Medicine Group . 2022 March 11;3(3):246-263. doi: 10.37871/jbres1430.
Epigenetic Strategies to Discover Novel Fungal Secondary Metabolites
Komal Anjum1* and Ye Xuewei2
2Department of Basic Medical Sciences, Shulan International Medical College, Zhejiang Shuren University, Hangzhou, China
- Epigenetic approaches
- Novel secondary metabolites
- Genome mining strategies
- DNA methyltransferase inhibitors
- Histone deacetylase inhibitors
Abstract
Natural product search is an enduring revitalization upon the exploration of a huge already exotic potential for Secondary Metabolite (SM) production obscure in microbial genomes. Filamentous fungi genomes have an immense number of “orphan” SM gene clusters. Current evaluation indicates that only 5% of extant fungal species have been explored, thus the apparent for the disclosure of novel metabolites in fungi is extensive. In this situation, fungi burgeoning in severe environments are of special interest since they are distinguished producers of astonishing chemical structures. Genome mining strategies, more specifically epigenetic strategies are playing an important role in natural product discovery. This review has been organized and written to focus on available epigenetic approaches, targeting on DNA methyltransferase and histone deacetylase inhibitors along with reported novel secondary metabolites. To the best of our knowledge, this review article is the first attempt to incorporate the facts regarding DNA methyltransferase inhibitors and histone deacetylase inhibitors along with reported novel secondary metabolites with their recorded bioactivities.
Introduction
In the repetitive research by pharmacists for new products, natural selection is superior to combinatorial chemistry for discovering novel substances that have the potential to be developed into this era [1]. Search for natural products is undergoing rebirth upon the discovery of a huge previously unknown potential for Secondary Metabolite (SM) production hidden in microbial genomes. Many fungi can effectively produce many natural products, more specifically bioactive natural products. Fungi are biosynthetically nature gifted organisms efficient of producing an extensive range of chemically diverse and biologically fascinating small molecules. The majority of scientific insight in fungal natural products has focused on their pharmaceutical applications, roles as mycotoxins, and diverse ecological functions. Unfortunately, characteristic fungal fermentation approaches such as an axenic shake or static culture strategies on artificially defined media are a poor replacement for mirroring an organism’s native habitat. The significance of these procedures is that only a subgroup of the biosynthetic pathways which encrypt for secondary metabolite production in fungi are ever expressed, thus restraining prospects for understanding the comprehensive drug discovery potential of these organisms [2].
DNA and Histone Methylation Among Fungi
It has been revealed that there is a biological relationship between the methylation of DNA and histone, which both are interrelated with diverse chemical reactions. This connection has a dynamic function in gene silencing from fungi to mammals [3-7]. The histone modification markers, such as H3K4me3, can work as indirect regulators to affect DNA methylation [8-10]. The primary methyltransferase of histone arginine methylation (H3R8me) is PRMT5, and its decreased levels can reduce the binding between DNMT3A and chromatin, decrease DNA methylation, and afterward facilitate genetic transcription [11]. Current data recommend that DNA and histone methylation commonly control fungal development and biosynthesis of toxic secondary metabolites [12]. Histone methylation is willingly reversible and usually precedes DNA methylation in N. crassa, whereas DNA methylation is comparatively stable and conduces to form a stable heterochromatic state [4,7].
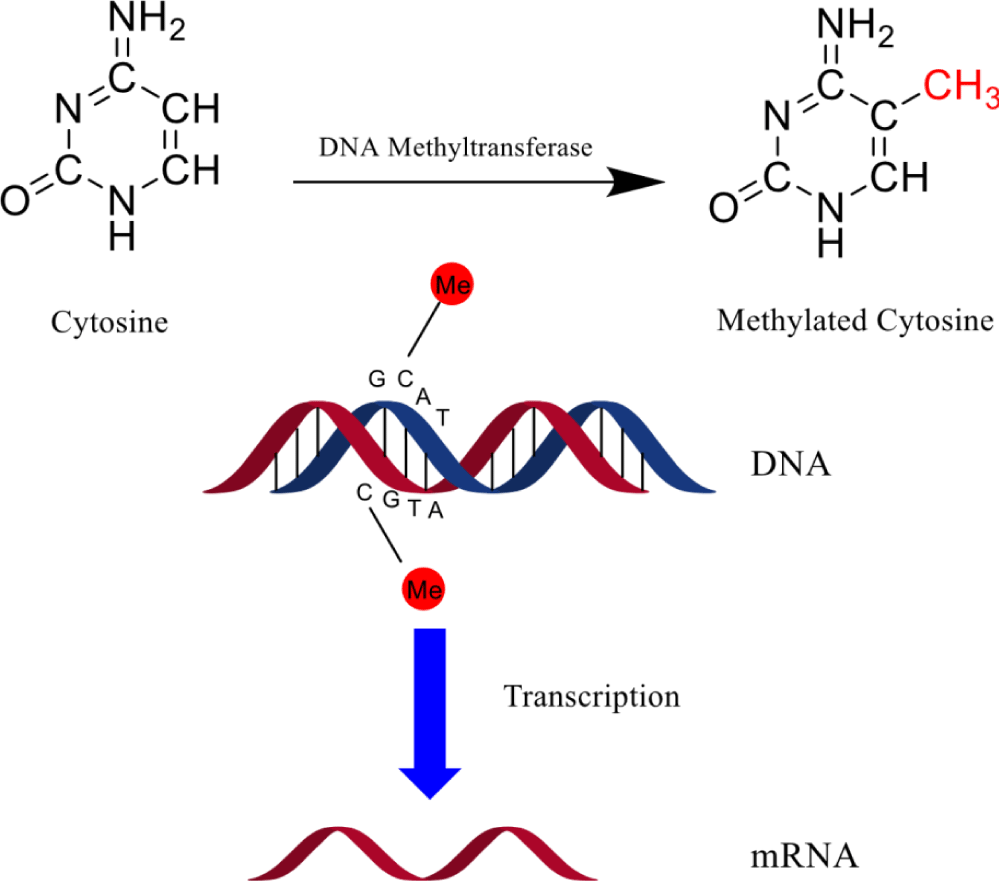
DNA methylation is an epigenetic mechanism comprising the transfer of a methyl group onto the cytosine C5 position to form 5-methylcytosine. DNA methylation controls gene expression by engaging proteins involved in gene repression or by hindering the binding of transcription factor(s) to DNA. In the course of development, the DNA methylation pattern in the genome changes as a result of a vigorous process comprising both de novo DNA methylation and demethylation. As a result, discriminated cells develop a steady and exceptional DNA methylation pattern that controls tissue-specific gene transcription [13].
Among fungi, DNA methylation consists of imperceptible levels i.e. ≤ 0.1% of cytosine residues [14] to low but detectable levels i.e. 0.2-4.3% of cytosine residues [15-17] to markedly high level i.e. 10-30% of cytosine residues [18]. Furthermore, the methylated locates are usually clustered away from principally unmethylated regions. Though the significance of DNA methylation in fungi is still uncertain, Ascobolus immersus and Neurospora crassa are two documented examples of DNA methylation that plays roles in genome protection. Current studies on the genome-wide methylation study specified that DNA methylation ensues in and around genes, and fungal epigenetic entities subsidized to fungal growth as well as genome protection [19,20]. Furthermore, while some functions of DNA methylation have been identified, its regulation is not well understood (Figure 1).
Histone Acetylation and Deacetylation
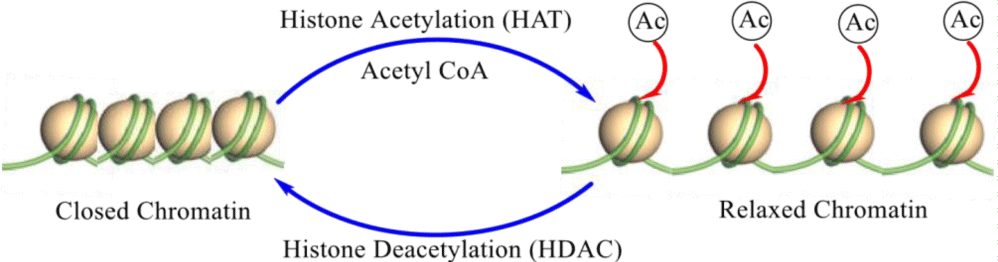
Histone acetylation and deacetylation are crucial processes of gene regulation. These reactions are naturally accelerated by an enzyme including "Histone Acetyltransferase" (HAT) or "Histone Deacetylase" (HDAC). The process of acetylation in terms of gene regulation is the transfer of an acetyl functional group from a molecule i.e. from Acetyl-Coenzyme A to histone. Whereas deacetylation is the reverse process of acetylation in which an acetyl group is removed from a histone molecule.
Acetylated histones which are octameric proteins organize chromatin into nucleosomes, basic structural units of the chromosomes and finally upper order structures characterize a type of epigenetic marker within chromatin. Acetylation reaction eliminates the positive charge on the histones, thus reducing the interaction of the N-terminal of histones with the negatively charged phosphate groups of DNA. As a result, the compressed chromatin is converted into a more relaxed structure that is concomitant with greater levels of gene transcription. This recreation can be inverted by HDAC activity. Relaxed, transcriptionally active DNA is stated as euchromatin. More compressed DNA is termed Compression can be brought about by processes including deacetylation and methylation [21] (Figure 2).
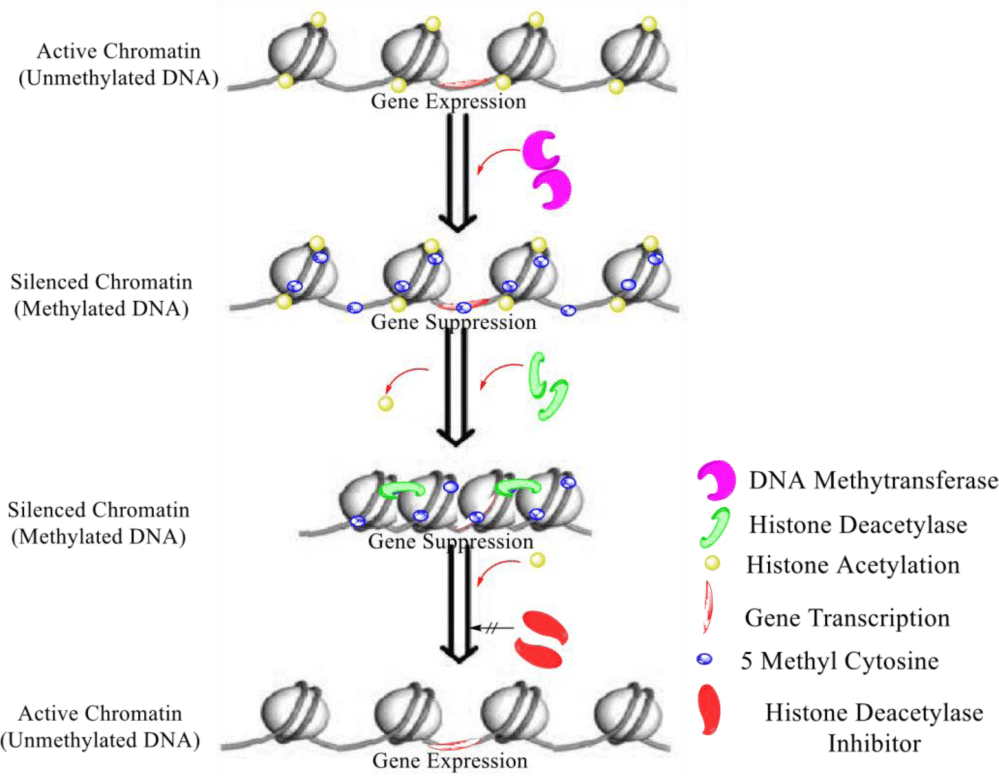
So far it can be said that both processes of DNA methylation and histone deacetylation among fungi are not biosynthetically essential. These processes can alter the transcription of many essential genes which may require either for defense mechanism [22] (Figure 3). Transcription of such genes usually not happened until such fungi have not been exposed to special modifiers. For that purpose, naturally or synthetically some modulators are available which were studied with different aspects including cancer treatment among eukaryotes [23]. These kinds of modulators are well known as epigenetic modulators. In the research of novel bioactive natural products by epigenetic strategies, these epigenetic modulators are serving as inducers for the induction of silent biosynthetic gene clusters in fungi [24,25]. In literature, several examples are available for the use of such epigenetic modifiers to be used for the induction of antimicrobial metabolites [26], toxicities induction [27], anticancer candidates [28], and others [29].
Epigenome Manipulation
Practicing epigenetic strategies with some examples
The term ‘epigenetic’ was invented by Waddington CH [30] and from that time; the definition of ‘epigenetic’ has developed. Epigenetics is the study of molecular procedures that affect the sequence of information between an adaptable gene expression patterns and a constant DNA sequence. The well-mannered definition of epigenetic is stated as the range of biochemical features that serve to modify the transcription of a gene or genes, nevertheless, do not straight forward change the conformation of DNA. Additionally, the epigenome functions as a biological filter that is accountable for controlling the availability of cells to ‘inbound’ interspecies and intraspecies signaling events. Likewise, the epigenome also can act as an ‘outbound’ filter that can chunk the DNA transcription and, in that way, successfully results signal generation [2,31].
