Medicine Group . 2022 April 08;3(4):323-326. doi: 10.37871/jbres1442.
A Conceptual Model of Nickel Content in the Bodies of Seafood Mollusks and their Gastrointestinal Absorption
Chee Kong Yap1*, Wen Siang Tan2,3, Wan Mohd Syazwan1, Noor Azrizal-Wahid1, Rosimah Nulit1, Muskhazli Mustafa1, Mohd Amiruddin Abdul Rahman4, Chee Wah Yap5, Franklin Berandah Edward6, Takaomi Arai7 and Wan Hee Cheng8
2Department of Microbiology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
3Laboratory of Vaccines and Biomolecules, Institute of Bioscience, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
4Department of Physics, Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
5MES SOLUTIONS, 22C-1, Jalan BK 5A/2A, Bandar Kinrara, 47100 Puchong, Selangor, Malaysia
6Natural Resources and Environment Board, Petra Jaya, 93050 Kuching, Sarawak, Malaysia
7Environmental and Life Sciences Programme, Faculty of Science, Universiti Brunei Darussalam, Jalan Tungku Link, Gadong, BE 1410, Brunei Darussalam
8Faculty of Health and Life Sciences, INTI International University, Persiaran Perdana BBN, Nilai 71800, Negeri Sembilan, Malaysia
- Seafood mollusks
- Human health risks
- Nickel potentially
Abstract
There are many biomonitoring studies of nickel in the mollusks, but to understand how the nickel is accumulated in the human body and the health risk posed by the metal is not a straightforward formula. Therefore, the aim of this paper is to draw a conceptual model of nickel content in the bodies of invertebrate mollusks and their gastrointestinal absorption of nickel. This model is useful to understand how nickel accumulation in the tissues of the mollusks could affect the nickel oral ingestion pathways. This conceptual model can shed some light on the mechanisms of nickel that may cause various toxicological risks and target cancer slope formulation in the future.
Introduction
The aim of this paper is to draw a conceptual model of nickel content in the bodies of invertebrate mollusks and their gastrointestinal absorption. This is due to the fact that consumption of seafood mollusks may be a significant pathway of seafood contaminants such as heavy metals [1]. In particular, mollusks such as cockles are filter feeders, making them a significant potential source of human exposure to the Potential Toxic Metals (PTM) [2]. Consequently, human health assessment of shellfish consumption is a fundamental component of seafood safety risk assessments. Such assessments involve accurately the estimation of shellfish consumption, and the elevated concentrations of the PTM of interest [3], which may contradict the multiple health benefits provided by fish and shellfish consumptions [4-9]. Nickel is found in many aquatic foods [10], and it binds to proteins and nucleic acids in fish and shellfish, and may cause toxicity by interfering with the Fe metabolism [6,11]. Even though there are many biomonitoring studies of nickel in the molluscs [4-9] but it is hard to understand how the nickel is accumulated in the human body. Consequently, the mechanisms of how the elevated nickel accumulated in the human body could pose a human health risk is still an enigma.
Despite the fact that there is no evidence that nickel has a physiological or nutritional advantage in humans [12], it has been identified as a crucial and major ingredient in a variety of microorganisms, plants, and animals [13-16]. Stainless steel, alloys, rubber and plastic industries, Ni-cadmium battery sectors, and electroplating industries all use nickel and nickel compounds [3,9-11]. However, due to the extensive spread of objects containing this metal, the production and use of nickel and its derivatives can expose persons and the environments to a variety of dangers, with its secondary products damaging the environments at all stages of manufacture, recycling, and disposal [10,11].
Human exposure to highly Ni-polluted environments can cause a range of pathological and toxicological effects, which is our primary public worry since it has been related to health issues in those who deal with the metal. Nickel and nickel compounds have been associated to a variety of unfavorable health consequences in humans, including lung fibrosis, renal illness, cardiovascular disease, and respiratory tract cancer [12,13]. Group 1 includes both soluble and insoluble nickel compounds (carcinogenic to people). Nickel and alloys, on the other hand, are classified by the International Agency for Research on Cancer (IARC) as Group 2B (possibly carcinogenic to humans) [14].
