Medicine Group . 2022 March 11;3(3):240-245. doi: 10.37871/jbres1429.
Compressive Non-Toxic Intrathoracic Goiter in Patient with Contraindication to Surgical Treatment: Case Report
Gustavo Cancela E Penna1*, Rachel Cardoso Lopes Rego2, Gabriela Malta Silva Diniz3, Gabriela Maciel Campolina Cardoso4, Rudolf Moreira Pfeilsticker5, Adelina Sanches6, Barbara Moreira Ribeiro Trindade dos Santos7 and Hans Graf8
2Endocrinologist, Clinical Care at Santa Casa de Belo Horizonte, Brazil
3Endocrinologist at Hospital Mater Dei, Brazil
4Radiologist at the Spectra Diagnostic Imaging Clinic and the Radiocentro Diagnostic Imaging Clinic, Brazil
5Radiologist at the Felício Rocho Hospital and the Spectra Diagnostic Imaging Clinic & Professor of Basic Medical Propaedeutics at the Faculty of Medical Sciences of Minas Gerais (CMMG), Brazil
6Nuclear medical at Santa Casa de Misericórdia of Bahia and Instituto Integrado de Endocrinologia, Brazil
7Medical student at Faculty of Medicine, Federal University of Minas Gerais, Brazil
8Associate Professor at Medicine Faculty, Federal University of Paraná, Brazil
- Goiter
- Thyroid nodule
Abstract
Introduction: Thyroidectomy is the treatment of choice for non-toxic compressive Multinodular Goiter (MNG). However, when surgery is contraindicated, other therapeutic options should be evaluated. In this case report, non-surgical therapeutic possibilities are reviewed, and the results obtained with Radioiodine Therapy (RAI) after stimulating with Recombinant Thyroid-Stimulating Hormone (rhTSH) are described.
Case report: A 92-year-old patient with multiple comorbidities, non-toxic MNG, and symptomatic compression of the trachea and esophagus. Accordingly, RAI was chosen due to the high surgical risk. Initially, pre-treatment with methimazole was performed to increase TSH, which was interrupted due to drug hepatitis. Then, RAI was chosen in the post-stimulus with a low dose of rhTSH, with significant and early reduction of goiter and symptoms.
Discussion: Although total thyroidectomy is the treatment of choice for non-toxic and symptomatic intrathoracic MNG; it does present risks, especially in patients with multiple comorbidities. Thus, interest in less invasive techniques is increasing. Thermal radio-ablations exhibit satisfactory results, however it is still an inaccessible technique. Radioiodine is an effective treatment option subsequent to the use of rhTSH or hypothyroidism methimazole-induced. RhTSH and methimazole can increase the level of radiation absorbed by the gland, which can lead to a reduction in the required I-131 dose.
Conclusion: It is possible to mitigate the symptoms and improve the quality of life of patients with non-toxic and symptomatic MNG and multiple comorbidities, without surgical intervention. In this case report we describe alternatives to surgical intervention and show the effectiveness and safety of RAI along with rhTSH in the management of MNG.
Introduction
The prevalence of thyroid nodules depends on the population studied and the methods used for its diagnosis. The incidence of thyroid nodules is higher in women, increases with age, and is more prominent in regions with deficient iodine nutrition and after exposure to radiation. Studies show a prevalence of 2-6% on palpation, 19-35% on Ultrasound (US) and 8-65% on autopsies [1]. Approximately 90% of thyroid nodules are benign, and 95% are asymptomatic [2]. Surgical resection can be recommended for aesthetic reasons, when there is a mass effect on adjacent structures or when there is suspicion of thyroid cancer through cytology [3]. However, in cases of non-toxic MNG with a compressive effect, the surgical procedure presents risks to the patient, requires general anesthesia, hospitalization and is expensive. On that account, for these cases, the interest in non-surgical and less invasive techniques is increasing.
Radioiodotherapy (RAI) is an alternative to surgery in non-toxic and large Multinodular Goiter (MNG) treatments, which is causing symptoms resulting from the compression of adjacent structures, such as trachea, esophagus, blood vessels and nerves [4]. Both rhTSH and methimazole have been previously in RAI to stimulate their absorption since the Radioactive Iodine Uptake (RAIU) in non-toxic MNGs is low and heterogeneous [4]. The pre-treatment with rhTSH can double the radiation dose absorbed by the gland and make the uptake more homogeneous, which can reduce the required I-131 dose [5]. However, it is questionable whether the use of rhTSH could increase the risk of permanent hypothyroidism or cause airway compression by transient enlargement of the goiter, especially in cases of more sizable goiters [6].