Fungal genome manipulation via chemical epigenetics
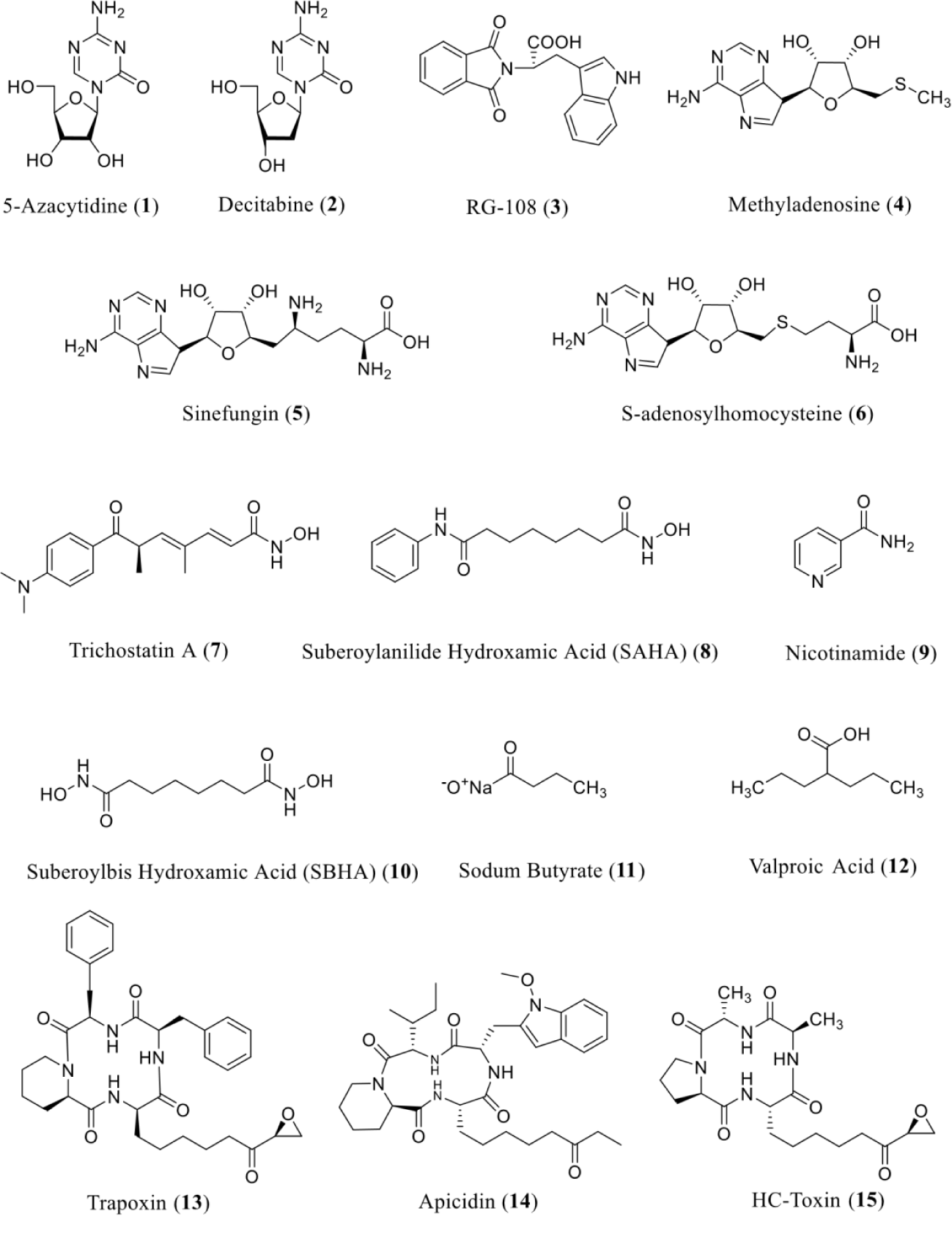
The scientific researcher community's abilities to an emerging collection of small-molecule tools has permitted the expansion of chemical epigenetic techniques [32] that are directed toward searching how epigenome features control biological processes. This includes the biosynthetic mechanisms regulating potential for secondary metabolite production [33]. A considerable body of information has accrued relating to the effects of epigenetic modulators on fungi. Maximum examples have engrossed on the use of DNMT inhibitors such as 5-azacytidine (1) and 5-aza-2′deoxycytidine (decitabine) (2), which have validated the capability to reduce the DNA methylation- interceded silencing of a phleomycin-resistance gene in Phanerochaete Chrysosporium [33] and hygromycin-resistance genes in N. crassa [34] and Schizophyllum commune [35]. Some of the data related to the role of 5-azacytidine (1) revealed the capability of this in induction of heritable epigenetic modifications in fungi with respect to the acquisition of new and mitotically stable phenotypic characteristics [36]. However, a mutation-inducing effect for 5-azacytidine as a result of its DNA integration cannot be completely ignored in some situations [34,35]. Another unusual DNMTi is RG-108 (3), reported in the article of Asai, et al. [37] with the isolation of novel secondary metabolites [38]. Other less common DNMTi are mathyladenosine (4), sinefungin (5), and S-adenosylhomocystein (6) [36] (Figure 4).
Chemical-epigenetic approaches and mutant studies were positively employed for the de novo or improved production of structurally diverse fungal natural products (e.g., mycotoxins, anthraquinones, nygerones, cladochromes, and lunalides) [36]. Some reported epigenetic modifiers apart of DNMTi are HDAC inhibitors (HDACi). Several research articles have described the differential effects of HDAC inhibitors on fungi. A chemical genetics approach retaining hydroxamic-acid-containing compounds such as trichostatin A (7) has also been used to reveal HDAC functions and universal transcriptional control mechanisms in Saccharomyces cerevisiae [39]. It is notable that Cochliobolus caronum, a fungal pathogen, which is responsible for the production of HC-toxin which is a potent HDAC inhibitor [40], synthesize a structurally modified HDAC that is unaffected to both trichostatin A (7). It is supposed that this exclusively adapted HDAC serves to defend C. caronum from the autotoxic effects of HC-toxin throughout the fungus's invasion and chemical attack upon its maize host [41]. A synthetic derivative of trichostatin A (7), suberoylanilide hydroxamic acid (SAHA) (8) also known as vorinostat, is another noteworthy examples of potent HDACi that have been effectively used as chemical epigenetic probes in a variety of eukaryotic systems, including filamentous fungi [10].
Other examples of amending media with HDACi are nicotinamide (9), suberoyl bishydroxamic acid (SBHA) (10), and sodium butyrate (11) in several studies directed not only to the improved production of compounds by fungi besides to the biosynthesis of novel compounds that were not produced without epigenetic modification [42,43]. Apart of that, some other unusual HDACi which uncommonly used are valproic acid (12), trapoxin (13), apicidin (14), and HC-toxin (15) [36] (Figure 4).
Different Epigenetic Modifiers as DNMTi and HDACi Tested on Fungi with Reported Secondary Metabolites (Table 1)
| Table 1: List of novel secondary metabolites stimulated by different DNMTi and HDACi with reported bioactivities. | |||||
| Species | Modulators | Class | Compounds | Reported Bioactivity | References |
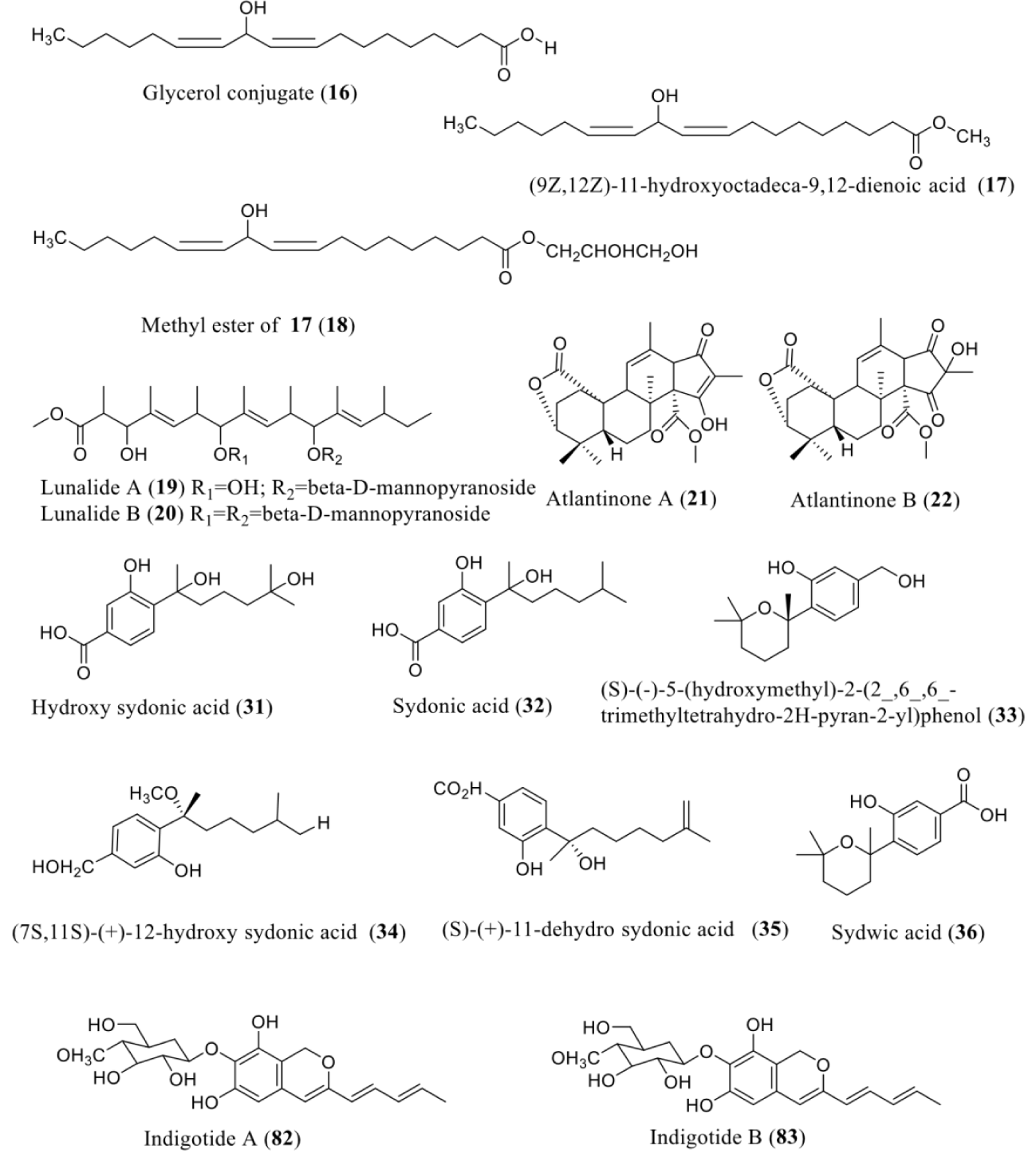
| Cladosporium cladosporioides | 5ʹ azacytidine | DNMTi | Oxylipins [glycerol conjugate (16), (9Z,12Z)-11-hydroxyoctadeca-9,12-dienoic acid (17), and its methyl ester (18)] | NT | [2,36] |
| Diatrype species | 5ʹ azacytidine | DNMTi | Lunalides A (19) and B (20) | NT | [2] |
| P. citrreonigrum | 5ʹ azacytidine | DNMTi | Meroterpenes [atlantinones A (21) and B (22)] | NA | [44] |
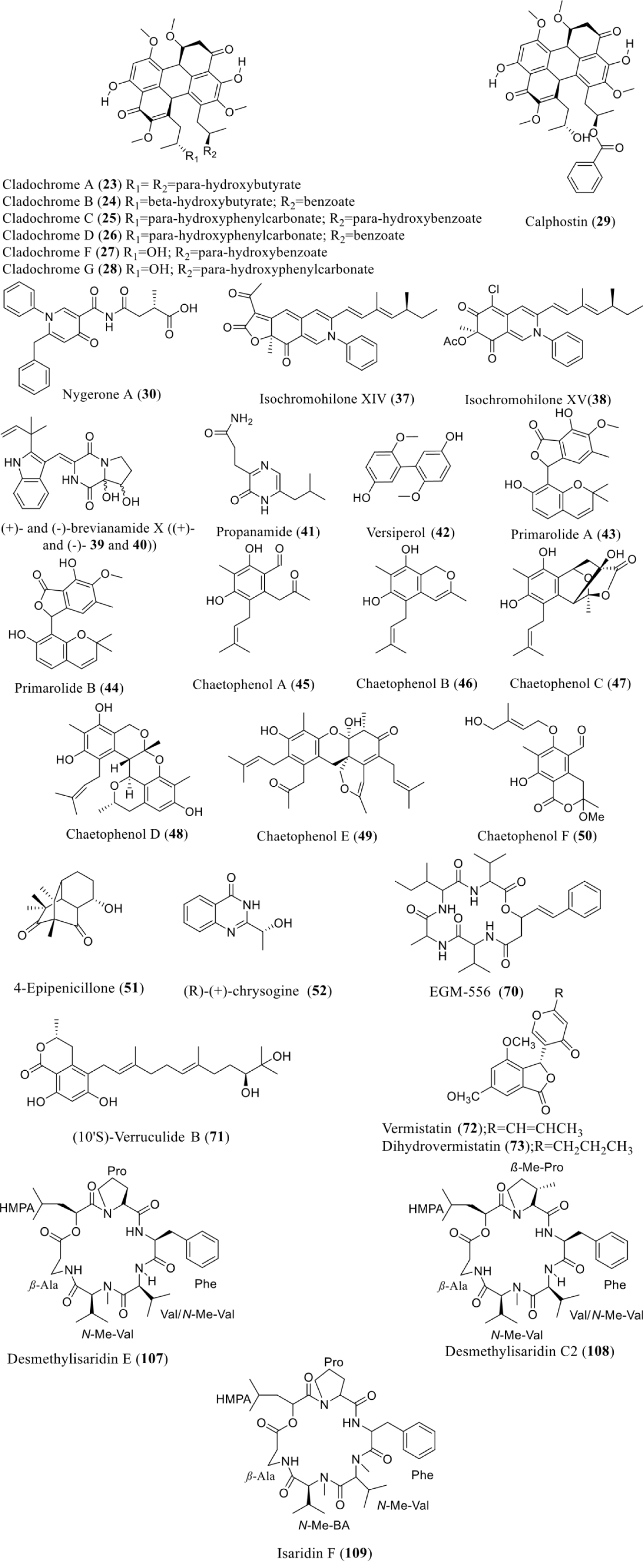
| C. cladosporioides | SAHA | HDACi | Cladochromes A- D, F, G (23-28), and calphostin B (29) | Pathogenesis | [2,36] |
| Neurospora crassa | 5ʹ azacytidine | DNMTi | Carotenoids | NT | [45] |
| A. alternate and Penicillium expansum | Trichostatin A | HDACi | Unidentified natural products | NT | [46] |
| Aspergillus niger | SAHA | HDACi | Nygerone A (30) | NT | [36] |
| Aspergillus sp. | 5ʹ azacytidine | DNMTi | Bisabolane-type sesquiterpenoids [(R)-(–)-hydroxy sydonic acid (31), (S)-(+)-sydonic acid (32), (S)-(–)-5-(hydroxy methyl)-2-(2-,6-,6--trimethyltetrahydro-2H-pyran-2-yl)phenol (33), (7S,11S)-(+)-12-hydroxy sydonic acid (34), (S)-(+)-11-dehydrosydonic acid (35), and (S)-(–)-sydowic acid (36)] | Antidiabetic, anti-inflammatory, antibacterial activity | [47-52] |
| Penicillium mallochii | SAHA | HDACi | Isochromophilone XIV (37) and isochromophilone XV (48) | NT | [53] |
| Aspergillus versicolor | SAHA | HDACi | (+)-brevianamide X ((+)−39), (−)−brevianamide X ((-)- 40), 3-[6-(2-methylpropyl)-2-oxo-1H-pyrazin-3-yl] propanamide (41), versiperol A (42) | NT | [54,55] |
| A. cruciatus | SAHA | HDACi | Primarolides A (43) and B (44) | NT | [56] |
| Chrysanthemum indicum | SAHA | HDACi | Prenylated aromatic polyketides, chaetophenols A-F (45- 50) | NT | [42] |
