Medicine Group . 2022 January 31;3(1):111-113. doi: 10.37871/jbres1409.
Haemolytic Anaemia Due to Early Failure of Mitral Valve Repair with Artificial Neochordae: A Technical Error
Fabrizio Ceresa1*, Antonino Salvatore Rubino1, Concetta Zito2 and Francesco Patane1
2Department of Clinical and Experimental Medicine, University of Messina, Italy
- Mitral valve replacement
- Haemolysis
- Cardiac surgery
Abstract
We report the case of an unexpected massive haemolysis occurred early after mitral valve repair. Potential underlying causes have been investigated, with review of the literature. After mitral valve replacement, haemolysis disappeared and the patient recovered, returning to normal life after 12 months.
Case Report
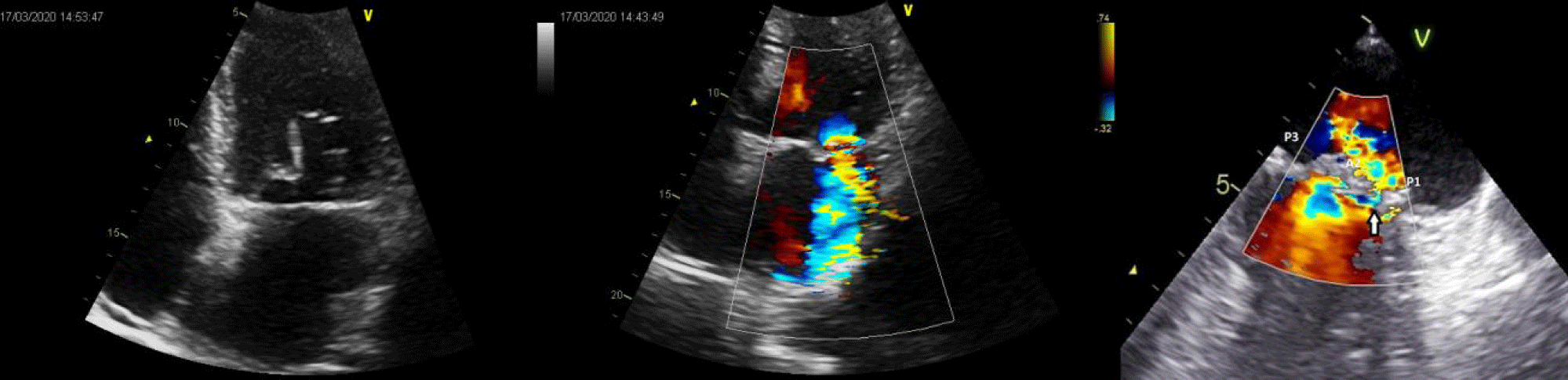
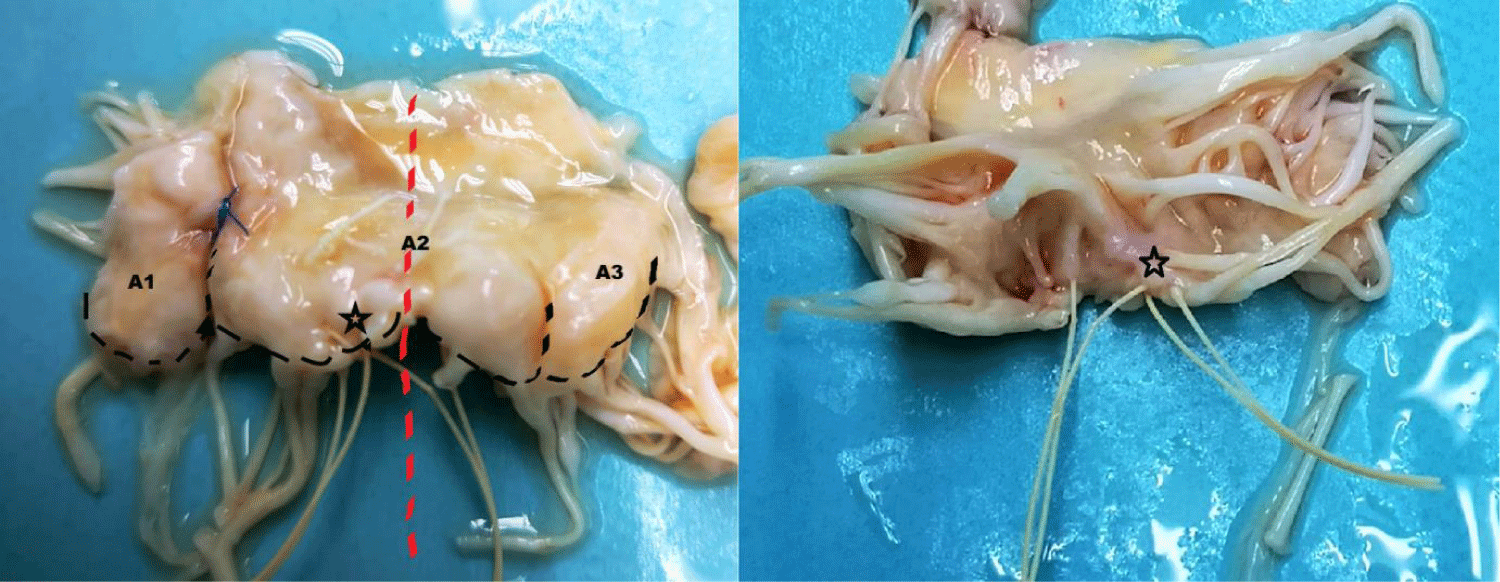
A 65-year-old man, with severe mitral regurgitation secondary to mitral valve prolapse, underwent mitral valve repair with two pairs of artificial neochordae to A2 and annuloplasty with semi-rigid ring in another hospital. After discharge, early post-operative course was characterized by fatigue on exertion, dyspnoea and the finding of a severe anaemia, requiring transfusion of 65 packed red cells. Laboratory findings were suggestive for a haemolytic aetiology (haemoglobin 5.2 g/dl; haptoglobin 9.2 mg/dl; reticulocytes count 6.3%, lactate dehydrogenase 657 U/L). Echocardiogram performed few months after the first operation showed severe recurrent mitral valve regurgitation with a high velocity jet between A2 and P1 (Figure 1). The anterior leaflet was pulled down, thereby reducing the coaptation surface. After a careful examination and multidisciplinary discussion of the case, the patient was scheduled for a second operation. Intraoperative valve examination showed poor leaflet quality, not suggestive for a second repair, and thus a biological mitral prosthesis was implanted. Intriguingly, we noticed the incorrect placement of previous implanted neochordae. In particular, those arising from posterior papillary muscle to A2 scallop crossed the midline of the valve (Figure 2 - red dashed line) and overlapped with that on the other side (Figure 2 - black star). Postoperative course was uneventful and patient was discharged home on the 7 postoperative days. Anaemia completely resolved and haemoglobin levels were stable at 12 month follow-up.
