall was intact, very regular, well differentiated and signs of inflammation or necrosis were not observed. On the other hand, an active and a diffuse colitis (RCH) were found in the negative control group which had not received any treatment. Morphological evaluation of the shape of the colons showed pathological changes represented by extensive morphological disorientation and a severely damaged colic wall as shown in figure 7.2.A, a loss of crypt due to vasodilation and increased local blood flow, subsurface edema causing increased blood pressure and capillary permeability [27].
A mucosa that is largely necrotic and ulcerated with a large dense and polymorphic inflammatory infiltrate. From figure 7.2.A we can also observe the destruction of cells and the presence of some cellular Ghosts as shown in figure 7.2.B. This infiltration is the result of the release of inflammatory mediators (histamine, serotonin, etc.) that activate the vascular endothelium which induces an increase in the adhesive capacity of neutrophils and facilitates their migration to the lesion site [27]. This proves that acetic acid causes massive intracellular acidification leading to a lesion of the epithelial cells and an inflammatory response leading to severe damages to the mucous membranes and cell infiltration [31].
From another side, the treatment with Dexamethasone did not show a significant reduction in the severity of the damage but only a discreet recovery of the mucous membrane surface with a slight cellular regeneration as shown in figure 7.3. The crypts are still shortened and less numerous as shown in figure 7.3.A. Also a presence of some proliferations, inflammatory foci and some cellular phantoms as shown in figure 7.3.B. This provides evidence that Dexamethasone decreases inflammation but does not effectively treat the cause of the disease; it only acts by alleviating symptoms.
The figure 7.4 shows that colons treated with β-Chitosane at a dose of 0.5 g/kg showed regenerative mucosa with epithelial cell reconstruction and recovery of goblet cells with fibrous connective tissue. Also a disappearance of inflammation and edema with some focus of fibrous reorganization is observed.
The mices treated with β-Chitosane at a dose of 0.75g showed a significant decrease in the damage of this colic inflammation which is observed in a disappearance of edema and a remarkable recovery of the mucosa. In addition, we observed a regeneration of the caliciform cells (Goblet cell) as shown in figure 7.5.A.
For the batch treated with a dose of 1g of β-Chitosane the microscopic evaluation revealed a total reduction in colic damage. The tissue has gained back its regular appearance, well differentiated as shown in figure 7.6.A similar to the normal appearance of the colic wall of the untreated group with acetic acid. A remarkable reconstruction of epithelial cells as well as caliciform cells is shown in figure 7.6.B, the crypts and villus are back and returned to their normal dimensions.
This result suggests that the dose of 1g/kg of β-Chitosane has an anti-inflammatory effect better than the dose of 0.5 g and that of the dose of 0.75 g which is close to the one provided by the reference drug (Dexamethasone). This result closely matches the results obtained in morphological observations and score evaluation.
Discussion
In our study, colitis were caused by rectal instillation of acetic acid. This well-established model is close in its pathogenic mechanism of IBD in humans, in the disruption of the epithelial barrier inducing an exaggerated inflammatory mucosal response. By disrupting the intestinal epithelial barrier (which allows exposure of immune cells to luminal bacteria). Rectal instillation of acetic acid induces significant erosion of the colonic epithelium, resulting in an acute intestinal lesions and subsequent inflammation [32].
Acute diarrhea caused by the induction of acetic acid, are due to a decrease or even an inability of the colon to perform its power of absorption of water, electrolytes and mineral salts. This functional disorder is caused by the lesion of the epithelial barrier resulting from phenomenon of apoptosis (programmed death of cells), systematically relative with the increase in TNF-α (necrosis factor) in the seat of the ignition [33].