As it happens with rhTSH, the use of methimazole to generate hypothyroidism can optimize the absorption of I-131 by the thyroid, which is an achievable pre-treatment alternative [5].
Case Report
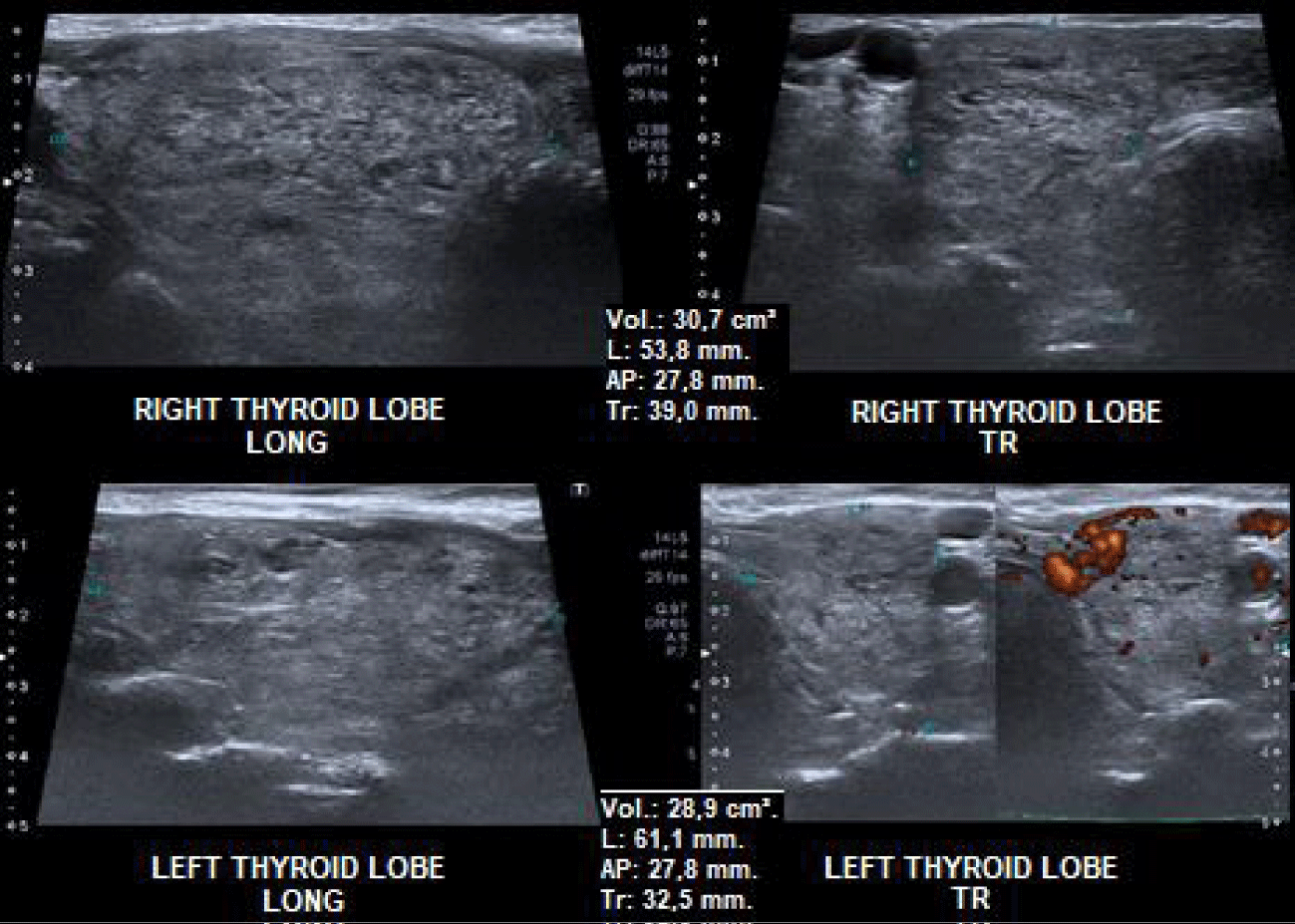
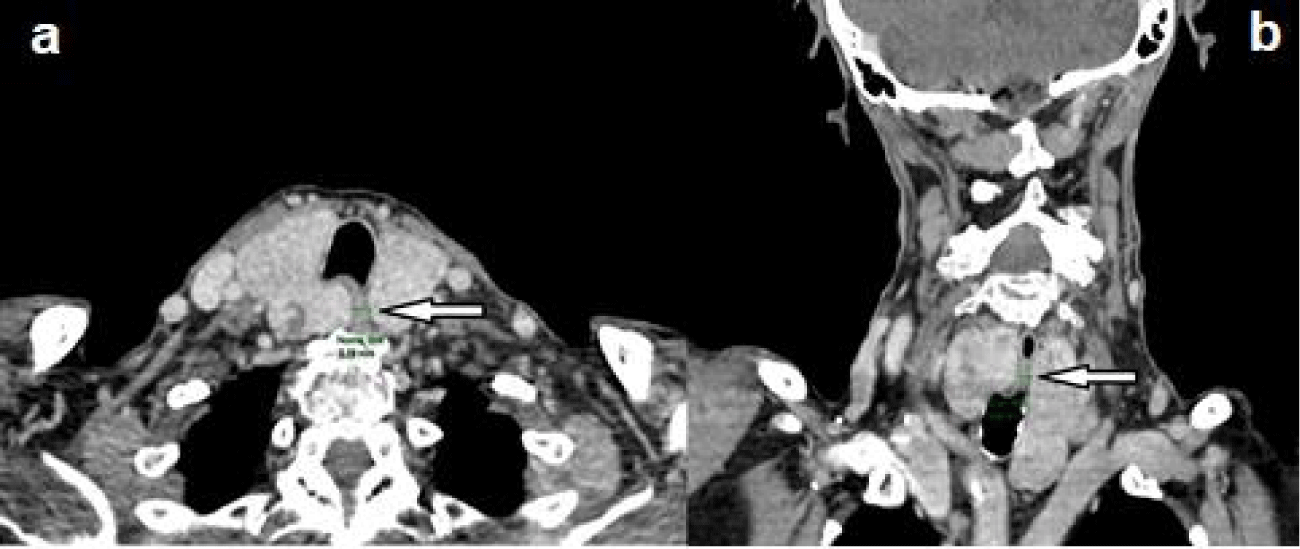
Patient L.I.Z, female, 92 years old, with multiple comorbidities, severe heart disease (arrhythmia and valvulopathy), attended the medical appointment complaining of dysphagia, frequent choking and cough. On cervical palpation, she presented an irregular goiter with significant rise (approximately four times the average size). Laboratory tests: TSH, Free Thyroxine (FT4), Anti-Thyroid Peroxidase (anti-TPO) and TSH-Receptor Autoantibodies (TRAb) within the reference values. As can be seen in figure 1, cervical Ultrasound (US) showed multinodular goiter, volume of 59.6 cm³, low-risk isoechoic nodules (ATA 2015), and the two nodules larger than 1.5 cm were submitted to Fine-Needle Aspiration (FNA), with cytology Bethesda II. The Computed Tomography (CT) of the neck (Figure 2) is compatible with an intrathoracic MNG with compression of the trachea and esophagus, in addition to the deviation of the esophagus.
Although thyroidectomy is the treatment of choice for symptomatic non-toxic MNG [1], the patient in question had a high surgical risk, with formal contraindication by the assistant cardiologist to any surgical intervention. Therefore, other treatment alternatives were considered.
Radiofrequency has been discarded, as it is still poorly available in our market and if of high cost. Hence, RAI was the option of choice. The preparation with methimazole was preferred due to the potentially severe side effect of cervical compression after RAI under rhTSH stimulation, as described in the literature [6].
