Environmental Sciences . 2022 April 13;3(4):327-331. doi: 10.37871/jbres1443.
A Comparative Study of Cadmium Levels in Brain Tissue of Mice Observed Over Two Generations after Exposure During their Perinatal Period: Modulation by Quercetin
Sumita Halder1*, Rajarshi Kar2, Sucharita Chakraborty3, Swapan K Bhattacharya4 and Basu D Banerjee2
2Department of Biochemistry, University College of Medical Sciences and G. T. B. Hospital, New Delhi 110095, India
3Geological Oceanographic Division, National Institute of Oceanograhy, Goa, Department of Geology and Geophysics, Indian Institute of Technology, Kharagpur, India
4Department of Pharmacology, North Delhi Municipal Corporation Medical College and Hindu Rao Hospital, New Delhi 110007, India
- Cadmium
- Perinatal period
- Quercetin
- Atomic absorption spectrophotometer
- Lactation
Abstract
The perinatal period is very critical as the embryo or the new born is more susceptible to Cd toxicity. This study was done to measure Cd levels in brain tissue of F1 and F2 generation mice whose mothers were exposed to Cd during lactation or during the entire period of gestation and lactation and also to investigate whether quercetin could modulate this effect. Dams were exposed to cadmium during lactation and during the entire perinatal period. F1 and F2 generations were reared till 100 days of age. After being sacrificed, their brains were extracted, and cadmium levels were estimated using Atomic absorption spectrophotometer. It was found that Cd levels in brain tissue were significantly higher in the F1 generation when animals were exposed in lactation. There was slight increase in Cd in brain tissue of animals exposed during gestation as well as lactation, but the change was not statistically significant. Quercetin reduced the Cd levels significantly in a dose dependent manner in lactation group. In the other two groups it reduced the Cd levels even lower than the controls. This study shows that Cd is passed on to the next generation more efficiently when exposed during lactation. Lesser transmission is seen when exposure is during gestation followed by lactation. Quercetin effectively reduces Cd levels in brain tissue irrespective of the type of exposure.
Introduction
Exposure to heavy metals and its associated harmful effects is something which the human race can no longer evade. Industrial efflux, farming products, mining and various such activities are actively contributing to the increasing heavy metal load in the environment. Cadmium is a heavy metal, whose source in the environment is from activities like manufacturing dyes, batteries, leather industry, and mining [1]. Not only those associated with these occupations are at higher risk of exposure, Cd finds its way to the general population too, through contaminated food and water [1]. Tobacco plant is known to assimilate more of this heavy metal than any other plant. Therefore, taking any form of tobacco increases the risk of Cd exposure manifold [2]. Once it enters the human body it starts accumulating in various tissues forming complexes with proteins, hence the harmful effects of Cd are seen in various organ systems, specially kidneys, liver and brain [3].
Cd has been shown to affect cognitive functions and derange neuronal development of children. The perinatal period is most critical as the embryo and the newborn is more susceptible to Cd toxicity due to lack of proper blood brain barrier [3]. There are conflicting reports about the amount of Cd that finds its way to the developing embryo through placenta. Some studies have reported Cd levels in cord blood were 50% that of maternal blood, indicating a huge load of Cd is passed on to the fetus [4]. Whereas, other studies have reported that the placenta might act as barrier [5]. Similar confusion exists in the role of breast milk causing Cd exposure in newborns. Some studies say that less cadmium is excreted in breast milk as lactation progresses [6]. However, one study has specifically shown that the level of breast milk Cd is at least 3-4 times than that of maternal blood which indicates that probably Cd is accumulated by the mammary glands [7].
Quercetin, a polyphenolic compound, is known for its antioxidant properties and has been shown to mitigate the effect of Cd [8]. Its beneficial effect on cognitive functions is even more pronounced and most studies have attributed this beneficial effect to its antioxidant properties [8,9]. It scavenges the superoxide ion directly inhibits the superoxide generating enzymes [10]. Quercetin also has shown ability to chelate heavy metals, and whether this property plays a role in modulating the Cd levels in brain needs to be evaluated [11].
