Medicine Group. 2021 November 23;2(11):1132-1135. doi: 10.37871/jbres1358.
Evaluation of Antimicrobial Activity of Acacia etbaica Water Extract Leafs against Some Pathogenic Microorganisms
Abdulmageed B Abdullah1, Abdulbaki Al-zaemey1, Rasheed Hasan Mudhesh Al-Husami1* and Mofeed Al-Nowihi2
2Department of microbiology, Faculty of Science, Sana'a University, Yemen
- Antimicrobial activity
- COVID-19
- Acacia etbaica
- Water extract
- Pathogenic microorganisms
Abstract
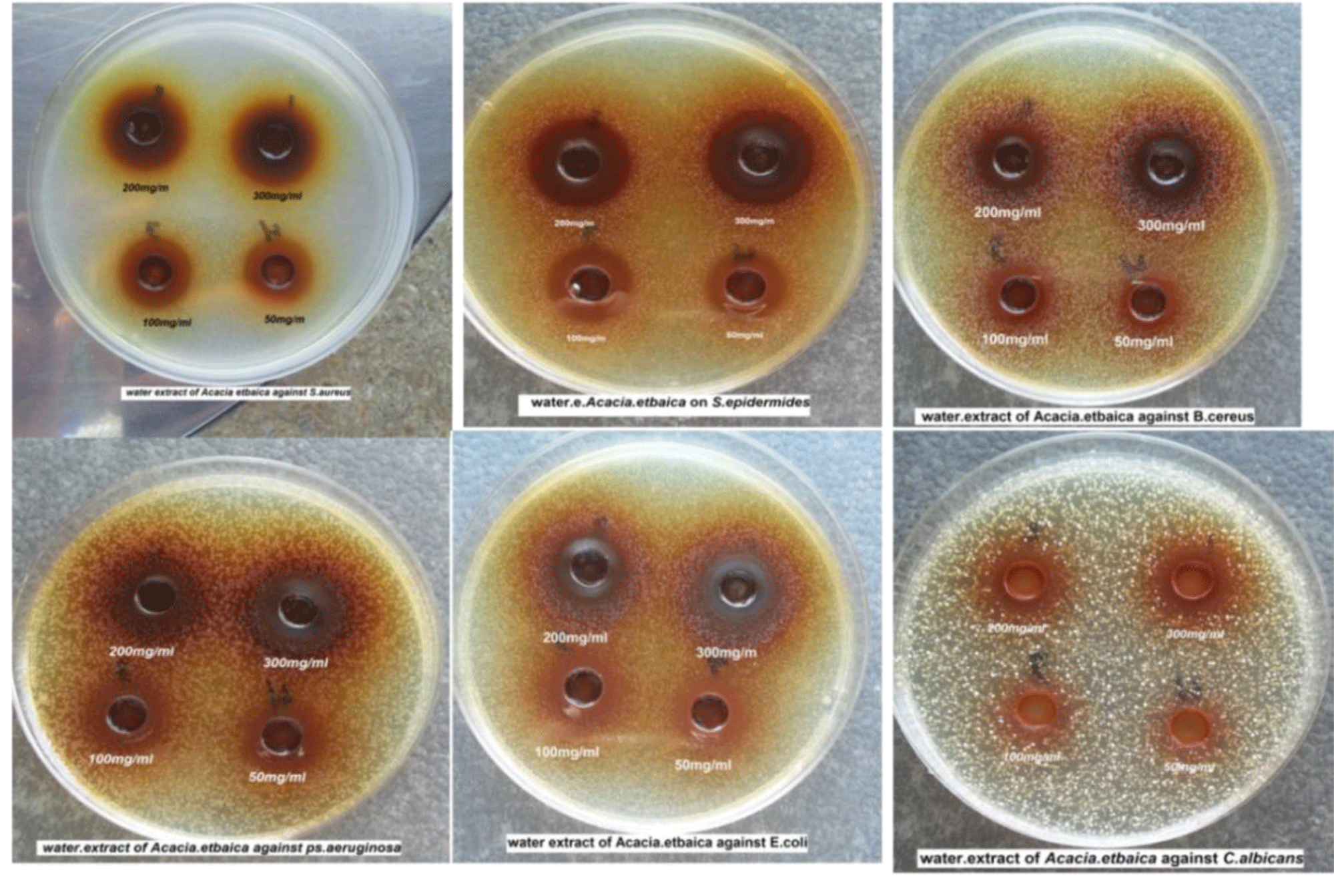
The antimicrobial activity about water extract of Acacia etbaica was examined by using agar well diffusion methods against five gram-positive and negative bacteria[Staphylococcus aureus (ATCC653-8), Pseudomonas aeruginosa MTCC2453, Bacillus cereus (ATCC6633), Escherichia coli MTCC739, and one local isolate (Staphylococcus epidermides)] in addition to Candida albicans (ATCC2019). this results designated that the water extract of Acacia etbaica possess antimicrobial efficacy against all tested microorganisms either (gram-positive and negative bacteria) or fungi (Candida albicans).Whereas the gram-positive bacteria (S. aureus, S. epidermides and B. cereus) with inhibition zones (21, 19.5 and 16.5) respectively was more sensitive than gram-negative bacteria (P. aeruginosa and E. coli) and C. albicans with inhibition zones 16 and 14.5 respectively. The antimicrobial effect was directly proportional with concentration of water extract where the highest inhibition zone at higher concentration 30% equal 21mm against S.aureus and the lower inhibition zone at lower concentration 5% equal 10mm against C. albicans
Introduction
The Acacia etbaica Schweinf subspecies etbaica, family: Leguminosae is a one of the most widespread plant in Yemen, locally known as ‘Qarad’ [1-3] has been reported that several Acacia species have been used to prepare disinfectant for microorganisms and for hands washes. Yemen has a rich culture of medicinal herbs, but only very few have been studied chemically and pharmacologically for their potential medicinal value [1]. Very few works has been carried out on the leaves of this plant toward documenting its ethnomedicinal uses and establishing its chemical constituents [4]. In Yemen it used as medicinal plants in traditional healthcare and well known in rural areas in many developing countries [5]. According to World Health Organization medicinal plants would be the best source to obtain a variety of drugs. About 80% of individuals from developed countries use traditional medicine, which has compounds