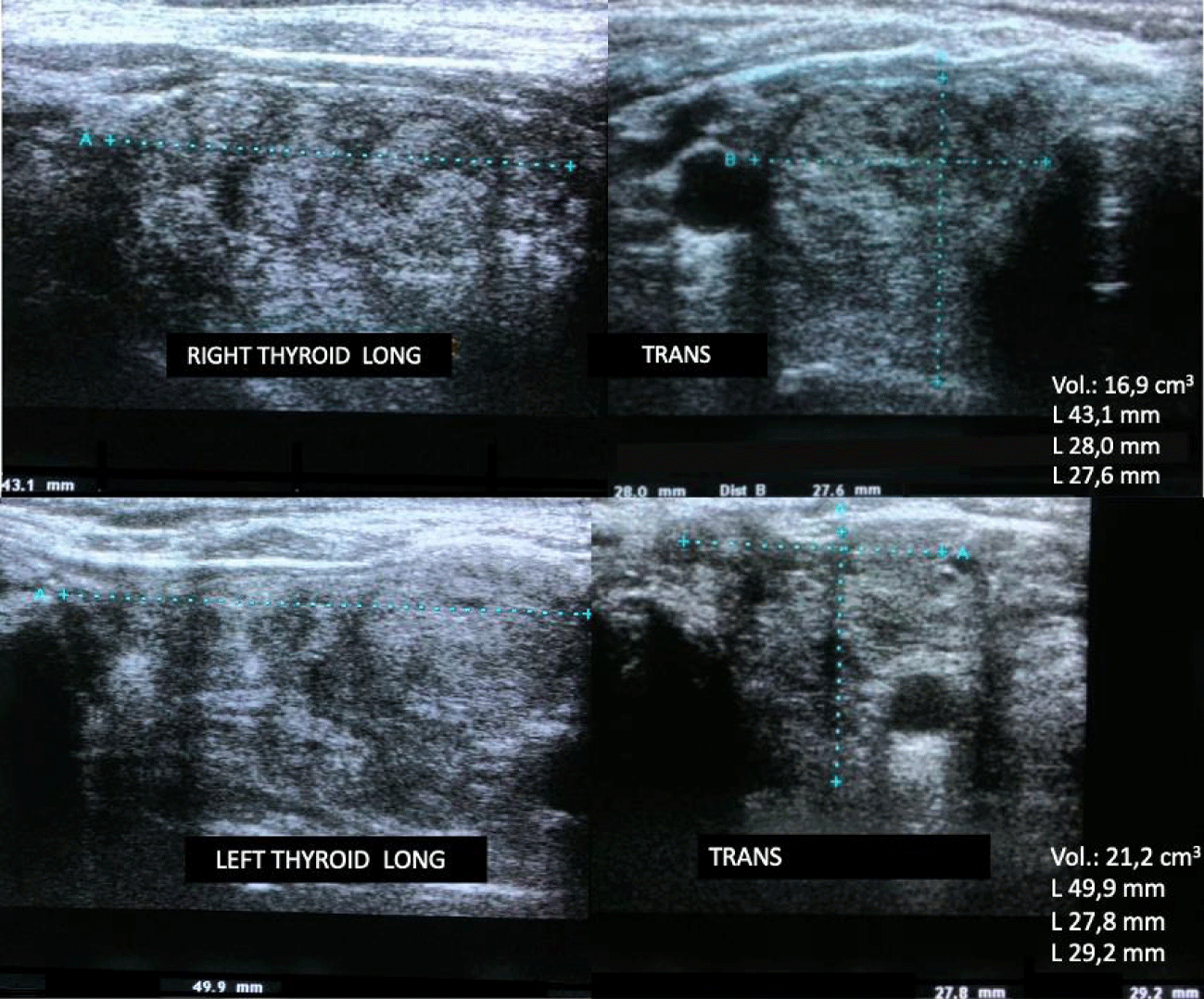
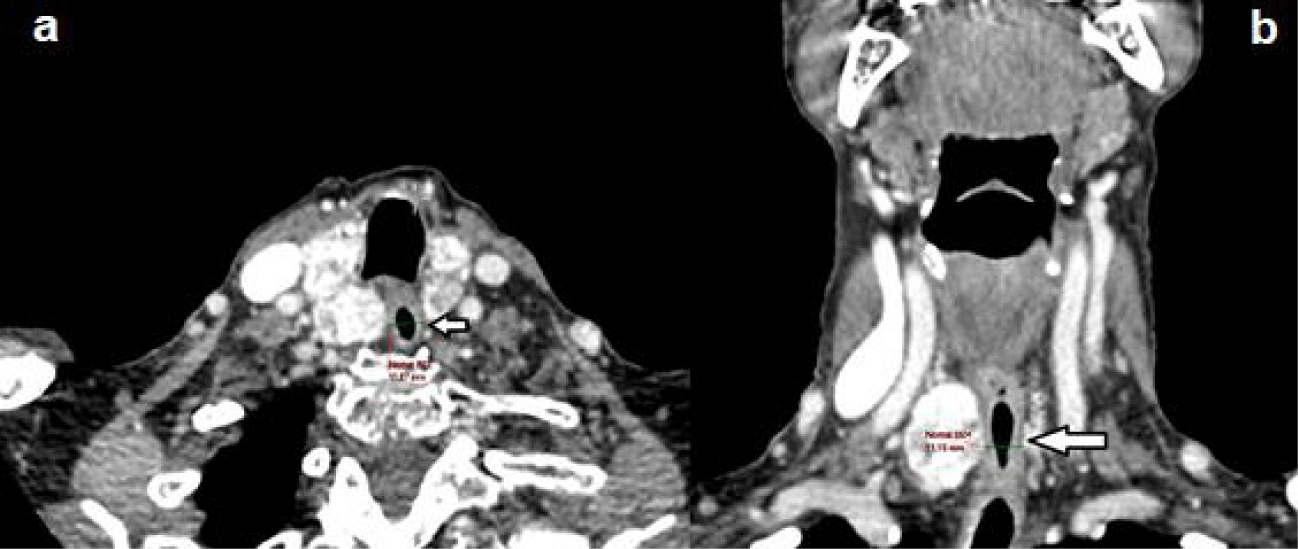
During the use of 10 mg of methimazole, the patient evolved with urticarial reactions and elevated transaminases (five times the reference value), requiring immediate suspension of medication. After symptom improvement and laboratory tests, the patient received an intramuscular dose of rhTSH 0.1 mg, followed by 1.1 GBq of I-131, 24 hours after the injection. No side effects, such as symptoms of thyrotoxicosis or tracheal compression, were observed. There was an early reduction – 6 months after RAI – of the thyroid volume to 38.1 cm³ (approximately 36%), seen in the cervical US (Figure 3) and in the control CT (Figure 4). Following the substantial initial result, the reduction may, presumably, still occur throughout the subsequent months. To date, the patient is euthyroid and reports significant improvement in symptoms, especially concerning the dysphagia and frequent choking initially presented.