Inflammation characteristics such as redness, swelling, pain and heat were observed in mices treated with acetic acid. These characteristics are the main events of an inflammatory reaction that result from vasodilatation, increased capillary permeability and migration of macrophages to the infected site through the endothelial wall, by diapedresis phenomenon, in order to stimulate the production of pro-inflammatory mediators (IL-1, IL-6, IL-8, and Tnfα). This explains the observations of the macroscopic and microscopic damages caused by acetic acid on the colons of the treated mices and the increase in the W/L ratio. Studies have shown that oxidative stress could be the major factor in tissue damage and fibrosis associated with a pathological inflammatory reaction, which characterizes this kind of intestinal disease, with high levels of nitric oxide (NO) during ulcerative colitis. This increases the formation of peroxynitrites that promote lipid oxidation. The increase in lipid peroxidation capacity due to the change in oxidation state, leads to the erosion of the mucosa, distortion and loss of crypts and decreased antioxidant defence capacity of the immune system [18,34,35].
IBD are associated with a disturbance of the bacterial flora in the intestine (the intestinal microbiota). In constant contact with mucus and intestinal epithelial cells, it probably plays a major role in the occurrence of intestinal lesions. In fact, it now seems well established that during IBD, microflora plays a pro-inflammatory deleterious role.
Many clinical and experimental arguments illustrate this fact. The presence of microflora in the colon aggravates all experimental colitis in animals and humans’ model, the topography of preferential ileal and colic lesions represents the segments with the highest bacterial concentrations.
Molecular studies have identified some intestinal microbiota abnormalities during IBD such as strong instability of the microbiota over time, the presence of about 30% unusual bacteria and an increase in the bacterial concentration of the mucosa like Escherichia. Coli. In the case of ulcerative colitis [36].
Observations of the batch treated with the reference drug are interpreted by the anti-inflammatory power of Dexamethasone. This corticoid (glucocortecoide) derived from the synthesis of hydrocortisone (or cortisol) has a slight positive impact on colic inflammation due to lipocortine-1 which is one of the proteins whose production is generated by Dexamethasone. The mechanism by which Dexamethasone exerts its anti-inflammatory power is summed up in this protein which blocks the production of phospholipase A2, a substance involved in the occurrence of inflammatory reactions. In addition, Dexamethasone causes NF-κB retention in cytoplasm [37].
But the benefits of corticotherapy in inflammatory bowel disease must be weighed against the risks of short- and long-term adverse effects such as: cushing syndrome with lunar face, central obesity, osteoporosis, acne and lower extremity edem [38].
Due to the adverse effects of corticosteroids and different drug classes, the molecules that treat IBD are becoming increasingly popular, the most common are biopolymers.
This study demonstrated that β-Chitosane administered after induction of colitis was particularly relevant for the application in the treatment of colitis, because it has reduced intestinal inflammation and suppressed the damage caused by it in mices with acute intestinal inflammation, also a recovery of the mucosa through a regeneration of the epithelial wall was observed after the administration of this biopolymer.
This finding supports future prospects for the therapeutic utility of β-Chitosane in the treatment of IBD [39] and reported that, since β-Chitosane oligosaccharide is resistant to degradation by digestive enzymes. Ingested it could reach the colon and provide protection against colitis by acting directly on the Intestinal Epithelial Cells. IECs have been shown to express Toll-type receptors (e.g. TLR4) and many other cytokine and chemokine receptors. LPS and TNF, the main stimulators of aberrant inflammation in IBD, interact with their respective receptors (TLR4 and TNFR 1 and 2) and cause the production of pro-cytokines inflammatory mediated by the transcription factor NF-κB in the IECs. In the case of IBD, an increased expression of TLR4 and TNFR in IECs has been linked to inflammatory mucosal responses induced by exaggerated IECs, therefore, a dysfunction of the intestinal epithelial barrier. Indeed, previous work has revealed that treatment with β-Chitosane oligosaccharide significantly attenuates the NF-κB activation stimulated by LPS and TNF as well as the production of TNF-α and IL-6 in human colon T84 epithelial cells. As it has recently been shown that TNF derived from IEC is sufficient to cause pathology similar to that of Crohn in mice, it is therefore suggested that the inhibitory effect of COS on the activation of NF-κB and pro-inflammatory cytokines production in the IECs could explain the anti-inflammatory effect of β-Chitosane observed in mouse colitis models.