A previous study in our lab has demonstrated that Cd levels attained in brain following exposure during gestation corelated with memory impairment in the subsequent generations. We had also tested that Cd exposure during lactation caused memory impairment in F1 generation [8-12]. To evaluate further, the present study was designed to measure Cd levels in brain tissue of F1 and F2 generation mice whose mothers had been exposed to Cd specifically during lactation or during the entire perinatal period. This work was meant to compare the Cd levels attained in brain tissue due to exposure during these crucial phases of the perinatal period. This study also intended to analyze the Cd levels in brain tissue when Cd and quercetin was co-administered.
Material and Methods
Animals
Animals (swiss albino mice) weighing approximately 20–25 g (8 weeks old) were used in the study. The animals were obtained from the Central Animal House of University College of Medical Sciences, Delhi. They were housed in cages in groups of three to four per cage with free access to pellet diet and water in a temperature-controlled environment (temperature:22 + 2°C, humidity: 50–55%, natural light/day cycle). Care of animals was done as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Govt. of India, New Delhi. The study was duly approved by the Institutional Animal Ethics Committee, University College of Medical Sciences, Delhi.
Preparation of animals
Nulliparous female mice were examined by vaginal cytology and their oestrous state was assessed. They were randomly divided into pairs (male and female) and were placed overnight for the purpose of mating. The start of gestation (i.e day 1 of gestation) was confirmed by presence of spermatozoa in vaginal smears on the subsequent morning. The impregnated female animals were then housed individually in separate breeding cages. The animals were thereafter divided into different groups.
For animals to be exposed during lactation period (first 7 days), following delivery the dams were divided into groups and treated with the respective drugs for 7 days. Following delivery, the F1 generation mice were reared till 100 days age and the brain tissue were collected. The F1 generation were also crossed among themselves to produce the F2 generation mice which were again raised till 100 days for collection of brain tissue.
For animals to be exposed during the entire perinatal period (last 7 days of gestation + first 7 days of lactation), the dams were divided into groups and treated with the respective drugs for 7 days during gestation (day 14-day21) and for one week during lactation (first 7 days). F1 and F2 animals were reared same as above to collect the brain tissue.
Plant material
Quercetin was purchased from M/s Sigma Aldrich (Product no. Q4952 CAS no.117-39-5, dated 8-03-2010) in the form of yellow amorphous powder. As per the literature provided by the manufacturer, the purity of the product as assessed by HPLC was found to be 98%. For the study, the quercetin powder was suspended in double distilled water to prepare solutions of the required doses (25, 50, 100 mg/kg). The suspension was made homogenous using an ultrasonic sonicator before administering each dose.
Drugs and dosing schedule
Cadmium chloride (M/s Sigma Aldrich, product no. 230723) was dissolved in distilled water (or vehicle) and was administered daily in dosages of 1.2 mg/kg/day intraperitoneally (i.p.). Quercetin was administered in doses of 25, 50, and 100 mg/kg/day, i.p. Control groups receiving equal volume of vehicle were run for different treatment groups. Thereafter, drug-dosing was stopped, and the newborn pups were kept with their dams for feeding. For the lactation group, animals in different groups, were treated with the drugs/vehicles for the first 7 days. Animals which were to be treated during the entire perinatal period were administered drugs/vehicles for total 14 days (7 days in gestation + 7 days in lactation). On Postnatal Day 21 (PND21) the litter mates were weaned, separated, housed together by sex, and were grouped in same laboratory conditions as their parents.
Estimation of cadmium in brain tissue
About 0.5 ml of homogenized brain tissue in phosphate buffer, was taken in triplicate. The homogenized tissue samples were taken in 100 ml digestion flask fitted with 30 cm long air condenser and 5.0 ml distilled nitric acid (HNO3) was added to each sample. The contents were heated at 80°C for 30 min. After cooling, 1.5 ml of concentrated perchloric acid (HClO4) (70%) was added and sample was heated again at 250°C with occasional shaking till white fumes evolved. Clear solution was cooled and transferred into a 10 ml measuring flask. The volume was made up to 10 ml with deionized water. Thus, obtained sample was filtered by using syringe filter of 0.45 micron pore size (RFCL Ltd, New Delhi) and the concentration of Cd was analyzed by Graphite Furnace Atomic Absorption Spectrophotometer (GFAAS) at CSIR-National Institute of Oceanography, Dona Paula, Goa (Model: PinAAcle 900T Manufacturer: Perkin Elmer, Country, Singapore). The analytical protocol of the measurement of Cd in GFAAS has been described previously [13,14].
Statistical analysis
Results of the above experiments were expressed as mean ± Standard Error of Mean (SEM), and the difference between means was analyzed by Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparisons test.