Discussion
Total thyroidectomy is still the treatment of choice for non-toxic intrathoracic MNG, with a mass effect [3]. However, when the surgical procedure is contraindicated or unwanted, other treatment strategies must be considered.
Thermal ablation is a relatively new and minimally invasive treatment modality, advisable for patients with solid or predominantly solid thyroid nodules. There are several ablation techniques available, including Radiofrequency (RFA) ablation, High-Intensity Focused Ultrasound (HIFU) ablation and laser ablation. The ablation with ethanol is very effective for thyroid cysts, and its recommendation for cases of recurrent cysts is well established. RFA can be used to treat symptomatic benign thyroid nodules and metastatic locoregional disease in well-differentiated thyroid carcinomas [7,8]. Before the patient is submitted to RFA, it is necessary to confirm the benignity of the thyroid nodule by at least two FNAs guided by US [9]. RFA induces necrosis and involution of the nodules. The reduction in nodular volume is slow and can take months to years [9]. However, this reduction can reach up to 74% after 6 months of ablation [10]. The procedure is associated with low complication rates when performed by trained professionals, where the main reported complications are local pain, hematoma and transient voice changes due to recurrent vagus or laryngeal nerve damage [11].
Laser technology directs light energy to a well-defined and precise tissue area. The absorption of energy destroys the tissue. Cell death continues for 72 hours after the procedure, due to microvessel coagulation and ischemic injury. The advantage of the laser over other thermal techniques is the fact that its fibers are thin and flexible, reaching the nodules easily and safely [12].
HIFU is an emerging thermal ablation technique that is rarely described in the literature [13]. The main advantage of HIFU over other ablation techniques is that it causes focal tissue destruction, without needle puncture or skin penetration [11]. A study published in 2017 by Lang, et al. [13] compared 22 patients with benign thyroid nodules submitted to HIFU, with 22 patients kept under active surveillance. After 12 months, the average volume reduction was statistically significant (68.87% ± 15.27%) only in the HIFU group. Additionally, there was an improvement in the score of compressive symptoms and quality of life.
RAI is also a therapeutic option available for non-toxic MNG. Despite being an effective treatment, the reduction in the size of the goiter after the application of RAI is not always observed in every patient. High doses of iodine are often necessary due to the large volume and low absortion of the radioactive by the gland [4]. Goiter reduction is directly related to the dose of I-131 and indirectly related to the initial size of the goiter [14].
Methimazole is a thioamide used in the treatment of hyperthyroidism, which main action is to inhibit the synthesis of thyroid hormones. Due to the synthesis block of triiodothyronine and thyroxine, TSH levels rise, stimulating iodine uptake by the Sodium-Iodide Symporter (NIS) [4]. Thus, methimazole could be an alternative as a pretreatment, presenting low cost and wide availability in our country. A non-randomized study published by Albino, et al. [4] included 9 patients with MNG (8 with subclinical hyperthyroidism). Initially, patients received between 10 to 20 mg of methimazole. TSH, FT4 and T3 measurements were performed monthly, and methimazole doses were adjusted to increase TSH levels. When TSH reached values higher than 6 mU/I, patients were instructed to follow a low iodine diet for ten days. After this period, the dose of methimazole was interrupted and, four days later, 1.11 GBq of I-131 was administered. Methimazole increased the 24-hour RAIU from 21.3% ± 8.1% to 78.3% ± 15.3% (p < 0.001), with a potential elevation in RAI’s effectiveness. After a year of follow-up, there was an average reduction in thyroid volume of 46.2% ± 17.8% (p = 0.012) [4].