As a result, previous results indicated that COS could suppress NF-κB activation in the IEC from the beginning of the inflammatory reaction and those by preventing binding of bacterial products (LPS) their membrane receptors (e.g. TLR4), leading to the attenuation of mucosal immune activation in colitis mouse models. In addition, they demonstrated that treatment with COS prevented the reduction of transepithelial electrical resistance induced by +LPS and TNF as well as TNF-induced apoptosis in T84 cells [39].
These results suggest that oral administration of β-Chitosane could preserve the integrity of the intestinal epithelial barrier by antioxidant, anti-apoptotic and antibacterial mechanisms, thus providing protection against the initiation and progression of colitis in vivo.
Another study demonstrated the efficacy of β-Chitosane in colon tissue regeneration. β-Chitosane degradation in vertebrates is ensured by lysozymes and bacterial enzymes found predominantly in the colon [40]. Indeed this mechanism has been reported in rats [41] and we also know that there is a great similarity between the human colic flora and that of the rodent [42].
β-Chitosane has a myriad of biological properties such as accelerated wound healing, stimulation of angiogenesis, collagen production, interleukin secretion (8) by fibroblasts and antimicrobial effects [43]. β-Chitosane has been shown to have an inhibitory effect on E. Coli and various pathogenic bacteria in the intestine at high AD, low molecular weight [44,45] and has a positive zeta potential. This is due to the high density of the amino groups –NH3+ which confers a positive charge on the polymer. This charge forms polyelectrolytic complexes with negative peptidoglycans of the bacterial wall and inhibits it [24].
In addition, previous work has shown that different molecular weights of β-Chitosane have a range of anti-inflammatory actions, including inhibition of production of TNF-α, IL-6, prostaglandin E2 (PGE2), cyclooxygenase-2 (COX-2), VCAM-1 and ICAM-1 in vitro [46]. Also, a decrease in oxidative tissue lesions, septic lesions, organ lesions, and cytokine levels mediated by this biomolecule was observed [47,48].
The results obtained are consistent with the data in the literature and suggest that oral administration of β-Chitosane could preserve the integrity of the intestinal epithelial barrier by anti-oxidants, anti-apoptotic and antibacterial mechanisms, thus providing protection against initiation and progression of colitis in vivo. From this fact it is concluded that β-Chitosane possesses a very important anti-inflammatory activity and can therefore serve as an excellent natural treatment for many diseases like ulcerative hemorrhagic colitis. β-Chitosane may stimulate the inflammatory response within a short six-day period at a dose of 1 g for the entire treatment.
Conclusion
Based on the results obtained and the literature data we found that β-Chitosane derived from squid feather has an anti-inflammatory therapeutic effect against ulcerative rectocolitis-hemmoragic by inhibiting the production of inflammation markers like TNF- α, IL-6, by exerting its antioxidant and antibacterial power against the harmful bacteria accompanying this disease and through its regenerative power on the destroyed tissues and cells.
References
- Louis, et al, Génétique et environnement dans les maladies inflammatoires chroniques de l’intestin. Renue Médicale de Liège. 2012;67: 298-304.
- Park KT, Ehrlich OG, Allen JI, Meadows P, Szigethy EM, Henrichsen K, Kim SC, Lawton RC, Murphy SM, Regueiro M, Rubin DT, Engel-Nitz NM, Heller CA. The Cost of Inflammatory Bowel Disease: An Initiative From the Crohn’s & Colitis Foundation. Inflamm Bowel Dis. 2020 Jan 1;26(1):1-10. doi: 10.1093/ibd/izz104. Erratum in: Inflamm Bowel Dis. 2020 Jun 18;26(7):1118. PMID: 31112238; PMCID: PMC7534391.
- Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang T, Chen H, Zhu W, Sartor RB, Boedeker EC, Harpaz N, Pace NR, Li E. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2011 Jan;17(1):179-84. doi: 10.1002/ibd.21339. Epub 2010 Sep 13. PMID: 20839241; PMCID: PMC3834564.
- Welcker, et al, Augmenntation de la perméabilité intestinale chez les patients atteints d’une maladie inflammatoire de l’intestin. European Journal of Medical Research. 2004;9:456-460.
- Pizarro TT, Cominelli F. Cytokine therapy for Crohn’s disease: advances in translational research. Annu Rev Med. 2007;58:433-44. doi: 10.1146/annurev.med.58.121205.100607. PMID: 17217333.
- Bouhnik Y, Manifestations extradigestives des maladies inflammatoires. 2006.
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003 Jul;3(7):521-33. doi: 10.1038/nri1132. PMID: 12876555.
- Vidal A, Gómez-Gil E, Sans M, Portella MJ, Salamero M, Piqué JM, Panés J. Life events and inflammatory bowel disease relapse: a prospective study of patients enrolled in remission. Am J Gastroenterol. 2006 Apr;101(4):775-81. doi: 10.1111/j.1572-0241.2006.00476.x. Epub 2006 Feb 22. PMID: 16494590.
- Cortot A, et al. Inflammatory bowel disease: Genetir or environmental diseases? Gastroenterologie clinique et biologique. 2008;33(8-9):681-691.
- Sagheer A, et al. characterization of chintin and chitosan from marinesources in Arabian Gulf Carbohydrate. Polymers. 2009;77:410-419.
- Seng JM. Chitine, Chitosane et dérivés; de nouvelles perspectives pour l’industrie. Biofuture. 1988;9 :40‐44.
- Aimin C, Chunlin H, Juliang B, Tinyin Z, Zhichao D. Antibiotic loaded chitosan bar. An in vitro, in vivo study of a possible treatment for osteomyelitis. Clin Orthop Relat Res. 1999 Sep;(366):239-47. PMID: 10627741.
- No HK, Park NY, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002 Mar 25;74(1-2):65-72. doi: 10.1016/s0168-1605(01)00717-6. PMID: 11929171.
- Jarmila V, Vavríková E. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities--a review. Curr Pharm Des. 2011;17(32):3596-607. doi: 10.2174/138161211798194468. PMID: 22074429.
- Xianomin Wang, LZ, Tianzhao Han, Shaofei Chen, Jialing Wang. Protective effects of 2,3,5,4’ tetrahydroxystilbene -2-O-beta-D-glucoside, an active component of polygonum multiflorum Thumb, on experimental colitis in mice. European Journal of Pharmacology. 2008.
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993 Aug;69(2):238-49. PMID: 8350599.
- Deshmukh CD, Pawar AT, Bantal V. Protective effect of Emblica officinalis fruits extract on acetic acid induced colitis in rats. Research Journal of Medicinal Plants. 2010;4(3):141-148. doi: 10.3923/rjmp.2010.141.148
- Sotnikova R, Nosalova V, Navarova J. Efficacy of quercetin derivatives in prevention of ulcerative colitis in rats. Interdiscip Toxicol. 2013 Mar;6(1):9-12. doi: 10.2478/intox-2013-0002. PMID: 24170973; PMCID: PMC3795315.
- Vllasaliu D, Exposito-Harris R, Heras A, Casettari L, Garnett M, Illum L, Stolnik S. Tight junction modulation by chitosan nanoparticles: comparison with chitosan solution. Int J Pharm. 2010 Nov 15;400(1-2):183-93. doi: 10.1016/j.ijpharm.2010.08.020. Epub 2010 Aug 19. PMID: 20727955.
- Lamprecht A, Schäfer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res. 2001 Jun;18(6):788-93. doi: 10.1023/a:1011032328064. PMID: 11474782.