| Penicillium sp. HS-11 | SAHA | HDACi | 4-epipenicillone B (51) and chrysogine (52) | NA | [57] |
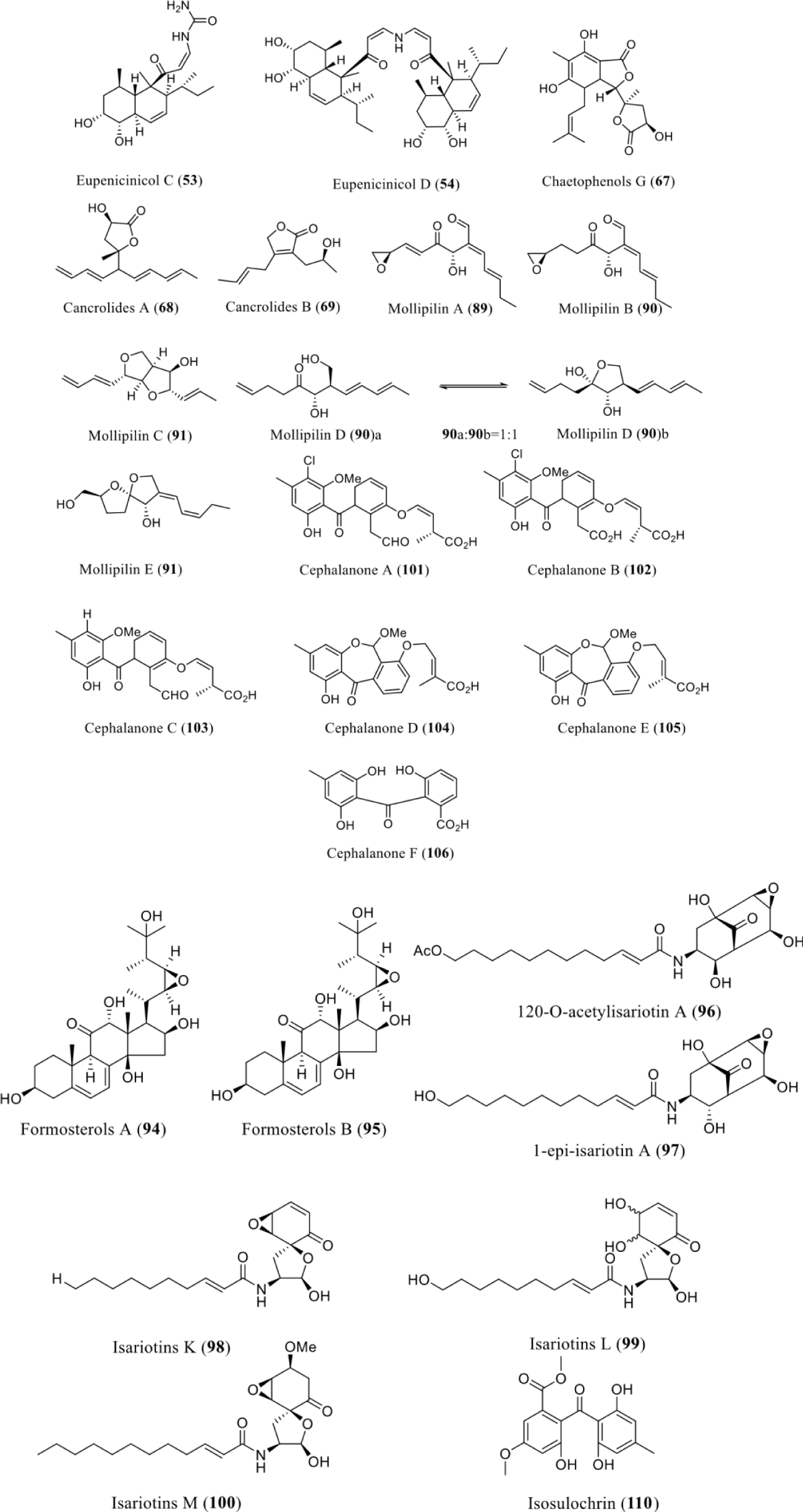
| Eupenicillium sp. LG41 | Nicotinamide | HDACi | Eupenicinicols C and D (53) and (54) | 53 has antibacterial and cytotoxic while 54 has antibacterial activity | [58] |
| Aspergillus terreus OUCMDZ-2739 | Trichostatin A | HDACi | Meroterpenoids [(4S)-4 decarboxylflavipesolide C (55), 1-(2,2- dimethylchroman-6-yl)-3-(4 hydroxyphenyl)propan-2-one (56), (R,E)-3-(2,2-dimethyl chroman- 6-yl)-4-hydroxy-5-((2-(2 hydroxypropan-2-yl)-2,3 dihydrobenzofuran-5-yl)methylene) furan- 2(5H)-one (57), methyl (R)-2 (2-(2-hydroxypropan-2-yl)-2,3 dihydrobenzofuran-5-yl) acetate (58) | 55 possessed α-glucosidase inhibitory activity | [59] |
| Torrubiella luteorostrata | SBHA | HDACi | Tryptophan analogs [ luteorides A–C (59–61) | NT | [60] |
| Phomopsis sp. | SBHA | HDACi | 13-angeloyloxy-diplosporin (62) | NA | [61] |
| Cochliobolus lunatus | Sodium butyrate | HDACi | 14-membered resorcylic acid lactones [ 5-bromozeaenol (63) and 3,5-dibromozeaenol (64)] | NA | [62] |
| R1 strain from Datura stramonium L. | SBHA | HDACi | Fusaric acid derivatives [5-butyl-6-oxo-1,6-dihydropyridine-2-carboxylic acid (65) and 5-(but-9-enyl)- 9 6-oxo-1,6-dihydropyridine-2-carboxylic acid (66)] |
NA | [63] |
| Chaetomium cancroideum | Nicotinamide | HDACi | Chaetophenols G (67) and cancrolides A (68) and B (69) | NA | [64] |
| Microascus sp. | SAHA | HDACi | EGM-556 (70) | NT | [65] |
| Phoma sp. nov. LG0217 | SAHA | HDACi | (10ʹS)-verruculide B (71), vermistatin (72) and dihydrovermistatin (73) | Protein tyrosine phosphatases (PTPs) 1B (PTP1B), Src homology 2-containing PTP 1 (SHP1) and T-cell PTP (TCPTP) inhibitory activity | [66] |
| Alternaria sp. | SBHA | HDACi | Alternariol (74), alternariol-5-O-methyl ether (75), 3′-hydroxyalternariol-5-O-methyl ether (76), altenusin (77), tenuazonic acid (78), and altertoxin II (79) | NT | [67] |
| Pestalotiopsis crassiuscula | 5ʹ Azacytidine | DNMTi | Courmarin (80) | NA | [68] |
| Isaria tenuipes | SBHA | HDACi | Tenuipyrone (81) | NT | [38] |
| Cordyceps indigotica | 5ʹ Azacytidine | DNMTi | Indigotide A (82) and indigotide B (83) | NT | [69] |
| Cordyceps annullata, | SBHA | HDACi | Annullatins A–E (84–88) | Cannabinoid receptor ligand | [37] |
| Chaetomium mollipilium | Nicotinamide | HDACi | Mollipilin A-E (89-93) | Anticancer activity | [70] |
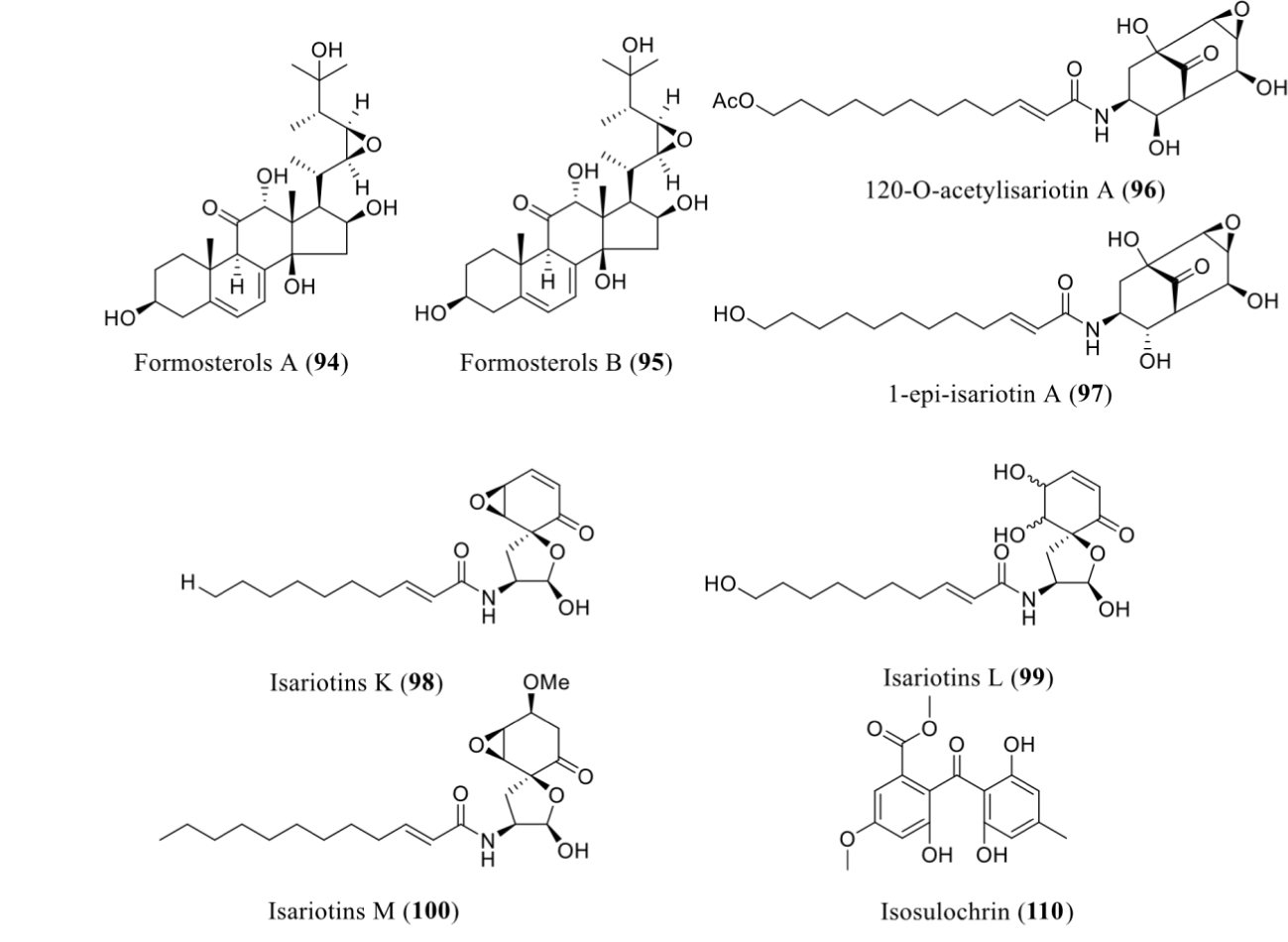
| Gibellula formosana | RG-110/SBHA | DNMTi/HDACi | Formosterols A (94) and B (95), 12ʹ-O-acetylisariotin A (96), 1-epi-isariotin A (97), and isariotins K-M (98-100), | NT | [71] |
| Graphiopsis chlorocephala | Nicotinamide | HDACi | Cephalanones A-F (101- 106) | NT | [72] |
| Beauveria felina | SAHA | HDACi | Desmethylisaridin E (107), desmethylisaridin C2 (108), and isaridin F (109) | Anti-inflammatory | [73] |
| Chaetomium sp | SAHA/5ʹ azacytidine | HDACi/DNMTi | Isosulochrin (110) | NA | [74] |
| Leucostoma persoonii | Sodium butyrate | HDACi | Cytosporone R (111) | NA | [75] |
DNA Methyltransferase Inhibitors (DNMTi)
5ʹ Azacytidine: A chemical analog of cytidine, azacitidine and its deoxy derivative, decitabine which is also known as 5-aza-2′-deoxycytidine are used in the treatment of the myelodysplastic syndrome. Czechoslovakia firstly synthesized both of these drugs which were used as potential chemotherapeutic agents for cancer [76]. Perturbation of 5-azacytidine to Cladosporium cladosporioides can stimulate the production of numerous oxylipins including a glycerol conjugate (16), (9Z, 12Z)-11-hydroxyoctadeca-9,12-dienoic acid (17), and its methyl ester (18). Treatment of a Diatrype species with 5-azacytidine elicited the formation of lunalides A (19) and B (20) [2]. Yang and co-workers also reported the production of aflatoxins by Aspergillus flavus via 5azacytidine treatment [12].
Chemical epigenetic manipulation of Penicillium citreonigrum directed to profound changes in the secondary metabolic profile of its guttate. Fungi treated with 50 µM 5-azacytidine results in the 2 new metabolites production, meroterpenes atlantinones A (21) and B (22). Both metabolites from the P. citreonigrum guttate were tested for antimicrobial activity in a disk diffusion assay but found to be inactive [44]. Aspergillus sp. XS-20090066 was treated with a DNA methyltransferase inhibitor, 5-azacytidine, resulted the production of six bisabolane-type sesquiterpenoids, including (R)-(–)-hydroxy sydonic acid (31) [48], (S)-(+)-sydonic acid (32) [48], (S)-(–)-5-(hydroxymethyl)-2-(2_,6_,6_-trimethyltetrahydro-2H-pyran-2-yl)phenol (33) [51,52], (7S,11S)-(+)-12-hydroxy sydonic acid (34) [47], (S)-(+)-11-dehydro sydonic acid (35) [50], and (S)-(–)-sydowic acid (36) [51]. It has been supposed that 5-azacytidine may defeat DNA methyltransferase and consequently activate genes that express the bisabolane-type sesquiterpenoids. All compounds were tested for their antibacterial activities against various pathogenic bacteria strains. Compounds 31-36 showed broad spectrum activities against tested bacteria, while the others had weak or no antibacterial activities. In particular, (31) showed pronounced antibacterial activity against S. aureus with a MIC value of 3.13 µM, which was close to the positive control ciprofloxacin (MIC = 2.5 µM). N. crassa was treated with 5-azacytidine and result in the production of new carotenoids while its bioactivity was not tested [45].