Hertel, et al. [15] provided a comprehensive list of nickel environmental health standards. The indirect genotoxic and epigenetic mechanisms are the threshold paradigm for nickel carcinogenicity [16]. Das, et al. [17] examined Ni-induced genotoxicity, carcinogenicity, immunotoxicity, and toxicity in metabolically active tissues. A study of the chemical characteristics of nickel in people, and the causes of nickel toxicity were reported by Genchi, et al. [18]. All of the abovementioned facts clearly demonstrated the importance and significance of nickel in terms of environmental and human health.
Conceptual model of nickel content in the bodies of invertebrate mollusks and their gastrointestinal absorption
In the risk evaluation of metals, estimating Gastrointestinal Absorption (GA) is a difficult task. Given the uncertainty of calculating metal absorption in humans, Diamond, et al. [19] examined GA of lead by presenting the physiological mechanisms involved in metal absorption into health risk assessment. In the framework of this model, the GA of lead is discussed [19]. Metal (such as lead) is primarily ingested and absorbed through the gastrointestinal tract in the population [20].
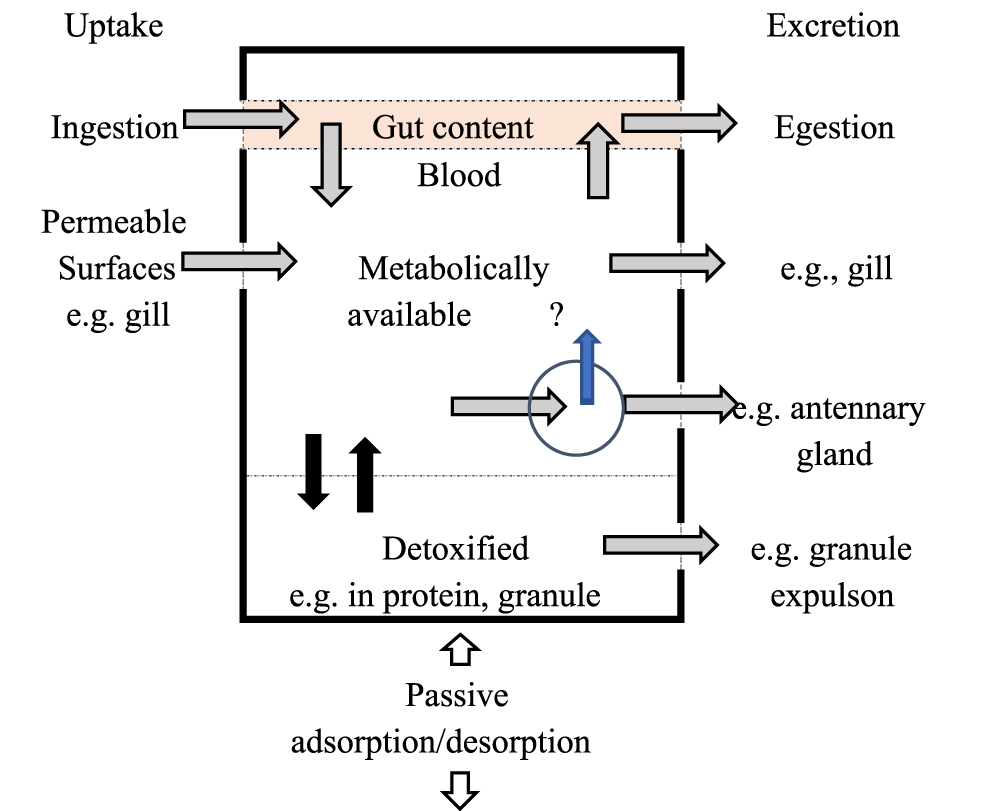
The accumulation of nickel by invertebrate mollusks is depicted schematically in figure 1, which is adapted from the model of a decapod crustacean [21,22]. Nickel adsorbed on the surface of a mollusk's mantle, gill, gonad, foot, and shell (and potentially desorbed when dissolved circumstances alter) would add to the mollusk's total body concentration of nickel. After uptake from solution through permeable ectodermal surfaces or across the endoderm of the gut, nickel will be metabolically available that is, it will have the ability to bind to molecules in the receiving cell or elsewhere in the body after internal transit via the hemolymph (Figure 1).