- MARIO F. Systèmes multiparticules par voie orale dans les maladies inflammatoires chroniques de l’intestin. 2012.
- Briant J. Phénomènes d’interface agents de surface: Principes et modes d’action. 1989.
- Kaci G. Caractérisation des propriétés anti-inflammatoires de souches commensales de Streptococcus salivarius. Paris; 2012. p.11.
- Sadeghi AM, Dorkoosh FA, Avadi MR, Saadat P, Rafiee-Tehrani M, Junginger HE. Preparation, characterization and antibacterial activities of chitosan, N-Trimethyl Chitosan (TMC) and N-Diethylmethyl Chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation mothods. International Journal of Pharmaceutics. 2007 Dec 4;355(1-2):299-306. doi: 10.1016/j.ijpharm.2007.11.052
- Qi L, Xu Z, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res. 2004 Nov 15;339(16):2693-700. doi: 10.1016/j.carres.2004.09.007. PMID: 15519328.
- Schulzke JD, Bojarski C, Zeissig S, Heller F, Gitter AH, Fromm M. Disrupted barrier function through epithelial cell apoptosis. Ann N Y Acad Sci. 2006 Aug;1072:288-99. doi: 10.1196/annals.1326.027. PMID: 17057208.
- Stevens ALBJ, Young. Anatomie pathologique. 4éme édition. de Boeck Supérieur; 2004. p.10-11.
- Yougbaré-Ziébrou, Ouédraogo N, Lompo M, Bationo H, Yaro B, Gnoula C, Sawadogo WR, Guissou IP. Anti-inflammatory, analgesic and antioxidant activities of an aqueous extract of Saba senegalensis Pichon stems with leaves (Apocynaceae) Pharmacognosie. 2015 October 4;14:213-219. doi : 10.1007/s10298-015-0992-5
- Topcu-Tarladacalisir Y, Akpolat M, Uz YH, Kizilay G, Sapmaz-Metin M, Cerkezkayabekir A, Omurlu IK. Effects of curcumin on apoptosis and oxidoinflammatory regulation in a rat model of acetic acid-induced colitis: the roles of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. J Med Food. 2013 Apr;16(4):296-305. doi: 10.1089/jmf.2012.2550. PMID: 23566056.
- Zeng C, Xiao JH, Chang MJ, Wang JL. Beneficial effects of THSG on acetic acid-induced experimental colitis: involvement of upregulation of PPAR-γ and inhibition of the Nf-Κb inflammatory pathway. Molecules. 2011 Oct 12;16(10):8552-68. doi: 10.3390/molecules16108552. PMID: 21993246; PMCID: PMC6264228.
- Otari KV, Gaikwad PS, Shete RV, Upasani CD. Protective effect of aqueous extract of Spinacia oleracea leaves in experimental paradigms of inflammatory bowel disease. Inflammopharmacology. 2012 Oct;20(5):277-87. doi: 10.1007/s10787-011-0114-4. Epub 2012 Jan 11. PMID: 22234676.
- Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995 Oct;109(4):1344-67. doi: 10.1016/0016-5085(95)90599-5. PMID: 7557106.
- Touboul J. Diarrhée de l’épisode aigu infectieux au trouble foncionnel chronique. Actuelitéhérapeutique. 2007;10:164-167.
- V VP, C G. Protective effect of marine mangrove Rhizophora apiculata on acetic acid induced experimental colitis by regulating anti-oxidant enzymes, inflammatory mediators and nuclear factor-kappa B subunits. Int Immunopharmacol. 2014 Jan;18(1):124-34. doi: 10.1016/j.intimp.2013.11.007. Epub 2013 Nov 21. PMID: 24269623.
- Bounihi, Criblage phytochimique, Étude Toxicologique et Valorisation Pharmacologique de Melissa officinalis et de Mentha rotundifolia (Lamiacées). Thèse de Doctorat de Sciences du Médicament. 2016.