In 2020, a randomized study published by Szumowski, et al. [15] included 31 patients with non-toxic MNG. A group of participants received 10 mg of methimazole for six weeks, and RAI was performed four days after discontinuation of the medication. The second group received a placebo, followed by RAI. The dose of I-131 used was 0.8 GBq and was repeated every six months, when necessary, until the volume of the goiter was lower than 40 cm3. In the methimazole group, the results showed that the RAIU for 24 hours and 48 hours were approximately twice as high. Six months after the application of RAI, the reduction in the volume of the goiter was 34% higher in the methimazole group compared to the RAI applied alone. Moreover, the average time for the volume of the goiter to decrease to less than 40 cm3 was nine months in the methimazole group, and 18 months in the group with RAI alone. Finally, after two years of RAI, the incidence of hypothyroidism was not statistically different between the two groups. Despite the optimistic results, there is a lack of studies to assess whether the use of methimazole can raise the effectiveness of RAI in the treatment of non-toxic MNG, and what is the ideal TSH value for administering I-131.
The use of rhTSH in the pre-treatment increases RAIU and contributes to a more homogeneous uptake pattern, reducing the dose of I-131 necessary for effective treatment [16]. Fast, et al. [17] evaluated the long-term outcome of rhTSH before RAI in the treatment of non-toxic MNG. One group received 0.3 mg of rhTSH before RAI, and another group received a placebo. After 71 months of follow-up, the average reduction in the volume of the goiter was 69.7% ± 3.1% in the rhTSH group and 56.2% ± 3.6% in the RAI alone group. In a study of patients with non-toxic and toxic MNG, Cubas, et al. [18] observed a more significant reduction in thyroid volume in the groups that received rhTSH (37.2% with 0.1 mg and 39.3% with 0.005 mg) than in the group that received RAI alone (15.3%), after 24 months of follow-up. The authors also concluded that the lower dose of rhTSH of 0.005 mg is as effective as the one of 0.1 mg, also presenting mild and transient adverse effects. A meta-analysis [19] with nine Randomized Controlled Trials (RCTs), including 416 patients, compared the effectiveness of pre-treatment with rhTSH (high and low doses) and RAI alone in the treatment of benign MNG. After one year of follow-up, there was a significantly greater reduction in thyroid volume in the group that received pre-treatment with rhTSH followed by RAI (with an average difference of 14.42% in the group that received high doses of rhTSH versus 19.66% in the one that received low doses), compared with RAI applied alone. The results showed that rhTSH associated with RAI was more effective in reducing goiter than RAI alone. Also, there was no considerable difference between those who received high or low doses of rhTSH. Therefore, not only is the low dose of rhTSH safe for presenting a lower risk of inducing hypothyroidism, as it is also more effective in treating non-toxic MNG than RAI alone [19].
Treatment with rhTSH and RAI induces short-term thyrotoxicosis, which is more significant in patients with previous clinical and subclinical hyperthyroidism [16,20]. After 15 to 30 days, TSH and thyroid hormone levels tend to normalize progressively [16].
RAI applied alone may cause a transitory increase in goiter in up to 15% of patients in the first week after administration. Severe respiratory complications are rare, but worsening of pre-existing tracheal compression due to gland edema may occur [21]. Nielsen, et al. [22] reported a 35% increase in thyroid volume 48 hours after stimulation with an rhTSH very high dose of 0.9 mg and 24% after a dose of 0.3 mg [6]. Therefore, the possibility of stimulation with rhTSH in combination with RAI to intensify acute goiter edema and airway compression [22] is questioned. Nevertheless, Albino, et al. [23] found no significant difference in the Tracheal Cross-Sectional Area (TCA) before and after RAI in groups receiving a dose of rhTSH of 0.01 mg, 0.1 mg or placebo, suggesting that treatment with rhTSH is safe in lower doses. Hypothyroidism and continuous use of Levothyroxine (L-T4) are consequences of surgery and are also very common after RAI. Following the treatment with RAI, the risk of permanent hypothyroidism varies from 11 to 55%, after 1 to 8 years [24]. The use of rhTSH in high doses is associated with an increase in the rate of hypothyroidism; however, there was no significant difference in the incidence of hypothyroidism between the groups using low doses of rhTSH (< 0.1 mg) or RAI alone [19].