In the case of an essential nickel, it is available to bind to sites where it can play an important role (e.g., zinc in the enzyme carbonic anhydrase or copper in hemocyanin) or, if present in excess (due to excessive entry), to sites where it can cause toxic effects (e.g., zinc in the enzyme carbonic anhydrase or copper in hemocyanin). An excess of necessary nickel must be detoxified, tied tightly to a binding site from which escape is limited, most likely in a storage organ beyond the absorption site. The nickel has now entered the detoxified storage (Figure 1), which can be transient or permanent. Depending on the nickel accumulation pattern of a given mollusk species (cockles, gastropods, and mussels), nickel taken up into the body may or may not be expelled, either from the metabolically accessible component or from a detoxified store (Figure 1) [22,23].
Following the nickel accumulated in the detoxified forms and metabolically available nickel in the body of mollusks (seafood), the seafood to be consumed will follow the schematic pathway to the GA of nickel. It starts with seafood consumption in the human mouth with mechanical breakdown into smaller pieces ending at the nickel’s GA (such as duodenum).
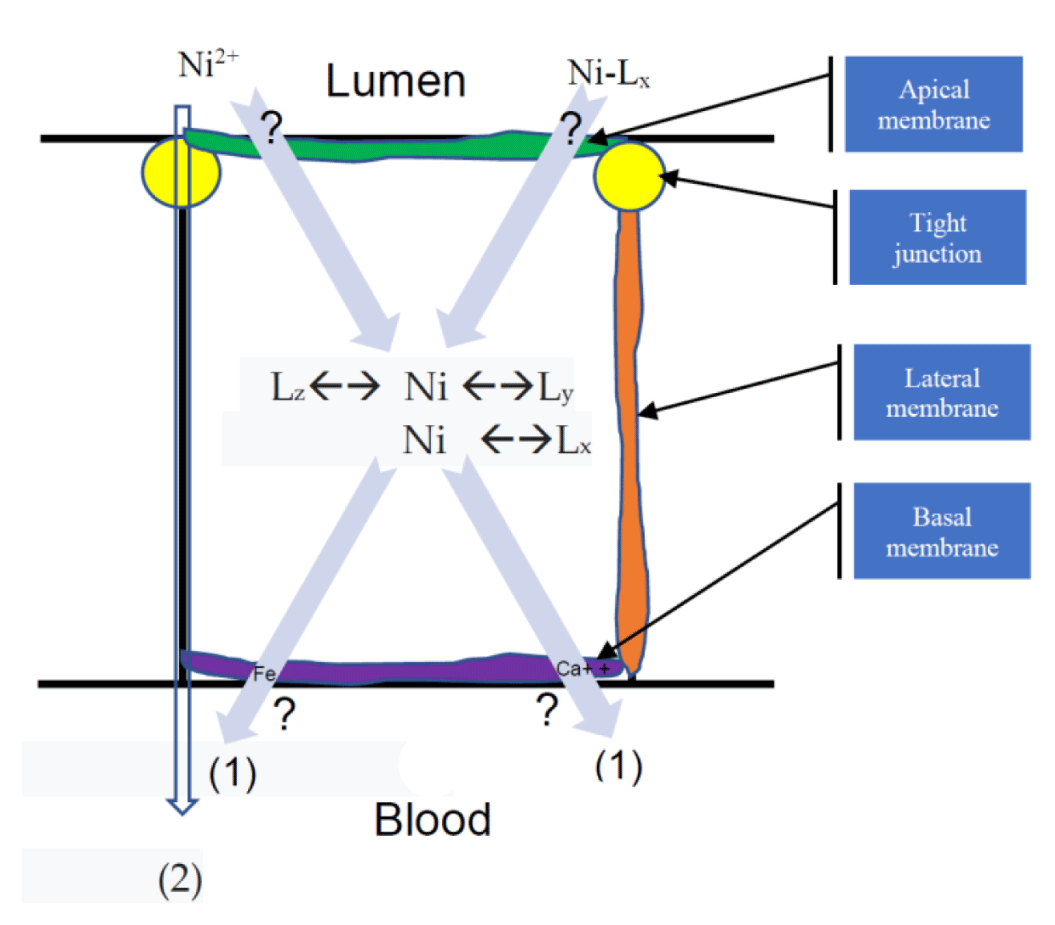
Metal (such as nickel) absorption in the gastrointestinal tract is assumed to occur predominantly in the duodenum [19]. Nickel absorption has a specific mechanism that is well understood. However, a theoretical model of nickel's GA can be considered. The existence of saturable and non-saturable pathways in the duodenum was demonstrated (based on the Pb absorption model [19]), with the saturable pathway involving some mechanisms associated with serosal transfer presumable transport mechanisms within the cell or the basal and lateral membranes. Nickel ion (Ni2+) complexes with one or more ligands may transport nickel out of the lumen and through the cell's basolateral membrane (Figure 2).