- Elodie Q, Philippe S. Intestinal microbiota: From antibiotic-associated diarrhea to inflammatory bowel diseases. La Presse Médicale. 2012 January;42(1):45-51. doi : 10.1016/j.lpm.2012.09.017
- Lee A, De Mei C, Fereira M, Marotta R, Yoon HY, Kim K, Kwon IC, Decuzzi P. Dexamethasone-loaded Polymeric Nanoconstructs for Monitoring and Treating Inflammatory Bowel Disease. Theranostics. 2017 Aug 23;7(15):3653-3666. doi: 10.7150/thno.18183. PMID: 29109767; PMCID: PMC5667339.
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005 Oct 20;353(16):1711-23. doi: 10.1056/NEJMra050541. PMID: 16236742.
- Yousef M, Pichyangkura R, Soodvilai S, Chatsudthipong V, Muanprasat C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: therapeutic efficacy and possible mechanisms of action. Pharmacol Res. 2012 Jul;66(1):66-79. doi: 10.1016/j.phrs.2012.03.013. Epub 2012 Mar 28. PMID: 22475725.
- Jain SK, Jain A, Gupta Y, Ahirwar M. Design and development of hydrogel beads for targeted drug delivery to the colon. AAPS PharmSciTech. 2007 Jul 13;8(3):E56. doi: 10.1208/pt0803056. PMID: 17915806; PMCID: PMC2750443.
- Zhang H, Neau SH. In vitro degradation of chitosan by bacterial enzymes from rat cecal and colonic contents. Biomaterials. 2002 Jul;23(13):2761-6. doi: 10.1016/s0142-9612(02)00011-x. PMID: 12059026.
- Gulbake A, Jain SK. Chitosan: a potential polymer for colon-specific drug delivery system. Expert Opin Drug Deliv. 2012 Jun;9(6):713-29. doi: 10.1517/17425247.2012.682148. Epub 2012 Apr 24. PMID: 22530707.
- Azuma K, Izumi R, Osaki T, Ifuku S, Morimoto M, Saimoto H, Minami S, Okamoto Y. Chitin, chitosan, and its derivatives for wound healing: old and new materials. J Funct Biomater. 2015 Mar 13;6(1):104-42. doi: 10.3390/jfb6010104. Retraction in: J Funct Biomater. 2018 Jun 07;9(2): PMID: 25780874; PMCID: PMC4384104.
- Zheng LY, Zhu JF. Study on antimictobial activity of chitosan with different molecular weights. Carbohydr Polym. 2003;54(4):527-530. Doi : 10.1016/j.carbpol.2003.07.009
- Benbbou, R. Developpment Et Caracterisation De Films Antimicrobiens Pour La Biopréservation Des Produits Marins Prêts À Consommer. 2009.
- Qiao Y, et al. Synergistic Activation of inflammatory Cytokine Gene by interferon-g-induced Chromatin Remodeling and Toll-like receptor signaling, USA 5Graduate Program in Immunology and Microbial Pathogenesis, Weill Cornell Graduate School of Medical Sciences, New York, NY 10065, USA.
- Chou TC, Fu E, Shen EC. Chitosan inhibits prostaglandin E2 formation and cyclooxygenase-2 induction in lipopolysaccharide-treated RAW 264.7 macrophages. Biochem Biophys Res Commun. 2003 Aug 22;308(2):403-7. doi: 10.1016/s0006-291x(03)01407-4. PMID: 12901883.
- Citgez B, Cengiz AN, Akgun I, Uludag M, Yetkin G, Bahat N, Ozcan O, Polat N, Akcakaya A, Karatepe O. Effects of chitosan on healing and strength of colonic anastomosis in rats. Acta Cir Bras. 2012 Oct;27(10):707-12. doi: 10.1590/s0102-86502012001000007. PMID: 23033132.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.