Incorporation of 5-azacytidine in endophytic fungus Pestalotiopsis crassiuscula culture can change the metabolic profile with biosynthesizing a novel coumarin (80) that was confirmed by NMR spectra. Compound 80 was reported to not possess antifungal activity [77].
Two new aromatic polyketide glycoside indigotide A (82) and indigotide B (83), were isolated from the culture broth of the entomopathogenic fungus, Cordyceps tenuipes culture broth with the treatment of 5-azacytidine with no tested bioactivity [69] (Figure 5).
Histone Deacetylase Inhibitors (HDACi)
Suberanilohydroxamic Acid (SAHA): Suberanilohydroxamic Acid (SAHA) also known as vorinostat with trade name Zolinza is a member of a larger class of compounds that inhibit Histone Deacetylases (HDAC). Histone deacetylase inhibitors have a broad spectrum of epigenetic activities. The compound was introduced by Ronald Breslow, the Columbia University chemist and Paul Marks, A Memorial Sloan-Kettering researcher [78,79]. The first Histone Deacetylase Inhibitor (HDACi) was vorinostat that was approved by the U.S. Food and Drug Administration (FDA) for the treatment of Cutaneous T-cell lymphoma (CTCL) on October 6, 2006 [78].
SAHA as an epigenetic modifying agent used by many researchers to stimulate the production of many secondary metabolites. Fungi, C. cladosporioides, treated SAHA resulted in the production of a complex series of perylenequinones including cladochromes A-D, F, and G (23-28) and calphostin B (29). The Cichewicz group also isolated nygerone A (30) from Aspergillus niger when culturing with suberoylanilide hydroxamic acid [79]. Metabolic profiles of P. mallochii CCH01 were reported to be changed by SAHA treatment. Two new natural sclerotioramine derivatives, isochromophilone XIV (37) and isochromophilone XV (38) were purified with no reported bioactivity [53]. Two new compounds, (+)- and (-)-brevianamide X ((+)- and (-)- 39 and 40)), in addition to a new naturally occurring one, 3-[6-(2-methylpropyl)-2-oxo1H-pyrazin-3-yl] propanamide (41) were purified from the chemical-epigenetic cultures of Aspergillus versicolor OUCMDZ-2738 with 10 µM vorinostat (SAHA) [54]. Similarly, another research reported about SAHA significance to improve the diversity of secondary metabolites of Aspergillus versicolor by the production of a new biphenyl derivative, named versiperol A (42) [10]. Two novel polyketides from a fermentation broth of A. cruciatus named primarolides A (43) and B (44) and were purified when treated with SAHA in combination with sodium chloride (NaCl). Bioactivity of 39, 40, 41, 42, 43, and 44 were not reported [80].
SAHA has been used to suppress HDAC in Chaetomium indicum, giving rise to six novel prenylated aromatic polyketides, chaetophenols A-F (45-50). Among these, compounds 48, 49, and 50 contained extraordinary polycyclic skeletons [42]. More recently, chemical analysis of the culture broth of the plant endophyte Penicillium sp. HS-11 in the modified Martin’s medium improved with SAHA, led to the isolation and identification of one novel chemical structure, 4epipenicillone B (51) and one previously undescribed polyketide with a rarely occurring tricycle [5.3.1.03,8] undecane skeleton (R)-(+)-chrysogine (52). Acquisition of 4-epipenicillone B (51) enriched the chemical diversities of fungal natural products possessing a tricyclo [5.3.1.03,8] undecane skeleton. The cytotoxic activity of 52 was also evaluated [57]. Vervoort, et al. in 2010 reported that culturing marine sediment-derived fungus Microascus sp. in the presence of SAHA can lead to the biosynthesis of EGM-556, a new cyclodepsipeptide (70) of hybrid biosynthetic origin [65]. Although bioactivity of that metabolite has not performed it was reported that the 16 atom peptolide center of 70 is rare; the solitary additional examples are the antimicrobial unnarmicins from a marine-derived Photobacterium sp. MBIC06485 [81] and the histone deacetylase inhibitory/ antitumor active FK228 (FR901228, 4) from Chromobacterium violaceum No. 968 [82].
Similarly, incorporation of SAHA to a culture broth of the endophytic fungus Phoma sp. nov. LG0217 isolated from Parkinsonia microphylla altered its metabolite profile and give rise to the production of (10ʹS)-verruculide B (71), vermistatin (72) and dihydrovermistatin (73). Compound 71 repressed the activity of Protein Tyrosine Phosphatases (PTPs) 1B (PTP1B), Src homology 2-containing PTP 1 (SHP1) and T-cell PTP (TCPTP) with IC50 values of 13.7 ± 3.4, 8.8 ± 0.6, and 16.6 ± 3.8 μM, respectively [94]. The addition of SAHA to a culture of the filamentous fungus Beauveria felina was reported to significantly changed its secondary metabolite profile and results in the isolation of three new compounds including cyclodepsipeptides, desmethylisaridin E (107), desmethylisaridin C2 (108), and isaridin F (109). The anti-inflammatory activity of these compounds was evaluated by assessing their effect on superoxide anion production and elastase release by FMLP-induced human neutrophils. Among all three compounds, desmethylisaridin E (107) repressed superoxide anion production and desmethylisaridin C2 (108) repressed elastase release, with IC50 values of 10.00 ± 0.80 and 10.01 ± 0.46 μM, respectively [73] (Figure 6).
Trichostatin A
An organic compound, trichostatin A is an antifungal antibiotic that selectively inhibits the class I and II histone deacetylase families of enzymes, but not class III HDACs among mammals. Researchers are using trichostatin A as an epigenetic modifying agent to selectively effect on HDAC machinery of fungus to stimulate its secondary metabolite production. Four new meroterpenoids named as (4S)-4-decarboxylflavipesolide C (55), 1-(2,2- dimethylchroman-6-yl)3-(4-hydroxyphenyl)propan-2-one (56), (R,E)-3-(2,2-dimethyl chroman-6-yl)-4-hydroxy-5-((2(2-hydroxypropan-2-yl)-2,3-dihydrobenzofuran-5 yl)methylene) furan- 2(5H)-one (57), methyl (R)-2-(2-(2-hydroxypropan-2-yl)-2,3-dihydrobenzofuran-5-yl) acetate (58), were identified from a 10 µM trichostatin A treated strain of Aspergillus terreus OUCMDZ-2739. Compound 57 showed potent α-glucosidase inhibitory activity in comparison with others [59]. Treatment of A. alternate and P. expansum with trichostatin A results the synthesis of new unidentified compounds with untested bioactivity [46] (Figure 7).
Nicotinamide
Nicotinamide, also known as niacinamide, serves as a component of the coenzyme Nicotinamide Adenine Dinucleotide (NAD). Nicotinamide acts as a radio and chemosensitizing agent via increasing tumor blood flow as a result to reduce tumor hypoxia. Nicotinamide also inhibits poly (ADP-ribose) polymerases, enzymes involved in the rejoining of DNA strand breaks induced by radiation or chemotherapy.
Nicotinamide is a histone deacetylase inhibitor that also serves as epigenetic modulators to stimulate the secondary metabolites production among fungi. Epigenetic perturbation of the endophytic fungus led to enhanced production of two new decalin-containing compounds, eupenicinicols C and D (53) and (54). Compound 53 was active against Staphylococcus aureus with an MIC of 0.1 μg/mL and confirmed obvious cytotoxicity against the human acute monocytic leukemia cell line (THP-1) and compound 54 was active against Escherichia coli with a MIC of 5.0 μg/mL [36]. Nicotinamide can also induce the production of chaetophenols G (67) and cancrolides A (68) and B (69) when treated with the culture Chaetomium cancroideum [64]. Another species of the same genus i.e. Chaetomium mollipilium can produce five new C13polyketides, mollipilin A-E (89-93) when cultivated with nicotinamide. Mollipilin A (89) and B (90) showed moderate inhibitory activity on cell growth with GI50 values of 1.8 and 3.7 μM, respectively [71].
Asai, et al. [72] reported the addition of nicotinamide, to the culture medium of the endophytic G. chlorocephala, can significantly stimulate its benzophenone production. A set of new benzophenones, cephalanones A-F (101-106) were isolated with no tested bioactivities (Figure 7).
Suberoyl Bis-Hydroxamic Acid (SBHA)
Suberoyl Bis-Hydroxamic Acid (SBHA) is a Histone Deacetylase (HDAC) inhibitor that hinders the activity of HDAC1 and HDAC3. SBHA suppress the proliferation and brings apoptosis in several cancer cell lines. SBHA has been shown to trigger Notch signaling in Medullary Thyroid Carcinoma (MTC) cells.
SBHA can significantly effect on the secondary metabolism of an entomopathogenic fungus, Torrubiella luteorostrata by the production of three new prenylated tryptophan analogs, luteorides A–C (59-61) with no record of bioactivity [60]. Similarly, another new compound, named 13-angeloyloxy-diplosporin (62) was isolated from the endophytic Phomopsis sp. 0391 cultivated in the presence of SBHA. 62 was tested for lipase inhibitory activity but not found to be active [61].
By the treatment of SBHA to the culture of F. oxysporumiz can produce two new fusaric acid derivatives 5-butyl-6-oxo-1,6- dihydropyridine-2-carboxylic acid (65) and 5-(but-9-enyl)-6oxo-1,6-dihydropyridine-2-carboxylic acid (66). Antibacterial activities were tested but unfortunately, none of the compounds was reported to be bioactive [62]. The accumulation of the epigenetic modifier, specifically, SBHA to the culture medium of Alternaria sp. intensely altered the metabolic profile. Six new compounds named alternariol (74), alternariol-5-O-methyl ether (75), 3′-hydroxyalternariol-5-O-methyl ether (76), altenusin (77), tenuazonic acid (78), and altertoxin II (79) [67].
Screening of the entomopathogenic fungi Isaria tenuipes that were cultured in the presence of SBHA showed significant changes in the production of secondary metabolites. This approach led to the isolation of tenuipyrone (81), a novel skeletal polyketide with no tested bioactivity [68]. The secondary metabolite production of an entomopathogenic fungus Cordyceps annullata, was improved by the accumulation of SBHA to the culture medium. Four new 2,3-dihydrobenzofurans, annullatins A-D (84-87), and a new aromatic polyketide, annullatin E (88) were purified.
Dihydrobenzofurans serve as cannabinoid receptor ligands [37] (Figure 7).
Sodium butyrate
Sodium butyrate is the sodium salt of butyric acid. It has numerous effects on cultured mammalian cells comprising inhibition of proliferation, induction of differentiation and induction or repression of gene expression [43]. Sodium butyrate can be used in a lab to bring about any of these effects. Precisely, butyrate treatment of cells results in histone hyperacetylation, and butyrate itself hinders class I Histone Deacetylase (HDAC) activity [83], specifically HDAC1, HDAC2, HDAC3, and butyrate can be used in defining histone deacetylate in chromatin structure and function [84].
Sodium butyrate is also a histone deacetylase inhibitor, effective to stimulate the metabolite production of fungi by reverse the effect of histone acetylase. Treatment of sodium butyrate to an endophytic fungus Phomopsis species led to the isolation of two new 14-membered resorcylic acid lactones described with bromine substitution, 5-bromozeaenol (63) and 3, 5-dibromozeaenol (64). Both compounds were found to be inactive when tested for cytotoxicity, antifouling and zebrafish teratogenicity [62].
Epigenetic modifier sodium butyrate was incorporated into the culture medium Leucostoma persoonii and induced the new secondary metabolite Cytosporone R (111) with no reported activity [75] (Figure 7).
DNMTi and HDACi Combined Treatment to Stimulate the Secondary Metabolite Production
The combined treatment of DNMTi and HDACi has studied to activate those secondary metabolites which could produce neither usual laboratory condition nor single epigenetic modifier treatment. Asai and co-workers have applied that approach by using RG-108 and SBHA as DNMTi and HDACi, respectively. Both modifiers were added to the culture medium of G. formosana and found significant enhancement of the secondary metabolite production. Two types of natural products were isolated include highly oxidized ergosterols and isariotin analogs. Highly oxidized ergosterols, include formosterols A (94) and B (95), while five new isariotin analogs include 120O-acetylisariotin A (96), 1-epi-isariotin A (97), and isariotins K-M (98-100). None of these compounds were tested for any bioactivities [72]. Likewise, incorporation of 5-azacytidine or SAHA led to the induction of isosulochrin (110) in Chaetomium sp with no reported bioactivities [74] (Figure 8).