derived from medicinal plants. Therefore, such plants should be investigated to better understand their properties, safety and efficiency. For a long period of time, plants have been a valuable source of natural products for maintaining human health, especially in the last decade, with more intensive studies for natural therapies [6]. Medicinal plants produce a variety of compounds such as phenolics, flavonoids, alkaloids, glycosides, saponins essential oils, mucilages and tannins in all of their parts (root, stem, bark, leaves, seeds) which possess a healing physiological effect in the treatment of various diseases of animals and humans [7]. The tribal people use the plants in their unique ways for various purposes mostly for the treatment of various diseases [8]. It has been reported that the plant Acacia sp was used to treat swelling, ring worm infection, hemorrhoids, scabies, fire burn, eye infection of livestock and anthrax by the community of Kilte Awulaelo District of Tigray Region, Ethiopia [9]. The fresh plant parts of different Acacia species are considered as astringent, spasmolytic, demulcent, anthelmintic and abortifacient in Indian traditional medicine system [10,11]. The Acacia species used to treat a variety of disease including malaria, leprosy and most concerning cancer [12]. Acacias have been cultivated in many countries for various purposes whereas it have a valuable wood that can be used for industries, decorations and as an important sources for gum (Arabian gums), tannins, perfumes, ink, proteins and paint [13,14].
This research aimed to investigate the potential antimicrobial activity of Acacia etbaica to ascertain the rationale for its use in traditional medicine.
Materials and Methods
Preparation of sample
Sample collection: The Acacia etbaica leafs were collected from Yemeni rural - Bani Alhusam -Taize government and identified according to guideline of the [15,16]. The collected plant leafs were dried in an open air protected from direct sun light. The dried plant samples finely ground by using an electrical grinder to a fine powder and made ready for extraction.
Preparation of extract: The fine powder of Acacia etbaica was extracted by using 1:10 in purified water at room temperature for 48 hours with occasional shaking. Then filtered by using filter paper (Whatman No 1.5, whatman Ltd., England). The water extract was evaporated to dryness in oven at 45ºC to yield dry crude extract. The obtained crude extract was filled to amber tight closed bottle glass in refrigerator until uses.