Results
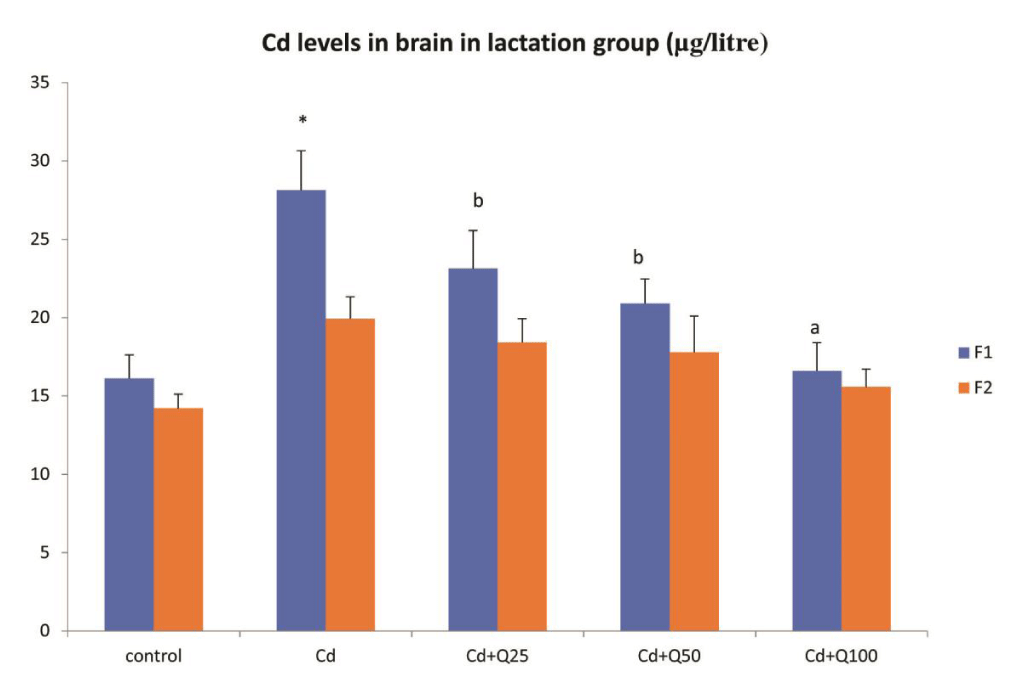
Cd levels in brain tissue in F1 and F2 generation when exposed during lactation period
Cadmium levels in brain tissue were significantly higher in F1 generation mice which were administered Cd alone, when compared to the control group (Figure 1). When Cd was administered along with quercetin, there was a significant decrease in Cd levels in F1 generation mice in a dose dependent manner when compared with Cd only group (Figure 1). However, the levels were still higher than control. Even though a similar trend was observed in F2 generation the difference was not statistically significant.
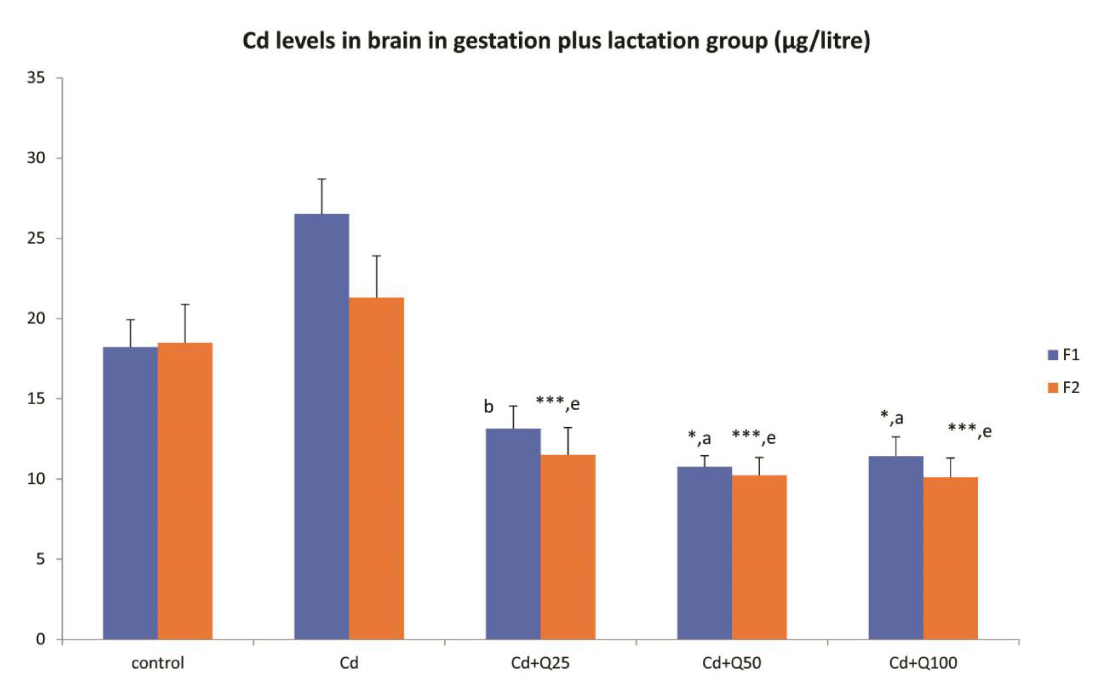
Cd levels in brain tissue in F1 and F2 generation when exposed during entire perinatal period
When exposed to Cd during the entire perinatal period, a similar trend was observed as seen in the gestation only group (Figure 2). The Cd levels in the group exposed to Cd only was not significantly higher than controls in both F1 and F2 generation. Co-treatment with Quercetin however brought about a significant decrease in Cd levels in both F1 and F2 generations in a dose independent manner (Figure 2).
Discussion
Cd exposure during the perinatal period is most hazardous as the embryo and the newborn has a poorly developed blood brain barrier. Therefore, they are at higher risk of neuronal damage caused by Cd toxicity [15]. Cd induced neuronal damage is more pronounced due to its ability to interfere with the signal transduction, increase oxidative stress, induce apoptosis in embryonic cells and even causing epigenetic changes which can be passed on to the next generation [5-15]. As there are conflicting reports about role of placental transfer and breast milk in passing on Cd to the next generation, this study was done to measure Cd levels in brain tissue of F1 and F2 generation mice whose dams were exposed to Cd during lactation alone or during the cumulative period of late gestation and early lactation. Our previous studies have shown that Cd is passed on to the next generation during gestation [8]. We have also observed in our lab that Cd exposure during lactation caused memory impairment in F1 generation [12]. Therefore, this comparative study focused mainly on the effect of exposure during lactation and compared it with that of exposure during entire perinatal period. As Cd is known to accumulate in various tissues and stay for long time it was imperative to analyze the level of Cd in the second generation as well. This was to evaluate whether the F1 generation which received Cd only through maternal sources could pass on the Cd to F2 generation as well.