Epigenetic as an Approach of Enhancement of Metabolite Production with Bioactivity
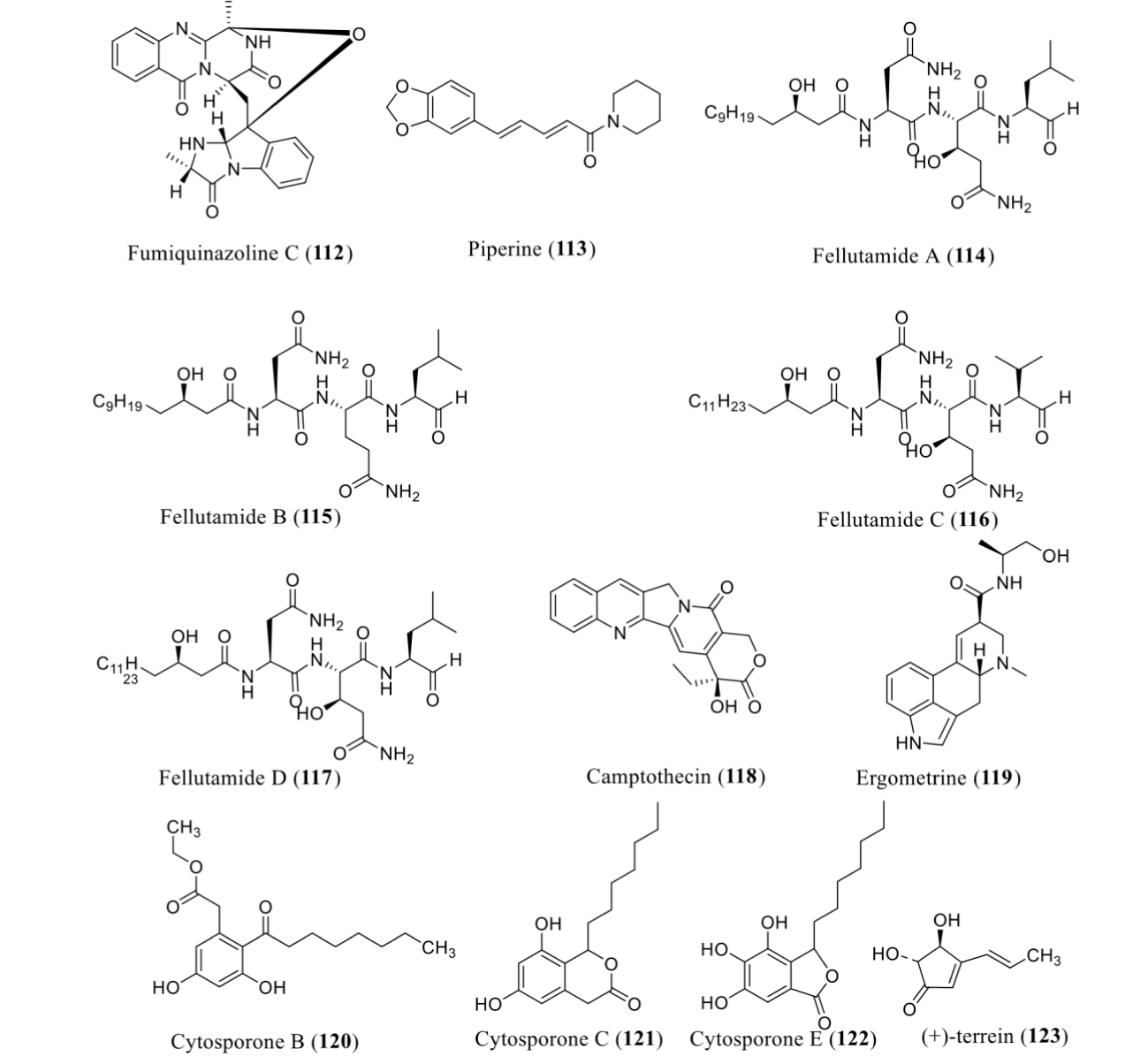
Valproic acid (valproate), an anticonvulsant and a mood stabilizer, is a potent Histone Deacetylase Inhibitor (HDACi). The addition of valproic acid in the culture medium can improved the metabolic profile of A. fumigatus (GA-L7) by the enrichment of fumiquinazoline C (112). This compound was produced in trace amounts under normal laboratory conditions. Fumiquinazolines are peptidyl alkaloids that are reported to possess substantial antitumor [85], antifungal [86] and antibacterial properties [87].
It was reported that endophytic fungal isolate, diaporthe sp. PF20. When exposing to epigenetic treatment along with previously characterized piperine producing Colletotrichum sp. and Mycosphaerella sp. from the Piper nigrum L. plant can overproduce piperine (113) by the use of SAHA. In this report, the epigenetic modulator (SAHA) mediated enrichment of phytochemical biosynthetic potential of endophytic fungi [47]. A. nidulans can overexpress the genes for fellutamides A-D (114-117), proteasome inhibitors when treated with SAHA, an HDACi [88]. Fungi, Botryosphaeria rhodina when cultured in the presence of 5-azacytidine can enhance the production of Camptothecin (CPT) (118) as compared to wild type. CPT was reported to have anticancer activity [89].
The histone deacetylase inhibitor SAHA was also reported to significantly improve the alkaloid productivity of the strain Claviceps purpurea Cp-1. Principally, the titers of total ergot alkaloids, ergometrine (119) were progressively improved with the increasing concentration of SAHA in the fermentation medium, and the maximum production of ergot alkaloids could be attained at the concentration of 500 µM SAHA. Particularly, the titers of ergometrine and total ergot alkaloids were as high as 95.4 mg/L and 179.7 mg/L, respectively, which were twice those of the control [90].
The histone deacetylase inhibitor sodium butyrate can lead to the enhancement of know bioactive secondary metabolites in Leucostoma persoonii including cytosporones B (120), C (121) and E (122). Cytosporone E (122) was reported to be the most bioactive, displaying an IC90 of 13 μM toward Plasmodium falciparum, with A549 cytotoxicity IC90 of 437 μM, demonstrating a 90% inhibition therapeutic index (TI90 = IC90 A459/IC90 P. falciparum) (Table 2). Including, cytosporone E (122) was active against MRSA with a Minimal Inhibitory Concentration (MIC) of 72 μM [75]. It was reported by Xiao, et al. in 2013 that SAHA exhibited a positive impact on (+) terrein (123) production in Aspergillus terreus strain PF26 which was resulting from endorsing the biosynthesis of 6-hydroxymellein, the precursor of (+)-terrein (123). (+)-terrein (123) has many reported bioactivities [91] (Figure 9).
| Table 2: List of known secondary metabolites enhanced by different DNMTi and HDACi with reported bioactivities. | |||||
| Species | Modulators | Class | Compounds | Reported Bioactivity/role | References |
| A. fumigatus (GA-L7) |
Valproic acid | HDACi | Fumiquinazoline C (112) | Antibacterial, antifungal, antitumor | [92] |
| Diaporthe sp. PF20 | SAHA | HDACi | Piperine (113) | Dietary supplement | [47] |
| Aspergillus nidulans | SAHA | HDACi | Fellutamides A-D (114-117) | Proteasome inhibitor | [88] |
| Botryosphaeria rhodina | 5ʹ azacitidine | DNMTi | Camptothecin (118) | Anticancer | [89] |
| Claviceps purpurea Cp-1 | SAHA | HDACi | Ergometrine (119) | Obstetrics | [90] |
| Leucostoma persoonii | Sodium butyrate | HDACi | Cytosporone B, C, and E (120-122) | Antimalarial and antibacterial | [75] |
| Aspergillus terreus | SAHA | HDACi | (+)-terrein (123) | Anti-inflammatory, melanin biosynthesis inhibition, antibiosis, weed inhibition, anti-tumor, improve Osseo integration | [91-100] |
Known Inhibitors of DNMTs from Natural Sources
Up to date above 500 compounds have been tested as inhibitors of DNMTs. Their structure and coverage in chemical space have been studied using chemoinformatic methods [101]. The DNMT inhibitors targets have been compared with inhibitors of other epigenetic targets [102]. Additionally, the Structure-Activity Relationships (SAR) of DNMT inhibitor using the idea of the activity landscape have been acknowledged [103] (Table 3).
| Table 2: List of known secondary metabolites enhanced by different DNMTi and HDACi with reported bioactivities. | |||||
| Species | Modulators | Class | Compounds | Reported Bioactivity/role | References |
| A. fumigatus (GA-L7) |
Valproic acid | HDACi | Fumiquinazoline C (112) | Antibacterial, antifungal, antitumor | [92] |
| Diaporthe sp. PF20 | SAHA | HDACi | Piperine (113) | Dietary supplement | [47] |
| Aspergillus nidulans | SAHA | HDACi | Fellutamides A-D (114-117) | Proteasome inhibitor | [88] |
| Botryosphaeria rhodina | 5ʹ azacitidine | DNMTi | Camptothecin (118) | Anticancer | [89] |
| Claviceps purpurea Cp-1 | SAHA | HDACi | Ergometrine (119) | Obstetrics | [90] |
| Leucostoma persoonii | Sodium butyrate | HDACi | Cytosporone B, C, and E (120-122) | Antimalarial and antibacterial | [75] |
| Aspergillus terreus | SAHA | HDACi | (+)-terrein (123) | Anti-inflammatory, melanin biosynthesis inhibition, antibiosis, weed inhibition, anti-tumor, improve Osseo integration | [91-100] |
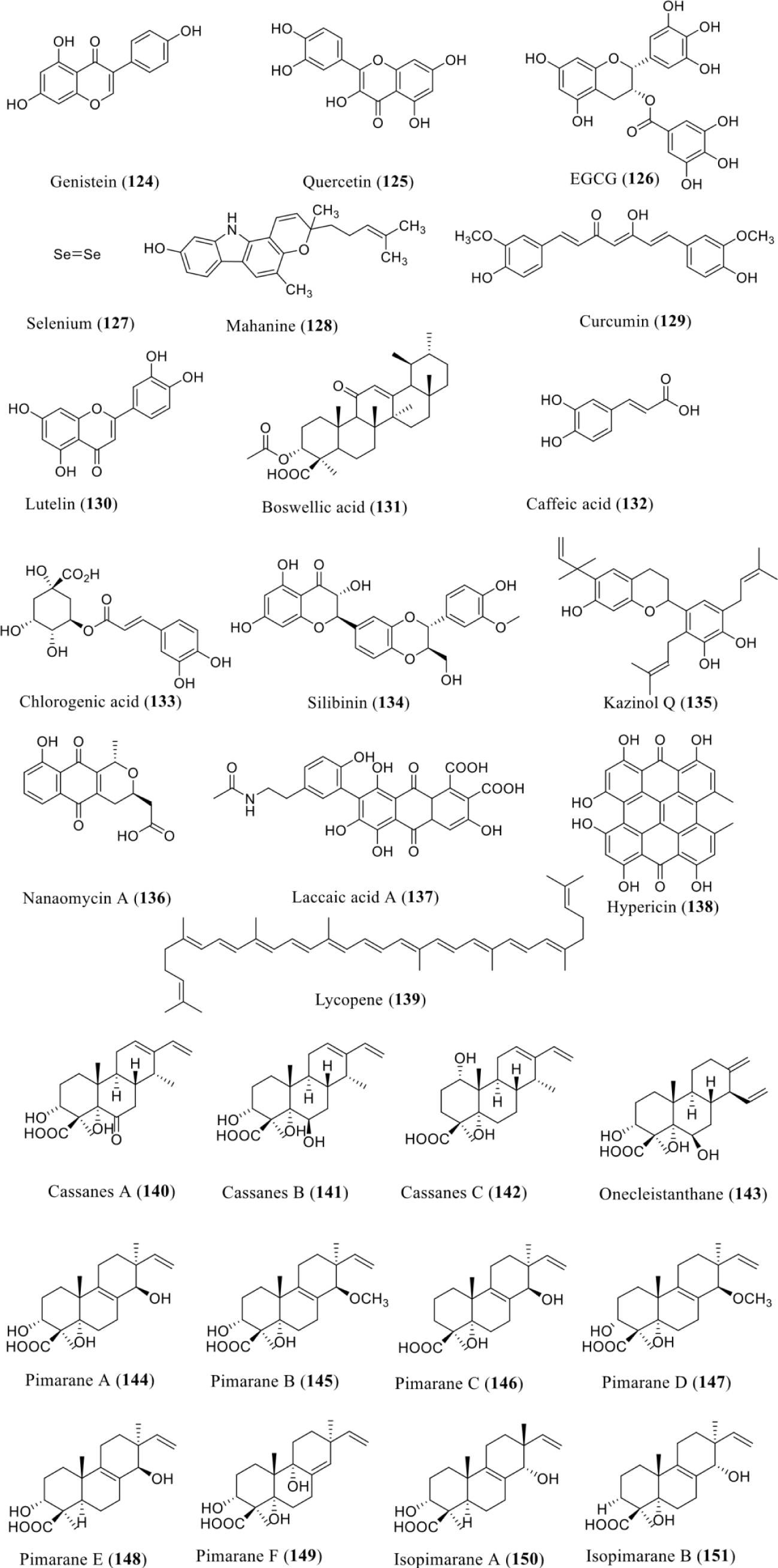
Several different strategies including virtual, organic synthesis, and high throughput screening were used to isolate DNA methyltransferase inhibitors [131]. For principal optimization, organic synthesis has been work in several instances [132,133]. Food chemicals and natural products have been serving as major sources of active compounds. Zwergel, et al. [104] have extensively reviewed the natural products as known DNMT inhibitors or demethylating agents. The natural products which were isolated belong to the class of flavonoids, polyphenols, anthraquinones, and others. We have collected some specific data from that review and accumulated here. Based on such DNMTi sources/origin and type/class, those DNMTi include flavonoids, genistein (124) from soybean Genista tinctoria, quercetin (125) from fruits, vegetables and beverages [104-108], luteolin (130) from Terminalia chebula [104,114,115], silibinin (134) from Silybum marianum, and kazinol Q (135) from Broussonetia kazinoki [104,119-122]. Including quinones, nanaomycin A (136) from Streptomyces, laccaic acid (137) from Kerria lacca, hypericin (138) from Hypericum [104,124-128]. Among polyphenols are (-)-epigallocatechin-3-gallate (EGCG) (126) from Camellia Sinensis (green tea) [104,109], curcumin (129) from Curcuma longa [21,133], caffeic acid (132), and chlorogenic acid (133) from Coffea Arabica [104,118]. Some other reported DNMTi were carbazole alkaloid, mahanine (128) from Micromelum minutum and Murraya koenigii, terpenoid, boswellic acid (131) from Boswellia serrata, nonmetal, selenium (127) is an essential trace element which also reported to act like DNMTi [104,110-111,116], and bright red carotene, lycopene (139) from lycopersicum [104,110-112,116,129]. The bioactive profile, mechanisms, and techniques of such natural products well described in the review of Zwergel, et al. [104] with their IC50 values (Figure 10).
Another study conducted by Wei, et al. in 2018 in which they examine the crude extract of C. arbuscula with deleted hdaA (ΔhdaA) strain resulted in the separation of twelve new diterpenoids including three cassanes A-C (140-142), onecleistanthane (143), six pimaranes A-F (144-149), and two isopimaranes A-B (150-151). Compounds 141 and 142 has reported to showed strong inhibitory effects on the expression of MMP1 and MMP2 (matrix metallo proteinases family) in human breast cancer (MCF-7) cells [130] (Figure 10).
Conclusion
More research on the effect of epigenetic modulators on fungi could be recommended to discover newly structured compounds in future studies. The rate of emerging diseases and infections is increasing day by day, and the efficacy and selectivity of available drugs are decreasing. However, many efforts still need to be devoted to addressing these strategies towards the infectious era. In this review, 15 DNMTi and HDACi are reported; 6 commonly used inhibitors are discussed in detail. A total of 96 new compounds with reported bioactivities from different research articles are mentioned, isolated from those 6 inhibitors, either in combination or a single effect. Epigenetic techniques have several noteworthy benefits related to presently available molecular or culture-dependent techniques. First and leading, it provides a needed tool for rapidly retrieving possible pools of cryptic fungal natural products in their natural hosts. Second, this approach can be readily implemented in most laboratories deprived of widespread retooling, giving it a varied scope of consumption. Third, this technique will suggestively reduce the cost and exertion of obtaining the products of silent secondary metabolic pathways since fungi do not need to be pre-screened using a multitude of culture conditions.