Discussion
Rapid acceleration of blood flow increases shear stress and may lead to haemolysis. Although more frequently observed after valve replacement, haemolytic anaemia is quite uncommon after mitral valve repair. Among the available surgical reparative techniques (e.g. quadrangular or triangular resection, chordal replacement, chordal transposition, patch enlargement), those addressing the mitral pathology at the chordal level might determine the occurrence of haemolysis, especially in case of recurrent re-gurgitation, with blood streaming at high velocity between narrowed orifices.
In an historical cohort of 13 patients operated on over a 11 year time span, Yeo et al identified “whiplash motion” of disrupted sutures, dehiscence of rings, paravalvular protrusion of suture materials determining fragmentation, non-endothelization of foreign materials and turbulent jets directed against the lateral atrial wall as potentially causes of haemolysis after mitral valve repair [1]. Similarly, Lam et al observed that haemolysis after mitral valve repair could be attributed to mechanical trauma in 19% out of 32 patients with recurrent mitral regurgitation after mitral valve repair [2]. Further chordal rupture has been reported as well [1-4]. In the case reported here, the incorrect placement of neochordae altered the geometry of the surface of coaptation, determining a leaflet malposition which eventually led to the occurrence of a high-velocity regurgitant jet into the left atrium. It could be supposed that the concomitant reoccurrence of mitral regurgitation, together with the altered rheology through the prosthetic material of the implanted neochordae, deter-mined the fragmentation of blood against sutures. Some critical steps need to be addressed accurately during reparative mitral valve surgery: first of all, a detailed transoesophageal description of the mechanisms leading to mitral insufficiency guides the surgeon towards the most appropriate surgical technique; second, leaflets should be inspected, to identify the prolapsing scallop and to judge if the amount of tissue might justify resection; third, whenever the valve is “respected rather than resected”, chordal length should be precisely determined, along with their appropriate placement on the free margin. In particular, when placing artificial neochordae, the loops (a) should never cross the imaginary midline of mitral valve and (b) should always arise from the papillary muscle facing the pro-lapsing leaflet [5]; fourth, an accurate and detailed transoesophageal assessment of the efficacy of the repair is mandatory before leaving the operative room, in order to avoid the occurrence of detrimental complications. Whenever anaemia is evidenced after valve surgery, common laboratory test should be routinely performed to help determining the aetiology (low haptoglobin, increased reticulocyte count and lactate dehydrogenase concentrations suggest haemolysis rather than unknown occult bleeding). A detailed echocardiography, with trans-oesophageal approach if needed, is mandatory to ascertain the potential putative valve or prosthesis aetiology of haemolysis.
Conclusion
Haemolytic anaemia is a clinical presentation of mitral valve repair failure and all potential underlying mechanisms should be investigated. Correct placement of artificial neochordae should be carefully assessed intraoperative. Replacement or re-repair yields favourable outcomes.
References
- Yeo TC, Freeman WK, Schaff HV, Orszulak TA. Mechanisms of hemolysis after mitral valve repair: Assessment by serial echocardiography. J Am Coll Cardiol. 1998 Sep;32(3):717-723. doi: 10.1016/s0735-1097(98)00294-0. PMID: 9741517.
- Lam BK, Cosgrove DM, Bhudia SK, Gillinov AM. Hemolysis after mitral valve repair: Mechanisms and treatment. Ann Thorac Surg. 2004 Jan;77(1):191-195. doi: 10.1016/s0003-4975(03)01455-3. PMID: 14726060.
- Hing JX, Macys A, Elshiekh MA, Momin A, Koertzen M, Punjabi PP. Haemolysis: The harbinger of recurrent mitral regurgitation after mitral valve repair. Perfusion. 2014 Mar;29(2):184-186. doi: 10.1177/0267659113505638. Epub 2013 Sep 16. PMID: 24043273.
- Nakaoka Y, Kubokawa S, Yamashina S, Yamamoto S, Teshima H, Irie HG, Kawai K, Hamashige N, Doi Y. Later rupture of artificial neochordae associated with hemolytic anemia. Journal of Cardiology Cases. 2017;16:123-125. https://tinyurl.com/2p8tb7dj
- Seeburger J, Borger MA, Falk V, Mohr FW. Gore-Tex loop implantation for mitral valve prolapse: The leipzig loop technique. Operative Techniques in Thoracic and Cardiovascular Surgery. 2008;13:83-90. https://tinyurl.com/yy6exykp
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.