In this study, Cd levels in brain tissue were significantly higher in the F1 generation of lactation only group. The values achieved were higher than when the dams were exposed during gestation only [8]. This indicates that Cd is passed on to the next generation most effectively when exposed during lactation period. The lower levels seen when exposed during gestation in our previous study [8] supports the earlier studies which stated that placenta acts as some sort of barrier for Cd [3-16], whereas, higher concentrations are achieved in breast milk [5-7]. However, it becomes difficult to explain why there is no significant increase in Cd levels in the group exposed during the whole perinatal period as this group was exposed for a longer period and should have at least achieved levels seen in lactation only group.
Studies have shown that Cd induces expression of various groups of metallothionein which can bind Cd and sequester it in various tissues. Placenta being an important site of metallothionein synthesis may offer protection to the growing fetus from maternal Cd levels [3-17]. Even though mammary glands also synthesize metallothionein, findings from this study indicate placenta plays a far more important role. That could also explain why higher levels were seen in lactation only group as compared to the gestation plus lactation group. As already stated, it was expected that the Cd levels in brain tissue of mice whose dams were administered Cd through the cumulative period of gestation as well as lactation should have been at least equal to the F1 generation in lactation only group if not more. One plausible explanation for the same could be that in the gestation plus lactation group the exposure was started when placenta was developing and the metallothionein synthesized by the placenta offered protection during the lactation period as well.
The significant difference in Cd levels in F1 and F2 generation seen only in lactation group further proves that the increase in Cd in this group was probably due to maternal exposure. The F1 generation did not pass on the Cd to the F2 generation. Therefore, it can be assumed that the Cd accumulated by the F2 generation was from environmental sources, probably the water supplied ad libitum. The Cd levels in the other two groups showed no difference in F1 and F2 generation irrespective of whether it was control, Cd only or in groups where Cd and quercetin was co-administered indicating environmental accumulation contributed more than maternal transmission.