Conflict of Interests
All the authors declared no potential conflict of interest.
References
- Schulz B, Boyle C, Draeger S, Römmert A. Endophytic fungi: A source of novel biologically active secondary metabolites. Published online by Cambridge University Press. 2002;106:996-1004. doi: 10.1017/S0953756202006342.
- Cichewicz R. Epigenetic regulation of secondary metabolite biosynthetic genes in fungi. In: Witzany G. editor. Biocommunication of Fungi. Springer: Dordrecht; 2012. doi:10.1007/978-94-007-4264-2_4.
- Cichewicz RH. Epigenome manipulation as a pathway to new natural product scaffolds and their congeners. Nat Prod Rep. 2010 Jan;27(1):11-22. doi: 10.1039/b920860g. Epub 2009 Oct 27. PMID: 20024091; PMCID: PMC2958777.
- Williams RB, Henrikson JC, Hoover AR, Lee AE, Cichewicz RH. Epigenetic remodeling of the fungal secondary metabolome. Org Biomol Chem. 2008 Jun 7;6(11):1895-7. doi: 10.1039/b804701d. Epub 2008 Apr 14. PMID: 18480899.
- Henrikson JC, Hoover AR, Joyner PM, Cichewicz RH. A chemical epigenetics approach for engineering the in situ biosynthesis of a cryptic natural product from Aspergillus niger. Org Biomol Chem. 2009 Feb 7;7(3):435-8. doi: 10.1039/b819208a. Epub 2008 Dec 11. PMID: 19156306.
- Zhang S, Fang H, Yin C, Wei C, Hu J, Zhang Y. Antimicrobial Metabolites Produced by Penicillium mallochii CCH01 Isolated From the Gut of Ectropis oblique, Cultivated in the Presence of a Histone Deacetylase Inhibitor. Front Microbiol. 2019 Oct 2;10:2186. doi: 10.3389/fmicb.2019.02186. PMID: 31632360; PMCID: PMC6783908.
- Liu W, Wang L, Wang B, Xu Y, Zhu G, Lan M, Zhu W, Sun K. Diketopiperazine and Diphenylether Derivatives from Marine Algae-Derived Aspergillus versicolor OUCMDZ-2738 by Epigenetic Activation. Mar Drugs. 2018 Dec 22;17(1):6. doi: 10.3390/md17010006. PMID: 30583513; PMCID: PMC6356248.
- Zhu JX, Ding L, He S. Discovery of a new biphenyl derivative by epigenetic manipulation of marine-derived fungus Aspergillus versicolor. Nat Prod Res. 2019 Apr;33(8):1191-1195. doi: 10.1080/14786419.2018.1465423. Epub 2018 Apr 23. PMID: 29683350.
- Igboeli HA, Marchbank DH, Correa H, Overy D, Kerr RG. Discovery of Primarolides A and B from Marine Fungus Asteromyces cruciatus Using Osmotic Stress and Treatment with Suberoylanilide Hydroxamic Acid. Mar Drugs. 2019 Jul 24;17(8):435. doi: 10.3390/md17080435. PMID: 31344982; PMCID: PMC6723326.
- Jasim B, Sahadevan N, Chithra S, Mathew J, Radhakrishnan EK. Epigenetic Modifier Based Enhancement of Piperine Production in Endophytic Diaporthe sp. PF20. Proc Natl Acad Sci India Sect B Biol Sci. 2019;89:671-677. doi: 10.1007/s40011-018-0982-0.
- Asai T, Yamamoto T, Shirata N, Taniguchi T, Monde K, Fujii I, Gomi K, Oshima Y. Structurally diverse chaetophenol productions induced by chemically mediated epigenetic manipulation of fungal gene expression. Org Lett. 2013 Jul 5;15(13):3346-9. doi: 10.1021/ol401386w. Epub 2013 Jun 14. PMID: 23767797.
- Wang X, Sena Filho JG, Hoover AR, King JB, Ellis TK, Powell DR, Cichewicz RH. Chemical epigenetics alters the secondary metabolite composition of guttate excreted by an atlantic-forest-soil-derived Penicillium citreonigrum. J Nat Prod. 2010 May 28;73(5):942-8. doi: 10.1021/np100142h. PMID: 20450206; PMCID: PMC2878378.
- Kudo S, Murakami T, Miyanishi J, Tanaka K, Takada N, Hashimoto M. Isolation and absolute stereochemistry of optically active sydonic acid from Glonium sp. (Hysteriales, Ascomycota). Biosci Biotechnol Biochem. 2009 Jan;73(1):203-4. doi: 10.1271/bbb.80535. Epub 2009 Jan 7. PMID: 19129640.
- Serra S. Bisabolane sesquiterpenes: Synthesis of (R)-(+)-sydowic scid and (R)-(+)- curcumene ether. Syn Lett. 2000;6:890. doi: 10.1055/s-2000-6698.
- Chung YM, Wei CK, Chuang DW, El-Shazly M, Hsieh CT, Asai T, Oshima Y, Hsieh TJ, Hwang TL, Wu YC, Chang FR. An epigenetic modifier enhances the production of anti-diabetic and anti-inflammatory sesquiterpenoids from Aspergillus sydowii. Bioorg Med Chem. 2013 Jul 1;21(13):3866-72. doi: 10.1016/j.bmc.2013.04.004. Epub 2013 Apr 13. PMID: 23647825.
- Lu Z, Zhu H, Fu P, Wang Y, Zhang Z, Lin H, Liu P, Zhuang Y, Hong K, Zhu W. Cytotoxic polyphenols from the marine-derived fungus Penicillium expansum. J Nat Prod. 2010 May 28;73(5):911-4. doi: 10.1021/np100059m. PMID: 20415462.
- Hamasaki T, Sato Y, Hatsuda Y, Tanabe M and Cary LW. Sydowic acid, a new metabolite from Aspergillus sydowic. Tetrahedron Lett. 1975;9:659. doi: 10.1016/S0040-4039(00)71947-2.
- Asai T, Yamamoto T, Oshima Y. Histone deacetylase inhibitor induced the production of three novel prenylated tryptophan analogs in the entomopathogenic fungus, Torrubiella luteorostrata. Tetrahedron Lett. 2011;52:7042-7045. doi: 10.1016/j.tetlet.2011.10.020.
- Zhang W, Shao CL, Chen M, Liu QA, Wang CY. Brominated resorcylic acid lactones from the marine-derived fungus Cochliobolus lunatus induced by histone deacetylase inhibitors. Tetrahedron Lett. 2014;55:4888-4891. doi: 10.1016/j.tetlet.2014.06.096.
- Ying YM, Li L, Yu HF, Xu YL, Huang L, Mao W, Tong CP, Zhang ZD, Zhan ZJ, Zhang Y. Induced production of a new polyketide in Penicillium sp. HS-11 by chemical epigenetic manipulation. Nat Prod Res. 2021 Oct;35(20):3446-3451. doi: 10.1080/14786419.2019.1709190. Epub 2020 Jan 3. PMID: 31899961.
- Sheng SL, Li YP, Xiang HY, Liu Y, Wang YD, Kong LP, Du G, Hu QF, Chen YJ, Wang WG. Histone deacetylase inhibitor induced lipase inhibitors from Endophytic Phomopsis sp. 0391. Rec Nat Prod. 2020;14:42-47. doi: 10.25135/rnp.134.19.01.1243.
- Waddington CH. The epigenotype. 1942. Int J Epidemiol. 2012 Feb;41(1):10-3. doi: 10.1093/ije/dyr184. Epub 2011 Dec 20. PMID: 22186258.
- Kritskiĭ MS, Filippovich SIu, Afanas'eva TP, Bachurina GP, Russo VE. Vliianie ingibitorov fermentativnogo metilirovaniia DNK na obrazovanie reproduktivnykh struktur i karotinogenez u Neurospora crassa [Effect of inhibitors of enzymatic DNA methylation on the formation of reproductive structures and carotenoid production in Neurospora crassa]. Prikl Biokhim Mikrobiol. 2001 May-Jun;37(3):279-84. Russian. PMID: 11443894.
- Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009 May;10(5):295-304. doi: 10.1038/nrg2540. PMID: 19308066.
- Migliori V, Phalke S, Bezzi M, Guccione E. Arginine/lysine-methyl/methyl switches: biochemical role of histone arginine methylation in transcriptional regulation. Epigenomics. 2010 Feb;2(1):119-37. doi: 10.2217/epi.09.39. PMID: 22122749.
- Li Y, He Y, Li X, Fasoyin OE, Hu Y, Liu Y, Yuan J, Zhuang Z, Wang S. Histone Methyltransferase aflrmtA gene is involved in the morphogenesis, mycotoxin biosynthesis, and pathogenicity of Aspergillus flavus. Toxicon. 2017 Mar 1;127:112-121. doi: 10.1016/j.toxicon.2017.01.013. Epub 2017 Jan 19. Erratum in: Toxicon. 2017 Aug;134:64. PMID: 28109854.
- Du J, Johnson LM, Jacobsen SE, Patel DJ. DNA methylation pathways and their crosstalk with histone methylation. Nat Rev Mol Cell Biol. 2015 Sep;16(9):519-32. doi: 10.1038/nrm4043. PMID: 26296162; PMCID: PMC4672940.
- Tamaru H, Zhang X, McMillen D, Singh PB, Nakayama J, Grewal SI, Allis CD, Cheng X, Selker EU. Trimethylated lysine 9 of histone H3 is a mark for DNA methylation in Neurospora crassa. Nat Genet. 2003 May;34(1):75-9. doi: 10.1038/ng1143. PMID: 12679815.
- Jurkowski TP, Jeltsch A. On the evolutionary origin of eukaryotic DNA methyltransferases and Dnmt2. PLoS One. 2011;6(11):e28104. doi: 10.1371/journal.pone.0028104. Epub 2011 Nov 30. PMID: 22140515; PMCID: PMC3227630.
- Migliori V, Phalke S, Bezzi M, Guccione E. Arginine/lysine-methyl/methyl switches: biochemical role of histone arginine methylation in transcriptional regulation. Epigenomics. 2010 Feb;2(1):119-37. doi: 10.2217/epi.09.39. PMID: 22122749.
- Stroka J. Food Additives & Contaminants: Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment. Foreword. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011 Mar;28(3):259. doi: 10.1080/19440049.2011.561599. PMID: 21360372.
- Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013 Jan;38(1):23-38. doi: 10.1038/npp.2012.112. Epub 2012 Jul 11. PMID: 22781841; PMCID: PMC3521964.
- Liu SY, Lin JQ, Wu HL, Wang CC, Huang SJ, Luo YF, Sun JH, Zhou JX, Yan SJ, He JG, Wang J, He ZM. Bisulfite sequencing reveals that Aspergillus flavus holds a hollow in DNA methylation. PLoS One. 2012;7(1):e30349. doi: 10.1371/journal.pone.0030349. Epub 2012 Jan 20. PMID: 22276181; PMCID: PMC3262820.
- Storck R, Nobles MK, Alexopou CJ. The nucleotide composition of deoxyribonucleic acid of some species of hymenochaetaceae and polyporaceae. Mycologia. 1971;63:38-49. doi: 10.1080/00275514.1971.12019080
- Antequera F, Tamame M, Villanueva JR, Santos T. DNA methylation in the fungi. J Biol Chem. 1984 Jul 10;259(13):8033-6. PMID: 6330093.
- Jeon J, Choi J, Lee GW, Park SY, Huh A, Dean RA, Lee YH. Genome-wide profiling of DNA methylation provides insights into epigenetic regulation of fungal development in a plant pathogenic fungus, Magnaporthe oryzae. Sci Rep. 2015 Feb 24;5:8567. doi: 10.1038/srep08567. PMID: 25708804; PMCID: PMC4338423.
- Zemach A, McDaniel IE, Silva P, Zilberman D. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science. 2010 May 14;328(5980):916-9. doi: 10.1126/science.1186366. Epub 2010 Apr 15. PMID: 20395474.
- Montanini B, Chen PY, Morselli M, Jaroszewicz A, Lopez D, Martin F, Ottonello S, Pellegrini M. Non-exhaustive DNA methylation-mediated transposon silencing in the black truffle genome, a complex fungal genome with massive repeat element content. Genome Biol. 2014 Jul 31;15(7):411. doi: 10.1186/s13059-014-0411-5. PMID: 25091826; PMCID: PMC4165359.
- Fernandez-de Gortari E, Medina-Franco JL. Epigenetic relevant chemical space: A chemoinformatic characterization of inhibitors of DNA methyltransferases. RSC Adv. 2015;5:87465-87476. doi: 10.1039/C5RA19611F.
- Naveja JJ, Medina-Franco JL. Activity landscape sweeping: Insights into the mechanism of inhibition and optimization of DNMT1 inhibitors. RSC Adv. 2015;5:63882-63895. doi: 10.1039/C5RA12339A.
- Naveja JJ, Medina-Franco JL. Insights from pharmacological similarity of epigenetic targets in epipolypharmacology. Drug Discov Today. 2018 Jan;23(1):141-150. doi: 10.1016/j.drudis.2017.10.006. Epub 2017 Oct 14. PMID: 29038074.
- Medina-Franco JL, Méndez-Lucio O, Dueñas-González A, Yoo J. Discovery and development of DNA methyltransferase inhibitors using in silico approaches. Drug Discov Today. 2015 May;20(5):569-77. doi: 10.1016/j.drudis.2014.12.007. Epub 2014 Dec 16. PMID: 25526932.
- Kabro A, Lachance H, Marcoux-Archambault I, Perrier V, Dore V, Gros C, Masson V, Gregoire JM, Ausseil F, Cheishvili D, Laulan NB, St-Pierre Y, Szyf M, Arimondo PB, Gagnon A. Preparation of phenylethylbenzamide derivatives as modulators of DNMT3 activity. Med Chem Comm. 2013;4:1562-1570. doi: 10.1039/C3MD00214D.
- Garella D, Atlante S, Borretto E, Cocco M, Giorgis M, Costale A, Stevanato L, Miglio G, Cencioni C, Fernández-de Gortari E, Medina-Franco JL, Spallotta F, Gaetano C, Bertinaria M. Design and synthesis of N-benzoyl amino acid derivatives as DNA methylation inhibitors. Chem Biol Drug Des. 2016 Nov;88(5):664-676. doi: 10.1111/cbdd.12794. Epub 2016 Jun 24. PMID: 27225604.
- Zwergel C, Valente S, Mai A. DNA Methyltransferases Inhibitors from Natural Sources. Curr Top Med Chem. 2016;16(7):680-96. doi: 10.2174/1568026615666150825141505. PMID: 26303417.