Antimicrobial assay
Microorganisms used include: The tested microorganisms have gotten from Science college, Sana’a university, Republic of Yemen include: Escherichia coli MTCC739 Mar.2001, Pseudomonas aeruginosa MTCC2453 Mar.2001, Staphylococcus aureus (ATCC653-8), local isolate (Staphylococcus epidermides), Bacillus cereus (ATCC6633) and Candida albicans(ATCC2019).
The bacterial strains were maintained on Trypton Soya Gar Medium (TSDA), and the fungal strains were maintained on Sabouraud dextrose agar (SDA) medium.
Antimicrobial and Antibiotic assay: The Antimicrobial and Antibiotic assay was performed by using Agar Well Diffusion Method according to (USDA, 2007 and Mahesh & Satish, 2008).
Antimicrobial assay: Four different concentrations of Acacia etbaica water extract 300 mg/ml (30%), 200 mg/ml (20%), 100 mg/ml(10%) and 50 mg/ml(5%) were dissolved in sterile purified water to be used in antimicrobial assay test. Extract solutions were prepared directly before the test. Antimicrobial activity of the extracts was determined by agar well diffusion method as described by (USDA, 2007 and Mahesh & Satish, 2008) on Tryptone soya Agar (TSA) (Figure 1).
In each of these plates four wells were cut out by using a standard cork borer (8 mm). About 60μl of each extract was added into different wells (triplicate each concentration). A positive control antibiotic disc was placed in the other plate. All the plates were incubated for 24h at 37°C. After incubation bioactivity was evaluated by measuring zone of inhibition. All the experiment was performed in triplicates.
Antibiotic assays: The Antibiotic assay was performed by using antibiotic standard disc diffusion. The antibiotic standard disc was get from HiMedia & oxoid company which are Aztreonam (AT30), Ceftazidin (CAZ30, Gentamycin (Gen10), Nystatin (Nys100IU), Norfloxacin (NOR5) and Vancomycin (VA30).
Results
Antimicrobial Activity of water extract
The results in present study showed that the water extract to Acacia etbaica possess high antimicrobial efficacy against tested microorganisms (gram positive and negative bacteria) in addition to C. albicans. results appeared that the tested gram-positive bacteria more sensitive than tested gram-negative bacteria and C. albicans where the highest effect was against S. aureus with inhibition zone equal 21 mm at high concentration(30%) followed by S. epidermides and B. cereus with inhibition zone 19.5 and 16.5mm respectively. whereas the gram negative bacteria (P. aeruginosa and E. coli) as well as C. albicans was low sensitive to these extract with inhibition zone 16 and 14.5mm respectively. results illustrated that the highest concentration (30%) was more effect against S. aureus and lower effect at lower concentration (5%) against C. albicans. these effected results showed in table 1.
| Table 1: Water extract activity of Acacia etbaica against tested microorganism at different concentrations. | ||||||||||
| Type of microorganism | Concentration % | Name of antibiotic standard | ||||||||
| 5% | 10% | 20% | 30% | AT30 | CAZ30 | NOR5 | Gen10 | VA30 | Nys100IU | |
| Zone of inhibition in mm | ||||||||||
| Staphylococcus aureus | 16 | 16.5 | 20 | 21 | 0 | 23 | 20 | 22 | 21 | NT |
| Staphylococcus epidermides | 15 | 16 | 19 | 19.5 | 0 | 19 | 20 | 23 | 22 | NT |
| Bacillus cereus | 12 | 12.5 | 15 | 16.5 | 0 | 24 | 30 | 27 | 30 | NT |
| Pseudomonas aeruginosa | 12 | 12.5 | 14 | 16 | 24 | 21 | 12.5 | 21 | 0 | NT |
| Escherichia coli | 11.5 | 12 | 15 | 16 | 29 | 27 | 26.5 | 19.5 | 12 | NT |
| Candida albicans | 10 | 12 | 14 | 14.5 | 0 | 0 | 0 | 0 | 0 | 13.8 |
Discussion
In water extract results showed a high antimicrobial activity at all concentrations against S.aureus. While, showed a low antimicrobial activity against E.coli at all concentrations. This results were agreed with previous study conducted by [17-19] who reported that the highest activity against S.aureus. Regarding to E.coli was agree with the results conducted by [18,19] while disagree with results by [17]. In recent study, B.cereus as well as P.aeruginosa showed sensitive to water extract of A.etbaica and this results agree with study conducted by [17] while disagree with results conduct by [18] who evaluated other species of Acacia (A. nilotica). Mechanism that the crude plant extract acting against gram-positive and gram-negative bacteria by changes in membrane potential