Quercetin was able to reduce the Cd levels significantly in a dose dependent manner in lactation group. In the other two groups it reduced the Cd levels even lower than the controls. This shows that the chelating properties of quercetin may play a role in reducing tissue levels of cadmium [18]. A study on chitosan, another chelating agent showed that it was able to reduce accumulation of Cd in rat tissues [19]. This aspect of quercetin has not been investigated yet as most studies focus on its antioxidant role. Cd is difficult to excrete and keeps accumulating in various tissues and organ systems. The fact that Cd was lower than controls indicate that quercetin may also play a role in Cd excretion which needs to be further evaluated. However, it is evident from this study that the protection of cognitive function is not due to antioxidant function of quercetin alone, but modulation of brain tissue levels also contributes to the same.
The limitation of the study is that we have not evaluated metallothionines in these samples and hope to address that in our future studies. Nevertheless, this study shows that Cd is passed on to the next generation more efficiently when exposed during lactation. Lesser transmission is seen when exposure is during gestation [8] or during the whole perinatal period (gestation followed by lactation). Quercetin effectively reduces Cd levels in brain tissue irrespective of the type of exposure.
Acknowledgment
The authors are grateful to the Metal Speciation Lab at CSIR, National Institute of Oceanography, Dona Paula, Goa, India for the estimation of metals in the samples.
Availability of data and material
All study data are available at request- contact to corresponding author.
Consent for publication
All authors gave their consent for publication.
Ethics approval
The study protocol was approved by the Institutional Animal Ethics Committee and has been carried out in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
Author contribution statement
SH and RK conceived and designed research, analyzed data and wrote the manuscript. SC conducted experiments to estimate the cadmium levels. SKB and BDB supervised the conduct of all experiments as well as contributed to new research and analytical tools. All authors read and approved the manuscript.
References
- Mezynska M, Brzóska MM. Environmental exposure to cadmium-a risk for health of the general population in industrialized countries and preventive strategies. Environ Sci Pollut Res Int. 2018 Feb;25(4):3211-3232. doi: 10.1007/s11356-017-0827-z. Epub 2017 Dec 12. PMID: 29230653.
- Richter P, Faroon O, Pappas RS. Cadmium and Cadmium/Zinc Ratios and Tobacco-Related Morbidities. Int J Environ Res Public Health. 2017 Sep 29;14(10):1154. doi: 10.3390/ijerph14101154. PMID: 28961214; PMCID: PMC5664655.
- Espart A, Artime S, Tort-Nasarre G, Yara-Varón E. Cadmium exposure during pregnancy and lactation: materno-fetal and newborn repercussions of Cd(ii), and Cd-metallothionein complexes. Metallomics. 2018 Oct 17;10(10):1359-1367. doi: 10.1039/c8mt00174j. PMID: 30221266.
- García-Esquinas E, Pérez-Gómez B, Fernández-Navarro P, Fernández MA, de Paz C, Pérez-Meixeira AM, Gil E, Iriso A, Sanz JC, Astray J, Cisneros M, de Santos A, Asensio Á, García-Sagredo JM, García JF, Vioque J, López-Abente G, Pollán M, González MJ, Martínez M, Aragonés N. Lead, mercury and cadmium in umbilical cord blood and its association with parental epidemiological variables and birth factors. BMC Public Health. 2013 Sep 12;13:841. doi: 10.1186/1471-2458-13-841. PMID: 24028648; PMCID: PMC3848449.
- Rebelo FM, Caldas ED. Arsenic, lead, mercury and cadmium: Toxicity, levels in breast milk and the risks for breastfed infants. Environ Res. 2016 Nov;151:671-688. doi: 10.1016/j.envres.2016.08.027. Epub 2016 Sep 10. PMID: 27619212.
- Chao HH, Guo CH, Huang CB, Chen PC, Li HC, Hsiung DY, Chou YK. Arsenic, cadmium, lead, and aluminium concentrations in human milk at early stages of lactation. Pediatr Neonatol. 2014 Apr;55(2):127-34. doi: 10.1016/j.pedneo.2013.08.005. Epub 2013 Nov 11. PMID: 24231114.