Conclusion
It is possible to improve the symptoms and quality of life of patients with non-toxic intrathoracic MNG and multiple comorbidities, without surgical intervention. Although dysphagia was not objectively evaluated, clinical changes along with imaging comparison indicated a dysphagia improvement. Pre-treatment with low doses of rhTSH is safe and more effective than using RAI alone. Methimazole appears to be an alternative adjunctive treatment to RAI; however, more studies are needed. Thermal ablations are effective treatment options for non-toxic MNG although, thus far, the access to them is limited. It is essential to highlight that we must continuously seek the best for each patient. As it was reported in this case of an elderly patient with a very high surgical risk, it was possible to find treatment and significantly improve her quality of life.
Declaration of Interest
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008 Dec;22(6):901-11. doi: 10.1016/j.beem.2008.09.019. PMID: 19041821.
- Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The Diagnosis and Management of Thyroid Nodules: A Review. JAMA. 2018 Mar 6;319(9):914-924. doi: 10.1001/jama.2018.0898. Erratum in: JAMA. 2018 Apr 17;319(15):1622. PMID: 29509871.
- Hegedüs L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003 Feb;24(1):102-32. doi: 10.1210/er.2002-0016. PMID: 12588812.
- Albino CC, Graf H, Sampaio AP, Vigário A, Paz-Filho GJ. Thiamazole as an adjuvant to radioiodine for volume reduction of multinodular goiter. Expert Opin Investig Drugs. 2008 Dec;17(12):1781-6. doi: 10.1517/13543780802501325. PMID: 19012495.
- Fast S, Nielsen VE, Bonnema SJ, Hegedüs L. Time to reconsider nonsurgical therapy of benign non-toxic multinodular goitre: focus on recombinant human TSH augmented radioiodine therapy. Eur J Endocrinol. 2009 Apr;160(4):517-28. doi: 10.1530/EJE-08-0779. Epub 2008 Dec 23. PMID: 19106244.
- Nielsen VE, Bonnema SJ, Hegedüs L. Transient goiter enlargement after administration of 0.3 mg of recombinant human thyrotropin in patients with benign nontoxic nodular goiter: a randomized, double-blind, crossover trial. J Clin Endocrinol Metab. 2006 Apr;91(4):1317-22. doi: 10.1210/jc.2005-2137. Epub 2006 Jan 24. PMID: 16434453.
- Baek JH, Kim YS, Sung JY, Choi H, Lee JH. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol. 2011 Aug;197(2):W331-6. doi: 10.2214/AJR.10.5345. PMID: 21785061.
- Monchik JM, Donatini G, Iannuccilli J, Dupuy DE. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg. 2006 Aug;244(2):296-304. doi: 10.1097/01.sla.0000217685.85467.2d. PMID: 16858194; PMCID: PMC1602167.
- Na DG, Lee JH, Jung SL, Kim JH, Sung JY, Shin JH, Kim EK, Lee JH, Kim DW, Park JS, Kim KS, Baek SM, Lee Y, Chong S, Sim JS, Huh JY, Bae JI, Kim KT, Han SY, Bae MY, Kim YS, Baek JH; Korean Society of Thyroid Radiology (KSThR); Korean Society of Radiology. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012 Mar-Apr;13(2):117-25. doi: 10.3348/kjr.2012.13.2.117. Epub 2012 Mar 7. PMID: 22438678; PMCID: PMC3303894.
- Ugurlu MU, Uprak K, Akpinar IN, Attaallah W, Yegen C, Gulluoglu BM. Radiofrequency ablation of benign symptomatic thyroid nodules: prospective safety and efficacy study. World J Surg. 2015 Apr;39(4):961-8. doi: 10.1007/s00268-014-2896-1. PMID: 25446486.
- Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, Lee D. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008 Jun;18(6):1244-50. doi: 10.1007/s00330-008-0880-6. Epub 2008 Feb 20. PMID: 18286289.
- Baek JH, Lee JH, Valcavi R, Pacella CM, Rhim H, Na DG. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol. 2011 Sep-Oct;12(5):525-40. doi: 10.3348/kjr.2011.12.5.525. Epub 2011 Aug 24. PMID: 21927553; PMCID: PMC3168793.
- Lang BH, Woo YC, Wong CKH. High-Intensity Focused Ultrasound for Treatment of Symptomatic Benign Thyroid Nodules: A Prospective Study. Radiology. 2017 Sep;284(3):897-906. doi: 10.1148/radiol.2017161640. Epub 2017 Apr 18. PMID: 28419814.