leading to hyperpolarization and decreasing cytoplasmic pH [20]. This changes that occurred in the internal pH and Hyperpolarization has been suggested to be one of the primary indicators of membrane damage of bacteria cells [21,22]. The differences in sensitivity of tested microorganisms may be return to different in concentration of extracts and microorganisms strains [19,23] was attributed the difference in the sensitivity of microorganisms to the differences in the ability of the active ingredients in the plant to penetrate through the cell wall and cell membrane of the tested microorganisms. In the former study by [4] on the phytochemical constituents of Acacia etbaica who evaluated that many of phytochemical compound like saponins, flavonoids, coumarins, tannins, triterpenes, sterols, amino acid and protein, sterols, triterpenes, carbohydrates and glycosides found in all extracts including water extract. This chemical compounds play essential role in its bioactivity. The antibacterial activity of flavonoids is probably due to their ability to complex with extracellular and soluble proteins and to complex with bacterial cell walls [24]. Terpenes are biologically active molecules and are considered to be part of plants defence systems and as such have been included in a large group of protective molecules found in plants named as ‘phytoprotectants’ [25]. The results of this study revealed that the gram-negative bacteria as well as C. albicans was more resistance to A. etbaica water extract than gram-positive extract these differences in sensitivity return to variations in chemical composition of microbial cell wall where the major components of the outer surface of gram-negative bacterial cell wall compositions is lipopolysaccharide layer with the combination of proteins and phospholipids, where heavy lipopolysaccharide layer hindered of most compounds from Access to the peptidoglycan layer of the cell wall [26,27] has reported that other genus of Acacia (Acacia nilotica) contain all the essential amino acids in good proportions comparable to egg protein. Mechanism of action plant may be the formation of ion channels in the microbial membrane through diverse molecular modes and bind to chitin or increasing the permeability of the fungal membrane and cell wall. This defense system of plants provide protection them against potential microbial invaders. Among these other plants secreted compounds several proteins or peptides and carbohydrates have a specific defense mechanism against phytopathogenic microbes by inhibiting their growth [24].
Conclusion
The high antimicrobial clear efficiency to water extract of Acacia etbaica leafs against many of microbial strains that causes different important diseases to human and animal confirming the traditional herbal medicines usages and provide a scientific basis for traditional medicine uses of water extract of Acacia etbaica leafs in primary health care.
References
- Wood J R I. Royal botanical gardens: Kew 1997;p169.
- Isaacs J. Bush Food: Aboriginal food and herbal medicine. Sydney: Lansdowne Publishing Pty Ltd; 1987.p.256.
- Cock IE. Medicinal and aromatic plants Australia, in Ethnopharmacology section, Biological, Physiological and Health Sciences, Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, EOLSS Publishers, Oxford, UK. https://tinyurl.com/d2f9ekbj
- Algfri SK, Algifri AN, Alshakka MA, Taleb M, Munaiem RT. Anatomical and phytochemical studies of the leaves of Acacia etbaica subspecies Etbaica. RJPBCS. 2014;5(4):809. https://tinyurl.com/y89c39nh
- Sandhu DS, Heinrich M. Phytotherapy Res. 2005;19:633-642.
- Nascimento GGF, Locatelli J, Freitas PC, Silva GL. Antibacterial Activity of Plant Extracts and Phytochemicals on Antibiotic resistant. BJM. 2000;31:247-256. doi: 10.1590/S1517-83822000000400003
- Adhikari BS, Babu MM, Saklani PL, Rawat GS. Medicinal plants diversity and their conservation status in Wildlife Institute of Indi (WII) Campus, Dehradun. Ethno-Botanical Leaflets. 2010;14: 46-56. https://tinyurl.com/uehcnw8h
- Kumar N, Wani ZA, Dhyani S. Ethnobotanical study of the plants used by the local people of Gulmarg and its allied areas. Int J Curr Res Biosci Plant Biol. 2015;(9):16-23. https://tinyurl.com/yun27y4b
- Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. 2013 Sep 8;9(1):65. doi: 10.1186/1746-4269-9-65. PMID: 24011232; PMCID: PMC3852788.