- Kippler M, Lönnerdal B, Goessler W, Ekström EC, Arifeen SE, Vahter M. Cadmium interacts with the transport of essential micronutrients in the mammary gland - a study in rural Bangladeshi women. Toxicology. 2009 Mar 4;257(1-2):64-9. doi: 10.1016/j.tox.2008.12.009. Epub 2008 Dec 14. PMID: 19126424.
- Halder S, Kar R, Chakraborty S, Bhattacharya SK, Mediratta PK, Banerjee BD. Cadmium level in brain correlates with memory impairment in F1 and F2 generation mice: improvement with quercetin. Environ Sci Pollut Res Int. 2019 Apr;26(10):9632-9639. doi: 10.1007/s11356-019-04283-2. Epub 2019 Feb 7. PMID: 30734250.
- Yuan Y, Ma S, Qi Y, Wei X, Cai H, Dong L, Lu Y, Zhang Y, Guo Q. Quercetin inhibited cadmium-induced autophagy in the mouse kidney via inhibition of oxidative stress. J Toxicol Pathol. 2016 Oct;29(4):247-252. doi: 10.1293/tox.2016-0026. Epub 2016 Aug 18. PMID: 27821909; PMCID: PMC5097967.
- Sanhueza J, Valdes J, Campos R, Garrido A, Valenzuela A. Changes in the xanthine dehydrogenase/xanthine oxidase ratio in the rat kidney subjected to ischemia-reperfusion stress: preventive effect of some flavonoids. Res Commun Chem Pathol Pharmacol. 1992 Nov;78(2):211-8. PMID: 1475527.
- Primikyri A, Mazzone G, Lekka C, Tzakos AG, Russo N, Gerothanassis IP. Understanding zinc(II) chelation with quercetin and luteolin: a combined NMR and theoretical study. J Phys Chem B. 2015 Jan 8;119(1):83-95. doi: 10.1021/jp509752s. Epub 2014 Dec 17. PMID: 25486072.
- Halder S, Kar R, Galav V, Mehta AK, Bhattacharya SK, Mediratta PK, Banerjee BD. Cadmium exposure during lactation causes learning and memory-impairment in F1 generation mice: amelioration by quercetin. Drug Chem Toxicol. 2016;39(3):272-8. doi: 10.3109/01480545.2015.1092042. Epub 2015 Oct 7. PMID: 26446883.
- Chakraborty P, Ramteke D, Chakraborty S, Chennuri K, Bardhan P. Relationship between the lability of sediment-bound Cd and its bioaccumulation in edible oyster. Mar Pollut Bull. 2015 Nov 15;100(1):344-351. doi: 10.1016/j.marpolbul.2015.08.027. Epub 2015 Sep 8. PMID: 26359116.
- Chakraborty P, Ramteke D, Gadi SD, Bardhan P. Linkage between speciation of Cd in mangrove sediment and its bioaccumulation in total soft tissue of oyster from the west coast of India. Mar Pollut Bull. 2016 May 15;106(1-2):274-82. doi: 10.1016/j.marpolbul.2015.12.025. Epub 2016 Feb 10. PMID: 26874748.
- Wang B, Du Y. Cadmium and its neurotoxic effects. Oxid Med Cell Longev. 2013;2013:898034. doi: 10.1155/2013/898034. Epub 2013 Aug 12. PMID: 23997854; PMCID: PMC3753751.
- Gundacker C, Hengstschläger M. The role of the placenta in fetal exposure to heavy metals. Wien Med Wochenschr. 2012 May;162(9-10):201-6. doi: 10.1007/s10354-012-0074-3. PMID: 22717874.
- Goyer RA, Cherian MG. Role of metallothionein in human placenta and rats exposed to cadmium. IARC Sci Publ. 1992;(118):239-47. PMID: 1303947.
- Ravichandran R, Rajendran M, Devapiriam D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014 Mar 1;146:472-8. doi: 10.1016/j.foodchem.2013.09.080. Epub 2013 Sep 23. PMID: 24176370.
- Kim MY, Shon WJ, Park MN, Lee YS, Shin DM. Protective effect of dietary chitosan on cadmium accumulation in rats. Nutr Res Pract. 2016 Feb;10(1):19-25. doi: 10.4162/nrp.2016.10.1.19. Epub 2015 Dec 11. PMID: 26865912; PMCID: PMC4742306.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.