- Watson JD, Baker TA, Gann A, Levine M, Losik R. Molecular biology of the gene 7th Ed. Boston: Pearson/CSH Press; 2014. https://tinyurl.com/99s65uu9
- Jeon J, Choi J, Lee GW, Park SY, Huh A, Dean RA, Lee YH. Genome-wide profiling of DNA methylation provides insights into epigenetic regulation of fungal development in a plant pathogenic fungus, Magnaporthe oryzae. Sci Rep. 2015 Feb 24;5:8567. doi: 10.1038/srep08567. PMID: 25708804; PMCID: PMC4338423.
- Garnaud C, Champleboux M, Maubon D, Cornet M, Govin J. Histone Deacetylases and Their Inhibition in Candida Species. Front Microbiol. 2016 Aug 5;7:1238. doi: 10.3389/fmicb.2016.01238. PMID: 27547205; PMCID: PMC4974301.
- Jasim B, Sahadevan N, Chithra S, Mathew J, Radhakrishnan EK. Epigenetic modifier based enhancement of piperine production in Endophytic Diaporthe sp. PF20. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. 2019;89:671-677. https://tinyurl.com/2p8vudsy
- Li G, Kusari S, Golz C, Laatsch H, Strohmann C, Spiteller M. Epigenetic Modulation of Endophytic Eupenicillium sp. LG41 by a Histone Deacetylase Inhibitor for Production of Decalin-Containing Compounds. J Nat Prod. 2017 Apr 28;80(4):983-988. doi: 10.1021/acs.jnatprod.6b00997. Epub 2017 Mar 23. PMID: 28333449.
- Bai J, Mu R, Dou M, Yan D, Liu B, Wei Q, Wan J, Tang Y, Hu Y. Epigenetic modification in histone deacetylase deletion strain of Calcarisporium arbuscula leads to diverse diterpenoids. Acta Pharm Sin B. 2018 Jul;8(4):687-697. doi: 10.1016/j.apsb.2017.12.012. Epub 2018 Feb 21. PMID: 30109192; PMCID: PMC6090014.
- Zhang S, Fang H, Yin C, Wei C, Hu J, Zhang Y. Antimicrobial Metabolites Produced by Penicillium mallochii CCH01 Isolated From the Gut of Ectropis oblique, Cultivated in the Presence of a Histone Deacetylase Inhibitor. Front Microbiol. 2019 Oct 2;10:2186. doi: 10.3389/fmicb.2019.02186. PMID: 31632360; PMCID: PMC6783908.
- Huang D, Cui L, Sajid A, Zainab F, Wu Q, Wang X, Yuan Z. The epigenetic mechanisms in Fusarium mycotoxins induced toxicities. Food Chem Toxicol. 2019 Jan;123:595-601. doi: 10.1016/j.fct.2018.10.059. Epub 2018 Oct 27. PMID: 30599843.
- Henrikson JC, Hoover AR, Joyner PM, Cichewicz RH. A chemical epigenetics approach for engineering the in situ biosynthesis of a cryptic natural product from Aspergillus niger. Org Biomol Chem. 2009 Feb 7;7(3):435-8. doi: 10.1039/b819208a. Epub 2008 Dec 11. PMID: 19156306.
- Fisch KM, Gillaspy AF, Gipson M, Henrikson JC, Hoover AR, Jackson L, Najar FZ, Wägele H, Cichewicz RH. Chemical induction of silent biosynthetic pathway transcription in Aspergillus niger. J Ind Microbiol Biotechnol. 2009 Sep;36(9):1199-213. doi: 10.1007/s10295-009-0601-4. Epub 2009 Jun 12. PMID: 19521728.
- Mooibroek H, Kuipers AG, Sietsma JH, Punt PJ, Wessels JG. Introduction of hygromycin B resistance into Schizophyllum commune: preferential methylation of donor DNA. Mol Gen Genet. 1990 Jun;222(1):41-8. doi: 10.1007/BF00283021. PMID: 1700269.
- Birch PR, Sims PF, Broda P. A reporter system for analysis of regulatable promoter functions in the basidiomycete fungus Phanerochaete chrysosporium. J Appl Microbiol. 1998 Sep;85(3):417-24. doi: 10.1046/j.1365-2672.1998.853468.x. PMID: 9750271.
- Cichewicz RH. Epigenome manipulation as a pathway to new natural product scaffolds and their congeners. Nat Prod Rep. 2010 Jan;27(1):11-22. doi: 10.1039/b920860g. Epub 2009 Oct 27. PMID: 20024091; PMCID: PMC2958777.
- Costa MA, Silva NC, Castro-Prado MA. Genetic and cytological characterization of a developmental mutant of Aspergillus nidulans induced by 5-azacytidine. Biol Res. 2001;34(2):91-8. doi: 10.4067/s0716-97602001000200012. PMID: 11715212.
- Bernstein BE, Tong JK, Schreiber SL. Genomewide studies of histone deacetylase function in yeast. Proc Natl Acad Sci U S A. 2000 Dec 5;97(25):13708-13. doi: 10.1073/pnas.250477697. Erratum in: Proc Natl Acad Sci U S A 2001 Apr 24;98(9):5368. PMID: 11095743; PMCID: PMC17640.
- Walton JD. HC-toxin. Phytochemistry. 2006 Jul;67(14):1406-13. doi: 10.1016/j.phytochem.2006.05.033. Epub 2006 Jul 12. PMID: 16839576.
- Brosch G, Dangl M, Graessle S, Loidl A, Trojer P, Brandtner EM, Mair K, Walton JD, Baidyaroy D, Loidl P. An inhibitor-resistant histone deacetylase in the plant pathogenic fungus Cochliobolus carbonum. Biochemistry. 2001 Oct 30;40(43):12855-63. doi: 10.1021/bi010508u. PMID: 11669622.
- Kritskiĭ MS, Filippovich SIu, Afanas'eva TP, Bachurina GP, Russo VE. Vliianie ingibitorov fermentativnogo metilirovaniia DNK na obrazovanie reproduktivnykh struktur i karotinogenez u Neurospora crassa [Effect of inhibitors of enzymatic DNA methylation on the formation of reproductive structures and carotenoid production in Neurospora crassa]. Prikl Biokhim Mikrobiol. 2001 May-Jun;37(3):279-84. Russian. PMID: 11443894.
- Shwab EK, Bok JW, Tribus M, Galehr J, Graessle S, Keller NP. Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot Cell. 2007 Sep;6(9):1656-64. doi: 10.1128/EC.00186-07. Epub 2007 Jul 6. PMID: 17616629; PMCID: PMC2043372.
- Li G, Kusari S, Golz C, Laatsch H, Strohmann C, Spiteller M. Epigenetic Modulation of Endophytic Eupenicillium sp. LG41 by a Histone Deacetylase Inhibitor for Production of Decalin-Containing Compounds. J Nat Prod. 2017 Apr 28;80(4):983-988. doi: 10.1021/acs.jnatprod.6b00997. Epub 2017 Mar 23. PMID: 28333449.
- Bai J, Mu R, Dou M, Yan D, Liu B, Wei Q, Wan J, Tang Y, Hu Y. Epigenetic modification in histone deacetylase deletion strain of Calcarisporium arbuscula leads to diverse diterpenoids. Acta Pharm Sin B. 2018 Jul;8(4):687-697. doi: 10.1016/j.apsb.2017.12.012. Epub 2018 Feb 21. PMID: 30109192; PMCID: PMC6090014.
- Sun K, Zhu G, Hao J, Wang Y, Zhu W. Chemical-epigenetic method to enhance the chemodiversity of the marine algicolous fungus, Aspergillus terreus OUCMDZ-2739. Tetrahedron. 2017;74:83-87. doi: 10.1016/j.tet.2017.11.039.
- Cihák A. Biological effects of 5-azacytidine in eukaryotes. Oncology. 1974;30(5):405-22. doi: 10.1159/000224981. PMID: 4142650.
- "Zolinza (vorinostat) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved. 2014. https://tinyurl.com/2p9f9pdr
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65-82. doi: 10.1007/BF00222695. PMID: 6174854.
- Candido EP, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105-13. doi: 10.1016/0092-8674(78)90305-7. PMID: 667927.
- Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003 Jul;133(7 Suppl):2485S-2493S. doi: 10.1093/jn/133.7.2485S. PMID: 12840228.
- Magotra A, Kumar M, Kushwaha M, Awasthi P, Raina C, Gupta AP, Shah BA, Gandhi SG, Chaubey A. Epigenetic modifier induced enhancement of fumiquinazoline C production in Aspergillus fumigatus (GA-L7): an endophytic fungus from Grewia asiatica L. AMB Express. 2017 Dec;7(1):43. doi: 10.1186/s13568-017-0343-z. Epub 2017 Feb 17. PMID: 28213885; PMCID: PMC5315648.
- Silva MG, Furtado NA, Pupo MT, Fonseca MJ, Said S, da Silva Filho AA, Bastos JK. Antibacterial activity from Penicillium corylophilum Dierckx. Microbiol Res. 2004;159(4):317-22. doi: 10.1016/j.micres.2004.06.003. PMID: 15646377.
- Belofsky GN, Anguera M, Jensen PR, Fenical W, Köck M. Oxepinamides A-C and fumiquinazolines H--I: bioactive metabolites from a marine isolate of a fungus of the genus Acremonium. Chemistry. 2000 Apr 14;6(8):1355-60. doi: 10.1002/(sici)1521-3765(20000417)6:8<1355::aid-chem1355>3.0.co;2-s. PMID: 10840958.
- Han XX, Xu XY, Cui CB, Gu QQ. Alkaloidal compounds produced by a marinederived fungus. Aspergillus fumigatus H1-04, and their antitumor activities. Chin J Med Chem. 2007;17:232-237. https://tinyurl.com/4w2emyvs
- Chen HJ, Awakawa T, Sun J, Wakimoto T, Abe I. Epigenetic modifier-induced biosynthesis of novel fusaric acid derivatives in endophytic fungi from Datura stramonium L. Nat Prod Bioprospect. 2013;3:20-23. doi: 10.1007/s13659-013-0010-2.
- Asai T, Morita S, Taniguchi T, Monde K, Oshima Y. Epigenetic stimulation of polyketide production in Chaetomium cancroideum by an NAD(+)-dependent HDAC inhibitor. Org Biomol Chem. 2016 Jan 14;14(2):646-651. doi: 10.1039/c5ob01595b. PMID: 26549741.
- Vervoort HC, Drašković M, Crews P. Histone deacetylase inhibitors as a tool to up-regulate new fungal biosynthetic products: isolation of EGM-556, a cyclodepsipeptide, from Microascus sp. Org Lett. 2011 Feb 4;13(3):410-3. doi: 10.1021/ol1027199. Epub 2010 Dec 21. PMID: 21174394; PMCID: PMC3031758.
- Oku N, Kawabata K, Adachi K, Katsuta A, Shizuri Y. Unnarmicins A and C, new antibacterial depsipeptides produced by marine bacterium Photobacterium sp. MBIC06485. J Antibiot (Tokyo). 2008 Jan;61(1):11-7. doi: 10.1038/ja.2008.103. PMID: 18305354.
- Sasakawa Y, Naoe Y, Inoue T, Sasakawa T, Matsuo M, Manda T, Mutoh S. Effects of FK228, a novel histone deacetylase inhibitor, on human lymphoma U-937 cells in vitro and in vivo. Biochem Pharmacol. 2002 Oct 1;64(7):1079-90. doi: 10.1016/s0006-2952(02)01261-3. PMID: 12234611.
- Albright JC, Henke MT, Soukup AA, McClure RA, Thomson RJ, Keller NP, Kelleher NL. Large-scale metabolomics reveals a complex response of Aspergillus nidulans to epigenetic perturbation. ACS Chem Biol. 2015 Jun 19;10(6):1535-41. doi: 10.1021/acschembio.5b00025. Epub 2015 Apr 3. PMID: 25815712; PMCID: PMC4475433.
- Gubiani JR, Wijeratne EM, Shi T, Araujo AR, Arnold AE, Chapman E, Gunatilaka AA. An epigenetic modifier induces production of (10'S)-verruculide B, an inhibitor of protein tyrosine phosphatases by Phoma sp. nov. LG0217, a fungal endophyte of Parkinsonia microphylla. Bioorg Med Chem. 2017 Mar 15;25(6):1860-1866. doi: 10.1016/j.bmc.2017.01.048. Epub 2017 Feb 3. PMID: 28202316; PMCID: PMC5362119.
- Sun J, Awakawa T, Noguchi H, Abe I. Induced production of mycotoxins in an endophytic fungus from the medicinal plant Datura stramonium L. Bioorg Med Chem Lett. 2012 Oct 15;22(20):6397-400. doi: 10.1016/j.bmcl.2012.08.063. Epub 2012 Aug 24. PMID: 22967766.
- Vasanthakumari MM, Jadhav SS, Sachin N, Vinod G, Shweta S, Manjunatha BL, Kumara PM, Ravikanth G, Nataraja KN, Uma Shaanker R. Restoration of camptothecine production in attenuated endophytic fungus on re-inoculation into host plant and treatment with DNA methyltransferase inhibitor. World J Microbiol Biotechnol. 2015 Oct;31(10):1629-39. doi: 10.1007/s11274-015-1916-0. Epub 2015 Aug 20. PMID: 26289161.
- Yang XL, Huang L, Ruan XL. Epigenetic modifiers alter the secondary metabolite composition of a plant endophytic fungus, Pestalotiopsis crassiuscula obtained from the leaves of Fragaria chiloensis. J Asian Nat Prod Res. 2014;16(4):412-7. doi: 10.1080/10286020.2014.881356. Epub 2014 Feb 5. PMID: 24498889.
- Asai T, Chung YM, Sakurai H, Ozeki T, Chang FR, Yamashita K, Oshima Y. Tenuipyrone, a novel skeletal polyketide from the entomopathogenic fungus, Isaria tenuipes, cultivated in the presence of epigenetic modifiers. Org Lett. 2012 Jan 20;14(2):513-5. doi: 10.1021/ol203097b. Epub 2011 Dec 27. PMID: 22201477.
- Asai, T, Yamamoto T, Chung YM, Chang FR, Wu YC, Yamashita K, Oshima Y. As Aromatic polyketide glycosides from an entomopathogenic fungus, Cordyceps indigotica. Tetrahedron Lett. 2012;53:277-280. doi: 10.1016/j.tetlet.2011.10.013.