- Duke JA, Godwin MJ, Cellier BJ, Duke PAK. Handbook of medicinal herbs. 2nd Ed. CRC PRESS Boca Raton London New York Washington: DC, USA. 2002.
- Khare CP. Indian Medicinal Plants, an illustrated dictionary. Springer Science, Business Media, LLC. New York, USA.
- Saini ML, Saini R, Roy S, Kumar A. Comparative pharmacognostical and antimicrobial studies of Acacia species (Mimosaceae). Journal of Medicinal Plants Research. 2008;2(12):378-386
- Seigler DS. Phytochemistry of Acacia sensulato. Biochem. Sys. Ecol. 2003;31(8):845-873.
- Arias ME, Gomez JD, Cudmani NM, Vattuone MA, Isla MI. Antibacterial activity of ethanolic and aqueous extracts of Acacia aroma Gill. ex Hook et Arn. Life Sci. 2004 May 28;75(2):191-202. doi: 10.1016/j.lfs.2003.12.007. PMID: 15120571.
- Ministry of tourism and environment. The general consortium for environment protection, wild plants from Yemen. Ministry of tourism and environment, Yemen. 2002.
- Al-Dubaie AS, Al-Khulaidi AA. Medical and Aromatic Plant in Yemen. Aubade center Sana'a-Republic of Yemen. 2005.
- Al-Fatimi M, Wurster M, Schröder G, Lindequist U. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J Ethnopharmacol. 2007 May 22;111(3):657-66. doi: 10.1016/j.jep.2007.01.018. Epub 2007 Jan 19. PMID: 17306942.
- Patel JD, Shrivatava AK, Kumar V. Evaluation of some medicinal plants used in traditional wound healing preparations for antibacterial property against some pathogenic bacteria. Journal of Clinical Immunology and Immunopathology Research. 2009;1(1):7-12. https://tinyurl.com/k6m6h44c
- Osman M. In vitro Antibacterial activity of Acacia nilotica (Quradh) extract against selected pathogenic bacteria. AJMSC. 2017;2(9). https://tinyurl.com/vyx842n
- Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M, Hatab SR. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front Microbiol. 2018 Jul 24;9:1639. doi: 10.3389/fmicb.2018.01639. PMID: 30087662; PMCID: PMC6066648.
- Sánchez E, García S, Heredia N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl Environ Microbiol. 2010 Oct;76(20):6888-94. doi: 10.1128/AEM.03052-09. Epub 2010 Aug 27. PMID: 20802077; PMCID: PMC2953017.
- Vanhauteghem D, Janssens GP, Lauwaerts A, Sys S, Boyen F, Cox E, Meyer E. Exposure to the proton scavenger glycine under alkaline conditions induces Escherichia coli viability loss. PLoS One. 2013;8(3):e60328. doi: 10.1371/journal.pone.0060328. Epub 2013 Mar 27. PMID: 23544135; PMCID: PMC3609748.
- Banso A. Phytohemical and antibacterial investigation of bark extracts of Acacia nilotica. J Med Plant Res.2009;3(2):82-85. https://tinyurl.com/4j7syccp
- Doss A, Vijayasanthi M, Parivuguna V, Venkataswamy R. Antimicrobial effects of the flavonoid fractions of Mimosa pudica L. leaves. J Pharmacy Res. 2011;4(5):1438-1439. https://tinyurl.com/3d76fexj
- Morrissey JP, Osbourn AE. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol Mol Biol Rev. 1999 Sep;63(3):708-24. doi: 10.1128/MMBR.63.3.708-724.1999. PMID: 10477313; PMCID: PMC103751.
- Naqvi SH, Dahot MU, Rafiq M, Khan MY, Imran I, Lashari KH, Asghar A, Korai AL. Anti-microbial efficacy and biochemical analysis from different parts of Acacia nilotica L. and Ricinus communis L. extracts. JMPR Vol. 2011;5(27):6299-6308. https://tinyurl.com/bmnt8r8n
- Barman K, Rai SN. Utilization of tanniniferous feeds: Chemical composition, amino acid profile, and tannin fractionation of certain Indian agro-industrial by products. Indian J. Anim. Sci. 2016;76:71-80. https://tinyurl.com/9wk2fc7m
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.