Nickel and its complexes can enter the cell by membrane carriers or channels at the apical membrane. Apart from diffusion through cation channels, Ni2+ can be transferred as a protein complex via pinocytosis or a compound with amino acids in this epithelial cell. However, there has been no direct evidence of this nickel complex transport. Nickel interacts with several intracellular ligands such as calcium-binding proteins once within the cell (Figure 2). Experiments on nickel GA should be conducted in the future. The mechanisms governing nickel absorption in the gastrointestinal tract would assist in making more accurate assessments of nickel's health concerns in humans.
Conclusion
This model could assist in understanding how the accumulation of nickel in the tissues of mollusks could affect the nickel oral ingestion pathways. Finally, this conceptual model can shed some light on the mechanisms of nickel that may cause various toxicological risks.
References
- Guy S, Beaven S, Gaw S, Pearson AJ. Shellfish Consumption and Recreational Gathering Practices in Northland, New Zealand. Reg Stud Mar Sci. 2021;47(1). doi: 10.1016/j.rsma.2021.101967.
- Venugopal V, Gopakumar K. Shellfish: Nutritive Value, Health Benefits, and Consumer Safety. Compr Rev Food Sci Food Saf. 2017 Nov;16(6):1219-1242. doi: 10.1111/1541-4337.12312. Epub 2017 Oct 25. PMID: 33371588.s
- Guidelines for Human Exposure Assessment Risk Assessment Forum. 223.
- Bosch AC, O'Neill B, Sigge GO, Kerwath SE, Hoffman LC. Heavy metals in marine fish meat and consumer health: a review. J Sci Food Agric. 2016 Jan 15;96(1):32-48. doi: 10.1002/jsfa.7360. Epub 2015 Sep 7. PMID: 26238481.
- Jahangir Sarker M, Naher Rima N, Sultana N. Human Health Risk Assessment with Reference to the Consumption of Shrimp and Marine Fish. Pak J Biol Sci. 2020 Jan;23(10):1291-1302. doi: 10.3923/pjbs.2020.1291.1302. PMID: 32981263.
- Yap CK, Al-Mutairi KA. Comparative Study of Potentially Toxic Nickel and Their Potential Human Health Risks in Seafood (Fish and Mollusks) from Peninsular Malaysia. Biology (Basel). 2022 Feb 27;11(3):376. doi: 10.3390/biology11030376. PMID: 35336750; PMCID: PMC8945417.
- Agah H. Ecological risk assessment of heavy metals in sediment, fish, and human hair from Chabahar Bay, Makoran, Iran. Mar Pollut Bull. 2021 Aug;169:112345. doi: 10.1016/j.marpolbul.2021.112345. Epub 2021 Jul 5. PMID: 34238565.
- Han JL, Pan XD, Chen Q, Huang BF. Health risk assessment of heavy metals in marine fish to the population in Zhejiang, China. Sci Rep. 2021 May 26;11(1):11079. doi: 10.1038/s41598-021-90665-x. PMID: 34040137; PMCID: PMC8155212.