- Asai T, Luo D, Obara Y, Taniguchi T, Monde K, Yamashita K, Oshima Y. Dihydrobenzofurans as cannabinoid receptor ligands from Cordyceps annullata, an entomopathogenic fungus cultivated in the presence of an HDAC inhibitor. Tetrahedron Lett. 2012;53:2239-2243. doi: 10.1016/j.tetlet.2012.02.088.
- Asai T, Morita S, Shirata N, Taniguchi T, Monde K, Sakurai H, Ozeki T, Oshima Y. Structural diversity of new C13-polyketides produced by Chaetomium mollipilium cultivated in the presence of a NAD(+)-dependent histone deacetylase inhibitor. Org Lett. 2012 Nov 2;14(21):5456-9. doi: 10.1021/ol302539s. Epub 2012 Oct 19. PMID: 23083076.
- Asai T, Chung YM, Sakurai H, Ozeki T, Chang FR, Wu YC, Yamashita K, Oshima Y. Highly oxidized ergosterols and isariotin analogs from an entomopathogenic fungus, Gibellula formosana, cultivated in the presence of epigenetic modifyin gagents. Tetrahedron. 2012;68:5817-5823. doi: 10.1016/j.tet.2012.05.020.
- Asai T, Otsuki S, Sakurai H, Yamashita K, Ozeki T, Oshima Y. Benzophenones from an endophytic fungus, Graphiopsis chlorocephala, from Paeonia lactiflora cultivated in the presence of an NAD+-dependent HDAC inhibitor. Org Lett. 2013 Apr 19;15(8):2058-61. doi: 10.1021/ol400781b. Epub 2013 Apr 11. PMID: 23578108.
- Chung YM, El-Shazly M, Chuang DW, Hwang TL, Asai T, Oshima Y, Ashour ML, Wu YC, Chang FR. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, induces the production of anti-inflammatory cyclodepsipeptides from Beauveria felina. J Nat Prod. 2013 Jul 26;76(7):1260-6. doi: 10.1021/np400143j. Epub 2013 Jul 3. PMID: 23822585.
- Chen JJ, Han MY, Gong T, Qiao YM, Yang JL, Zhu P. Epigenetic modification enhances ergot alkaloid production of Claviceps purpurea. Biotechnol Lett. 2019 Dec;41(12):1439-1449. doi: 10.1007/s10529-019-02750-x. Epub 2019 Oct 28. PMID: 31659576.
- Akone SH, Mandi A, Kurtan T, Hartmann R, Lin W, Daletos G, Proksch P. Inducing secondary metabolite production by the endophytic fungus Chaetomium sp. through fungal bacterial co-culture and epigenetic modification. Tetrahedron. 2016;72:6340-6347. doi: 10.1016/j.tet.2016.08.022.
- Fisch KM, Gillaspy AF, Gipson M, Henrikson JC, Hoover AR, Jackson L, Najar FZ, Wägele H, Cichewicz RH. Chemical induction of silent biosynthetic pathway transcription in Aspergillus niger. J Ind Microbiol Biotechnol. 2009 Sep;36(9):1199-213. doi: 10.1007/s10295-009-0601-4. Epub 2009 Jun 12. PMID: 19521728.
- Beau J, Mahid N, Burda WN, Harrington L, Shaw LN, Mutka T, Kyle DE, Barisic B, van Olphen A, Baker BJ. Epigenetic tailoring for the production of anti-infective cytosporones from the marine fungus Leucostoma persoonii. Mar Drugs. 2012 Apr;10(4):762-74. doi: 10.3390/md10040762. Epub 2012 Mar 28. PMID: 22690142; PMCID: PMC3366674.
- Xiao L, Yin Y, Sun W, Zhang F, Li Z. Enhanced production of (+)-terrein by Aspergillus terreus strain PF26 with epigenetic modifier suberoylanilide hydroxamic acid. Process biochem. 2013;48:1635-1639. doi: 10.1016/j.procbio.2013.08.007.
- Park SH, Kim DS, Kim WG, Ryoo IJ, Lee DH, Huh CH, Youn SW, Yoo ID, Park KC. Terrein: a new melanogenesis inhibitor and its mechanism. Cell Mol Life Sci. 2004 Nov;61(22):2878-85. doi: 10.1007/s00018-004-4341-3. PMID: 15558216.
- Park SH, Kim DS, Lee HK, Kwon SB, Lee S, Ryoo IJ, Kim WG, Yoo ID, Park KC. Long-term suppression of tyrosinase by terrein via tyrosinase degradation and its decreased expression. Exp Dermatol. 2009 Jun;18(6):562-6. doi: 10.1111/j.1600-0625.2009.00847.x. PMID: 19493001.
- Lee JC, Yu MK, Lee R, Lee YH, Jeon JG, Lee MH, Jhee EC, Yoo ID, Yi HK. Terrein reduces pulpal inflammation in human dental pulp cells. J Endod. 2008 Apr;34(4):433-7. doi: 10.1016/j.joen.2008.01.015. PMID: 18358890.
- Lee YH, Lee NH, Bhattarai G, Oh YT, Yu MK, Yoo ID, Jhee EC, Yi HK. Enhancement of osteoblast biocompatibility on titanium surface with Terrein treatment. Cell Biochem Funct. 2010 Dec 2;28(8):678-85. doi: 10.1002/cbf.1708. Epub 2010 Oct 29. PMID: 21104936.
- Malmstrøm J, Christophersen C, Barrero AF, Oltra JE, Justicia J, Rosales A. Bioactive metabolites from a marine-derived strain of the fungus Emericella variecolor. J Nat Prod. 2002 Mar;65(3):364-7. doi: 10.1021/np0103214. PMID: 11908979.
- Ghisalberti E, Narbey M, Rowland C. Metabolites of Aspergillus terreus antagonistic towards the take-all fungus. J Nat Prod. 1990;53:520-522. doi: 10.1021/np50068a043.
- Phattanawasin P, Pojchanakom K, Sotanaphun U, Piyapolrungroj N, Zungsontiporn S. Weed growth inhibitors from Aspergillus fischeri TISTR 3272. Nat Prod Res. 2007 Dec;21(14):1286-91. doi: 10.1080/14786410701766364. Erratum in: Nat Prod Res. 2008 Feb 15;22(3):285. PMID: 18075891.
- Arakawa M, Someno T, Kawada M, Ikeda D. A new terrein glucoside, a novel inhibitor of angiogenin secretion in tumor angiogenesis. J Antibiot (Tokyo). 2008 Jul;61(7):442-8. doi: 10.1038/ja.2008.60. PMID: 18776656.
- Liao WY, Shen CN, Lin LH, Yang YL, Han HY, Chen JW, Kuo SC, Wu SH, Liaw CC. Asperjinone, a nor-neolignan, and terrein, a suppressor of ABCG2-expressing breast cancer cells, from thermophilic Aspergillus terreus. J Nat Prod. 2012 Apr 27;75(4):630-5. doi: 10.1021/np200866z. Epub 2012 Feb 23. PMID: 22360613.
- Brosch G, Loidl P, Graessle S. Histone modifications and chromatin dynamics: a focus on filamentous fungi. FEMS Microbiol Rev. 2008 May;32(3):409-39. doi: 10.1111/j.1574-6976.2007.00100.x. Epub 2008 Jan 23. PMID: 18221488; PMCID: PMC2442719.
- Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005 Oct 1;11(19 Pt 1):7033-41. doi: 10.1158/1078-0432.CCR-05-0406. PMID: 16203797.
- Majid S, Kikuno N, Nelles J, Noonan E, Tanaka Y, Kawamoto K, Hirata H, Li LC, Zhao H, Okino ST, Place RF, Pookot D, Dahiya R. Genistein induces the p21WAF1/CIP1 and p16INK4a tumor suppressor genes in prostate cancer cells by epigenetic mechanisms involving active chromatin modification. Cancer Res. 2008 Apr 15;68(8):2736-44. doi: 10.1158/0008-5472.CAN-07-2290. PMID: 18413741.
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001 Oct;74(4):418-25. doi: 10.1093/ajcn/74.4.418. PMID: 11566638.
- Priyadarsini RV, Vinothini G, Murugan RS, Manikandan P, Nagini S. The flavonoid quercetin modulates the hallmark capabilities of hamster buccal pouch tumors. Nutr Cancer. 2011;63(2):218-26. doi: 10.1080/01635581.2011.523503. PMID: 21294050.
- Seelinger G, Merfort I, Wölfle U, Schempp CM. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008 Oct 22;13(10):2628-51. doi: 10.3390/molecules13102628. PMID: 18946424; PMCID: PMC6245397.
- Krifa M, Leloup L, Ghedira K, Mousli M, Chekir-Ghedira L. Luteolin induces apoptosis in BE colorectal cancer cells by downregulating calpain, UHRF1, and DNMT1 expressions. Nutr Cancer. 2014;66(7):1220-7. doi: 10.1080/01635581.2014.951729. Epub 2014 Sep 10. PMID: 25207720.
- Chi P, Allis CD, Wang GG. Covalent histone modifications--miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010 Jul;10(7):457-69. doi: 10.1038/nrc2876. PMID: 20574448; PMCID: PMC3262678.
- Kauntz H, Bousserouel S, Gossé F, Raul F. Epigenetic effects of the natural flavonolignan silibinin on colon adenocarcinoma cells and their derived metastatic cells. Oncol Lett. 2013 Apr;5(4):1273-1277. doi: 10.3892/ol.2013.1190. Epub 2013 Feb12.PMID:23599778;PMCID:PMC3629096.
- Ko HH, Yen MH, Wu RR, Won SJ, Lin CN. Cytotoxic isoprenylated flavans of Broussonetia kazinoki. J Nat Prod. 1999 Jan;62(1):164-6. doi: 10.1021/np980281c.PMID:9917310.
- Nass SJ, Herman JG, Gabrielson E, Iversen PW, Parl FF, Davidson NE, Graff JR. Aberrant methylation of the estrogen receptor and E-cadherin 5' CpG islands increases with malignant progression in human breast cancer. Cancer Res. 2000 Aug 15;60(16):4346-8. PMID: 10969774.
- Tanaka H, Marumo H, Nagai T, Okada M, Taniguchi K. Nanaomycins, new antibiotics produced by a strain of Streptomyces. III. A new component, nanaomycin C, and biological activities of nanaomycin derivatives. J Antibiot (Tokyo). 1975 Dec;28(12):925-30. doi: 10.7164/antibiotics.28.925. PMID: 1206004.
- Kuck D, Singh N, Lyko F, Medina-Franco JL. Novel and selective DNA methyltransferase inhibitors: Docking-based virtual screening and experimental evaluation. Bioorg Med Chem. 2010 Jan 15;18(2):822-9. doi: 10.1016/j.bmc.2009.11.050. Epub 2009 Nov 27. PMID: 20006515.
- Burwood R, Read G, Schofield K, Wright DE. 1133. The pigments of stick lac. Part I. Isolation and preliminary examination. J Chem Soc. 1965;6067-6073. doi: 10.1039/JR9650006067.
- Yoder JA, Soman NS, Verdine GL, Bestor TH. DNA (cytosine-5)-methyltransferases in mouse cells and tissues. Studies with a mechanism-based probe. J Mol Biol. 1997 Jul 18;270(3):385-95. doi: 10.1006/jmbi.1997.1125. PMID: 9237905.
- Blank M, Mandel M, Keisari Y, Meruelo D, Lavie G. Enhanced ubiquitinylation of heat shock protein 90 as a potential mechanism for mitotic cell death in cancer cells induced with hypericin. Cancer Res. 2003 Dec 1;63(23):8241-7. PMID: 14678981.
- Dror N, Mandel M, Lavie G. Unique anti-glioblastoma activities of hypericin are at the crossroad of biochemical and epigenetic events and culminate in tumor cell differentiation. PLoS One. 2013 Sep 16;8(9):e73625. doi:10.1371/journal.pone.0073625. PMID: 24066060; PMCID: PMC3774735.
- Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003 Nov 15;63(22):7563-70. PMID: 14633667.
- Liu Z, Xie Z, Jones W, Pavlovicz RE, Liu S, Yu J, Li PK, Lin J, Fuchs JR, Marcucci G, Li C, Chan KK. Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett. 2009 Feb 1;19(3):706-9. doi:10.1016/j.bmcl.2008.12.041. Epub 2008 Dec 14. PMID: 19112019.
- Lee WJ, Zhu BT. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis. 2006 Feb;27(2):269-77. doi: 10.1093/carcin/bgi206. Epub 2005 Aug 4. PMID: 16081510.
- Bhattacharya K, Samanta SK, Tripathi R, Mallick A, Chandra S, Pal BC, Shaha C, Mandal C. Apoptotic effects of mahanine on human leukemic cells are mediated through crosstalk between Apo-1/Fas signaling and the Bid protein and via mitochondrial pathways. Biochem Pharmacol. 2010 Feb 1;79(3):361-72. doi: 10.1016/j.bcp.2009.09.007. Epub 2009 Sep 12. PMID: 19751707.
- Shah BA, Qazi GN, Taneja SC. Boswellic acids: a group of medicinally important compounds. Nat Prod Rep. 2009 Jan;26(1):72-89. doi: 10.1039/b809437n. PMID: 19374123.
- Sun W, Iijima T, Kano J, Kobayashi H, Li D, Morishita Y, Okubo C, Anami Y, Noguchi M. Frequent aberrant methylation of the promoter region of sterile alpha motif domain 14 in pulmonary adenocarcinoma. Cancer Sci. 2008 Nov;99(11):2177-84. doi: 10.1111/j.1349-7006.2008.00965.x. Epub 2008 Sep 22. PMID: 18823374.
- Davis CD, Uthus EO. Dietary selenite and azadeoxycytidine treatments affect dimethylhydrazine-induced aberrant crypt formation in rat colon and DNA methylation in HT-29 cells. J Nutr. 2002 Feb;132(2):292-7. doi: 10.1093/jn/132.2.292. PMID: 11823593.
- Uthus EO, Ross SA. Dietary selenium affects homocysteine metabolism differently in Fisher-344 rats and CD-1 mice. J Nutr. 2007 May;137(5):1132-6. doi: 10.1093/jn/137.5.1132. PMID: 17449570.
- Fu LJ, Ding YB, Wu LX, Wen CJ, Qu Q, Zhang X, Zhou HH. The Effects of Lycopene on the Methylation of the GSTP1 Promoter and Global Methylation in Prostatic Cancer Cell Lines PC3 and LNCaP. Int J Endocrinol. 2014;2014:620165. doi: 10.1155/2014/620165. Epub 2014 Oct 20. PMID: 25389438; PMCID: PMC4217342.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.