- Le Moli R, Wesche MF, Tiel-Van Buul MM, Wiersinga WM. Determinants of longterm outcome of radioiodine therapy of sporadic non-toxic goitre. Clin Endocrinol (Oxf). 1999 Jun;50(6):783-9. doi: 10.1046/j.1365-2265.1999.00734.x. PMID: 10468951.
- Szumowski P, Abdelrazek S, Sykała M, Mojsak M, Żukowski Ł, Siewko K, Maliszewska K, Adamska A, Popławska-Kita A, Krętowski A, Myśliwiec J. Enhancing the efficacy of 131I therapy in non-toxic multinodular goitre with appropriate use of methimazole: an analysis of randomized controlled study. Endocrine. 2020 Jan;67(1):136-142. doi: 10.1007/s12020-019-02100-x. Epub 2019 Oct 4. PMID: 31586293; PMCID: PMC6969001.
- Giusti M, Caorsi V, Mortara L, Caputo M, Monti E, Schiavo M, Bagnara MC, Minuto F, Bagnasco M. Long-term outcome after radioiodine therapy with adjuvant rhTSH treatment: comparison between patients with non-toxic and pre-toxic large multinodular goitre. Endocrine. 2014 Mar;45(2):221-9. doi: 10.1007/s12020-013-9959-1. Epub 2013 Apr 26. PMID: 23619962.
- Fast S, Nielsen VE, Grupe P, Boel-Jørgensen H, Bastholt L, Andersen PB, Bonnema SJ, Hegedüs L. Prestimulation with recombinant human thyrotropin (rhTSH) improves the long-term outcome of radioiodine therapy for multinodular nontoxic goiter. J Clin Endocrinol Metab. 2012 Aug;97(8):2653-60. doi: 10.1210/jc.2011-3335. Epub 2012 May 10. PMID: 22577172.
- Cubas ER, Paz-Filho GJ, Olandoski M, Goedert CA, Woellner LC, Carvalho GA, Graf H. Recombinant human TSH increases the efficacy of a fixed activity of radioiodine for treatment of multinodular goitre. Int J Clin Pract. 2009 Apr;63(4):583-90. doi: 10.1111/j.1742-1241.2008.01904.x. Epub 2008 Sep 18. PMID: 18803554.
- Lee YY, Tam KW, Lin YM, Leu WJ, Chang JC, Hsiao CL, Hsu MT, Hsieh AT. Recombinant human thyrotropin before (131)I therapy in patients with nodular goitre: a meta-analysis of randomized controlled trials. Clin Endocrinol (Oxf). 2015 Nov;83(5):702-10. doi: 10.1111/cen.12654. Epub 2014 Dec 29. PMID: 25370124.
- Romão R, Rubio IG, Tomimori EK, Camargo RY, Knobel M, Medeiros-Neto G. High prevalence of side effects after recombinant human thyrotropin-stimulated radioiodine treatment with 30 mCi in patients with multinodular goiter and subclinical/clinical hyperthyroidism. Thyroid. 2009 Sep;19(9):945-51. doi: 10.1089/thy.2008.0394. PMID: 19678745.
- Bonnema SJ, Bertelsen H, Mortensen J, Andersen PB, Knudsen DU, Bastholt L, Hegedüs L. The feasibility of high dose iodine 131 treatment as an alternative to surgery in patients with a very large goiter: effect on thyroid function and size and pulmonary function. J Clin Endocrinol Metab. 1999 Oct;84(10):3636-41. doi: 10.1210/jcem.84.10.6052. PMID: 10523007.
- Nielsen VE, Bonnema SJ, Hegedüs L. Effects of 0.9 mg recombinant human thyrotropin on thyroid size and function in normal subjects: a randomized, double-blind, cross-over trial. J Clin Endocrinol Metab. 2004 May;89(5):2242-7. doi: 10.1210/jc.2003-031783. PMID: 15126548.
- Albino CC, Graf H, Paz-Filho G, Diehl LA, Olandoski M, Sabbag A, Buchpiguel C. Radioiodine plus recombinant human thyrotropin do not cause acute airway compression and are effective in reducing multinodular goiter. Braz J Med Biol Res. 2010 Mar;43(3):303-9. doi: 10.1590/s0100-879x2010007500001. PMID: 20401438.
- Bonnema SJ, Hegedüs L. A 30-year perspective on radioiodine therapy of benign nontoxic multinodular goiter. Curr Opin Endocrinol Diabetes Obes. 2009 Oct;16(5):379-84. doi: 10.1097/MED.0b013e32832ff2e1. PMID: 19623060.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.