- Younis EM, Abdel-Warith AA, Al-Asgah NA, Elthebite SA, Mostafizur Rahman M. Nutritional value and bioaccumulation of heavy metals in muscle tissues of five commercially important marine fish species from the Red Sea. Saudi J Biol Sci. 2021 Mar;28(3):1860-1866. doi: 10.1016/j.sjbs.2020.12.038. Epub 2020 Dec 31. PMID: 33732073; PMCID: PMC7938124.
- Rani S, Ahmed MK, Xiongzhi X, Keliang C, Islam MS, Habibullah-Al-Mamun M. Occurrence, spatial distribution and ecological risk assessment of trace elements in surface sediments of rivers and coastal areas of the East Coast of Bangladesh, North-East Bay of Bengal. Sci Total Environ. 2021 Dec 20;801:149782. doi: 10.1016/j.scitotenv.2021.149782. Epub 2021 Aug 20. PMID: 34467902.
- Strokes P. Nickel in Aquatic System. In Metal Ions in Biological Systems: Nickel and its Role in Biology. Sigel, H, Sigel A, Eds. New York and Basel: Routledge & CRC Press; 1988. p. 31-46.
- McGregor DB, Baan RA, Partensky C, Rice JM, Wilbourn JD. Evaluation of the carcinogenic risks to humans associated with surgical implants and other foreign bodies - a report of an IARC Monographs Programme Meeting. International Agency for Research on Cancer. Eur J Cancer. 2000 Feb;36(3):307-13. doi: 10.1016/s0959-8049(99)00312-3. PMID: 10708931.
- Seilkop SK, Oller AR. Respiratory cancer risks associated with low-level nickel exposure: an integrated assessment based on animal, epidemiological, and mechanistic data. Regul Toxicol Pharmacol. 2003 Apr;37(2):173-90. doi: 10.1016/s0273-2300(02)00029-6. Erratum in: Regul Toxicol Pharmacol. 2005 Feb;41(1):92-3. PMID: 12726752.
- IARC Nickel and Nickel Compounds. The International Agency for Research on Cancer. 2012.
- Nickel, Hertel RF, Maass T. International Programme on Chemical Safety, Eds. Environmental health criteria; World Health Organization: Geneva; 1991. ISBN 978-92-4-157108-1.
- Buxton S, Garman E, Heim KE, Lyons-Darden T, Schlekat CE, Taylor MD, Oller AR. Concise review of nickel human health toxicology and ecotoxicology. Inorganics. 2019;7(7). doi: 10.3390/inorganics7070089.
- Das KK, Reddy RC, Bagoji IB, Das S, Bagali S, Mullur L, Khodnapur JP, Biradar MS. Primary concept of nickel toxicity - an overview. J Basic Clin Physiol Pharmacol. 2018 Sep 4;30(2):141-152. doi: 10.1515/jbcpp-2017-0171. PMID: 30179849.
- Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A. Nickel: Human Health and Environmental Toxicology. Int J Environ Res Public Health. 2020;17(3). doi: 10.3390/ijerph17030679.
- Diamond GL, Goodrum PE, Felter SP, Ruoff WL. Gastrointestinal absorption of metals. Drug Chem Toxicol. 1997 Nov;20(4):345-68. doi: 10.3109/01480549709003892. Corrected and republished in: Drug Chem Toxicol. 1998 May;21(2):223-51. PMID: 9433663.
- Mushak P. Gastro-intestinal absorption of lead in children and adults: Overview of biological and biophysico-chemical aspects. Chem Speciat Bioavailab.1991;3(1):87-104. doi: 10.1080/09542299.1991.11083160.
- Edward T, Rainbow PS. Biology of Trace Metals. In Trace Metals in the Environment and Living Organisms: The British Isles as a Case Study. Ed Cambridge University Press Cambridge; 2018. p. 68-123. doi: 10.1111/ejss.12809.
- Rainbow PS. Trace metal bioaccumulation: models, metabolic availability and toxicity. Environ Int. 2007 May;33(4):576-82. doi: 10.1016/j.envint.2006.05.007. Epub 2006 Jun 30. PMID: 16814385.
- Rainbow PS. Biomonitoring of heavy metal availability in the marine environment. Mar Pollut Bull. 1995;31(1):183-192. doi: 10.1016/0025-326X(95)00116-5.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.